Pilot Study of Aerosolised Plus Intravenous Vancomycin in Mechanically Ventilated Patients with Methicillin-Resistant Staphylococcus Aureus Pneumonia
Abstract
1. Introduction
2. Materials and Methods
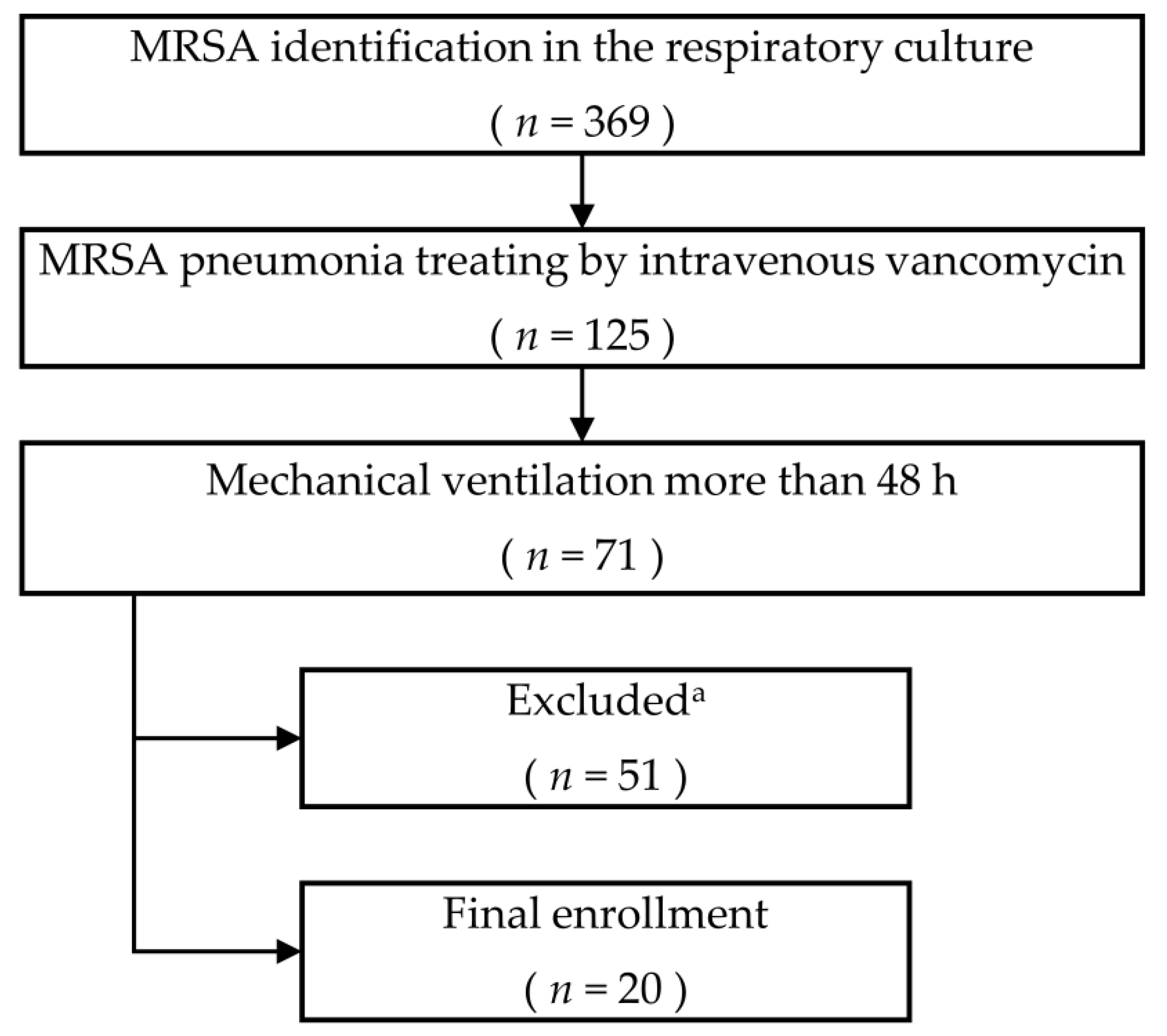
2.1. Patients and Settings
2.2. Intervention
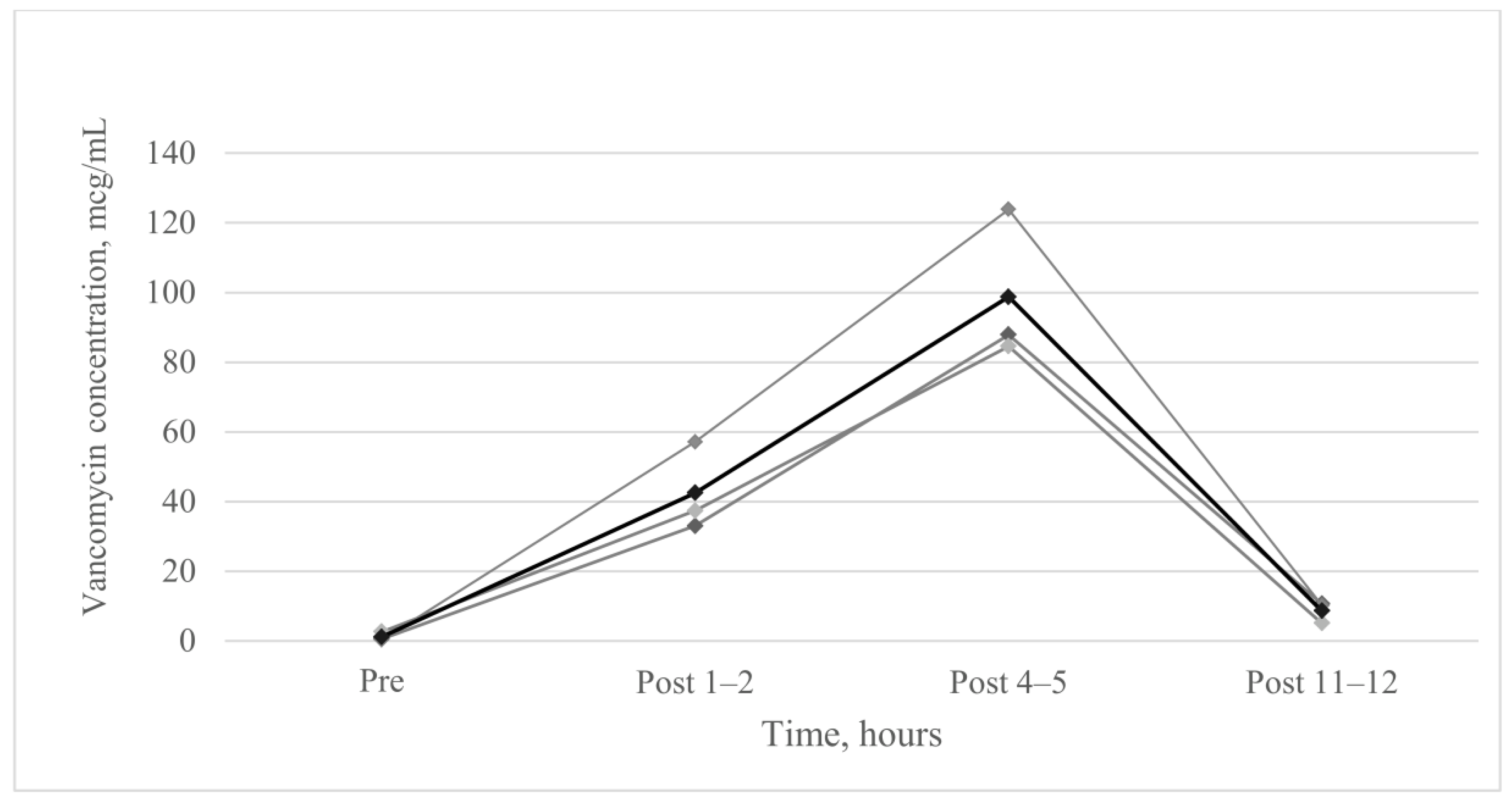
2.3. Vancomycin Concentration of Epithelial Lining Fluid
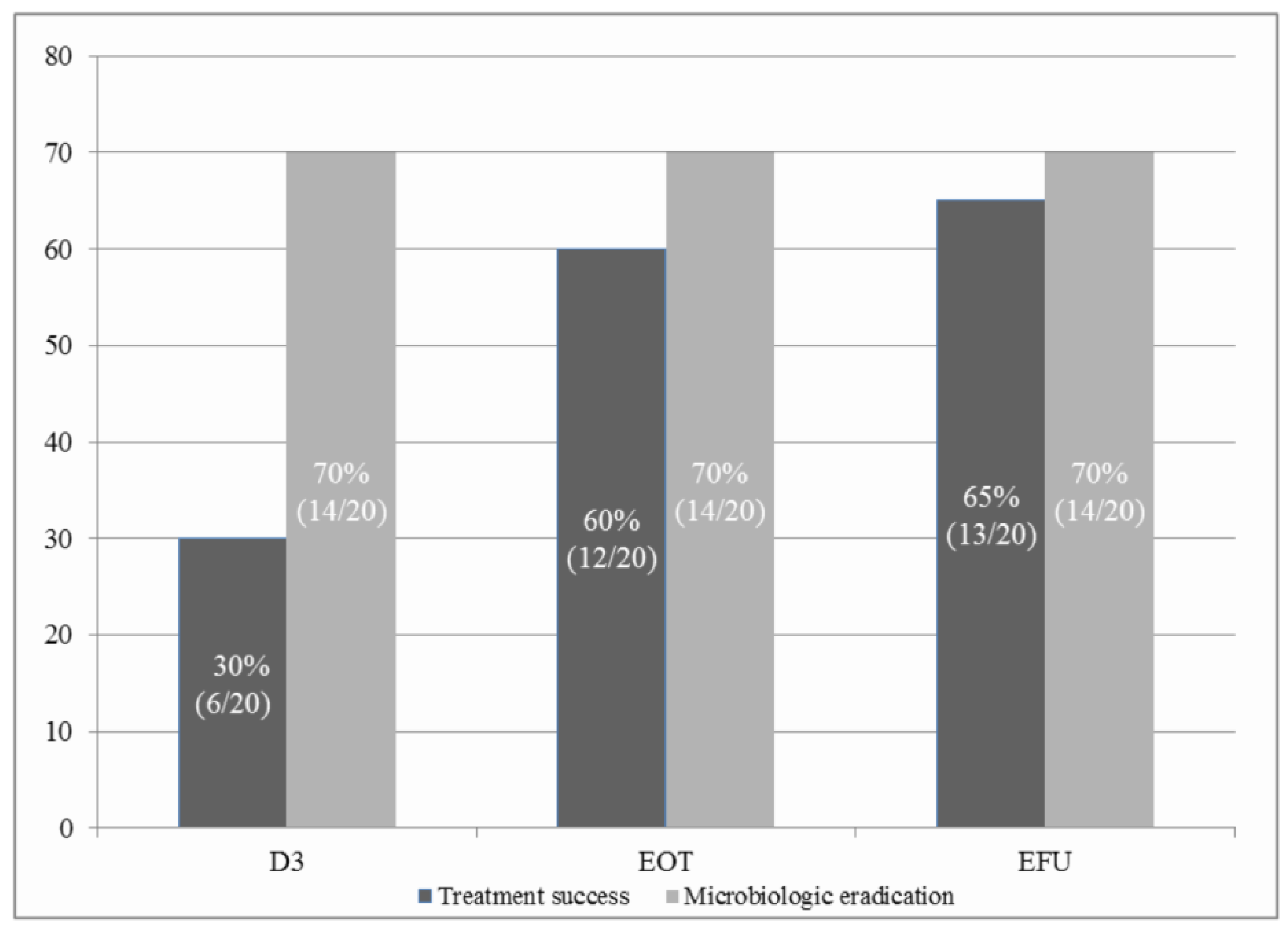
2.4. Outcomes and Follow-Up
2.5. Monitoring Side Effects
2.6. Statistical Analysis
2.7. Ethical Approval and Consent to Participate
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kalil, A.C.; Metersky, M.L.; Klompas, M.; Muscedere, J.; Sweeney, D.A.; Palmer, L.B.; Napolitano, L.M.; O’grady, N.P.; Bartlett, J.G.; Carratalà, J. Management of adults with hospital-acquired and ventilator-associated pneumonia: 2016 clinical practice guidelines by the Infectious Diseases Society of America and the American Thoracic Society. Clin. Infect. Dis. 2016, 63, e61–e111. [Google Scholar] [CrossRef] [PubMed]
- Kollef, M.H.; Shorr, A.; Tabak, Y.P.; Gupta, V.; Liu, L.Z.; Johannes, R. Epidemiology and outcomes of health-care–associated pneumonia: Results from a large US database of culture-positive pneumonia. Chest 2005, 128, 3854–3862. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zou, Y.; Xie, J.; Wang, T.; Zheng, X.; He, H.; Dong, W.; Xing, J.; Dong, Y. Linezolid versus vancomycin for the treatment of suspected methicillin-resistant Staphylococcus aureus nosocomial pneumonia: A systematic review employing meta-analysis. Eur. J. Clin. Pharmacol. 2015, 71, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Hidayat, L.K.; Hsu, D.I.; Quist, R.; Shriner, K.A.; Wong-Beringer, A. High-dose vancomycin therapy for methicillin-resistant Staphylococcus aureus infections: Efficacy and toxicity. Arch. Intern. Med. 2006, 166, 2138–2144. [Google Scholar] [CrossRef] [PubMed]
- Lamer, C.; De Beco, V.; Soler, P.; Calvat, S.; Fagon, J.; Dombret, M.; Farinotti, R.; Chastre, J.; Gibert, C. Analysis of vancomycin entry into pulmonary lining fluid by bronchoalveolar lavage in critically ill patients. Antimicrob. Agents Chemother. 1993, 37, 281–286. [Google Scholar] [CrossRef] [PubMed]
- Cruciani, M.; Gatti, G.; Lazzarini, L.; Furlan, G.; Broccali, G.; Malena, M.; Franchini, C.; Concia, E. Penetration of vancomycin into human lung tissue. J. Antimicrob. Chemother. 1996, 38, 865–869. [Google Scholar] [CrossRef] [PubMed]
- Kalil, A.C.; Murthy, M.H.; Hermsen, E.D.; Neto, F.K.; Sun, J.; Rupp, M.E. Linezolid versus vancomycin or teicoplanin for nosocomial pneumonia: A systematic review and meta-analysis. Crit. Care Med. 2010, 38, 1802–1808. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Berra, L.; Klompas, M. Should aerosolized antibiotics be used to treat ventilator-associated pneumonia? Respir. Care 2016, 61, 737–748. [Google Scholar] [CrossRef] [PubMed]
- Wenisch, C.; Laferl, H.; Szell, M.; Smolle, K.; Grisold, A.; Bertha, G.; Krause, R. A holistic approach to MRSA eradication in critically ill patients with MRSA pneumonia. Infection 2006, 34, 148–154. [Google Scholar] [CrossRef] [PubMed]
- Gradon, J.D.; Wu, E.H.; Lutwick, L.I. Aerosolized vancomycin therapy facilitating nursing home placement. Ann. Pharmacother. 1992, 26, 209–210. [Google Scholar] [CrossRef] [PubMed]
- Hayes, D., Jr.; Murphy, B.S.; Mullett, T.W.; Feola, D.J. Aerosolized vancomycin for the treatment of MRSA after lung transplantation. Respirology 2010, 15, 184–186. [Google Scholar] [CrossRef] [PubMed]
- Weathers, L.; Riggs, D.; Santeiro, M.; Weibley, R.E. Aerosolized vancomycin for treatment of airway colonization by methicillin-resistant Staphylococcus aureus. Pediatric Infect. Dis. J. 1990. [Google Scholar] [CrossRef] [PubMed][Green Version]
- de Jesus Valle, M.J.; Lopez, F.G.; Hurle, A.D.; Navarro, A.S. Pulmonary versus systemic delivery of antibiotics: Comparison of vancomycin dispositions in the isolated rat lung. Antimicrob. Agents Chemother. 2007, 51, 3771–3774. [Google Scholar] [CrossRef] [PubMed]
- Pugin, J.; Auckenthaler, R.; Mili, N.; Janssens, J.-P.; Lew, P.D.; Suter, P.M. Diagnosis of ventilator-associated pneumonia by bacteriologic analysis of bronchoscopic and nonbronchoscopic “blind” bronchoalveolar lavage fluid. Am. Rev. Respir. Dis. 1991, 143, 1121–1129. [Google Scholar] [CrossRef] [PubMed]
- Wunderink, R.G.; Niederman, M.S.; Kollef, M.H.; Shorr, A.F.; Kunkel, M.J.; Baruch, A.; McGee, W.T.; Reisman, A.; Chastre, J. Linezolid in methicillin-resistant Staphylococcus aureus nosocomial pneumonia: A randomized, controlled study. Clin. Infect. Dis. 2012, 54, 621–629. [Google Scholar] [CrossRef] [PubMed]
- Jennings, M.; Boyle, M.; Bucur, C.; Konstan, M.; Dasenbrook, E. Pharmacokinetics and safety of inhaled vancomycin in patients with cystic fibrosis. Pediatr. Pulmonol. 2012, 47, 320. [Google Scholar]
- Wunderink, R.G.; Rello, J.; Cammarata, S.K.; Croos-Dabrera, R.V.; Kollef, M.H. Linezolid vs vancomycin*: Analysis of two double-blind studies of patients with methicillin-resistant Staphylococcus aureus nosocomial pneumonia. Chest 2003, 124, 1789–1797. [Google Scholar] [CrossRef]
| Variables | Total (n = 20) |
|---|---|
| Age, years | 74.7 ± 8.9 |
| Sex, male | 13 (65) |
| Co-morbidities | |
| Hypertension | 9 (45) |
| Diabetes mellitus | 6 (30) |
| Dyslipidaemia | 2 (10) |
| Dementia | 2 (10) |
| Parkinson’s disease | 3 (15) |
| Stroke or haemorrhage | 5 (25) |
| Ischaemic heart diseases | 4 (20) |
| History of malignancy, not active | 7 (35) |
| History of orthopaedic surgery | 4 (20) |
| Pneumonia type | |
| Community acquired pneumonia | 2 (10) |
| Healthcare related acquired pneumonia | 3 (15) |
| Hospital-acquired pneumonia | 11 (55) |
| Ventilator-associated pneumonia | 4 (20) |
| MRSA bacteraemia | 4 (20) |
| Gram-negative bacteria respiratory infection | 13 (65) |
| Bronchoalveolar lavage | 14 (70) |
| APACHE II score | 21.8 ± 7.0 |
| SOFA score | 7.0 ± 3.6 |
| CPISa | 7.8 ± 1.2 |
| Median MIC, mcg/mL (range) | 1.0 (0.75-1.0) |
| Total duration of intravenous vancomycin, days | 15.5 ± 6.2 |
| Extracorporeal membrane oxygenation | 3 (15) |
| Renal replacement therapy | 7 (35) |
| Tracheostomy | 17 (85) |
| Outcomes | Total (n = 20) |
|---|---|
| Mortality | |
| In-intensive care unit | 6 (30) |
| In-hospital | 7 (35) |
| 28-day | 5 (25) |
| 90-day | 7 (35) |
| Ventilator-free day a | 11.0 ± 10.0 |
| Adverse events (any causes) | 2 (10) |
| Systemic | 0 |
| Localised b | 2 (10) |
| Unexpected events | 3 (15) |
| Death due to Gram-negative sepsis | 1 (5) |
| Mechanical airway obstruction | 1 (5) |
| Accidental T-cannula dislodgement | 1 (5) |
| Emergence of vancomycin-resistant strains | 3 (15) |
| Vancomycin intermediate Staphylococcus aureus c | 1 (5) |
| Vancomycin-resistant Enterococcus d | 2 (10) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cho, J.Y.; Kim, H.-S.; Yang, H.-J.; Lee, Y.J.; Park, J.S.; Yoon, H.I.; Kim, H.B.; Yim, J.-J.; Lee, J.-H.; Lee, C.-T.; et al. Pilot Study of Aerosolised Plus Intravenous Vancomycin in Mechanically Ventilated Patients with Methicillin-Resistant Staphylococcus Aureus Pneumonia. J. Clin. Med. 2020, 9, 476. https://doi.org/10.3390/jcm9020476
Cho JY, Kim H-S, Yang H-J, Lee YJ, Park JS, Yoon HI, Kim HB, Yim J-J, Lee J-H, Lee C-T, et al. Pilot Study of Aerosolised Plus Intravenous Vancomycin in Mechanically Ventilated Patients with Methicillin-Resistant Staphylococcus Aureus Pneumonia. Journal of Clinical Medicine. 2020; 9(2):476. https://doi.org/10.3390/jcm9020476
Chicago/Turabian StyleCho, Jun Yeun, Hyung-Sook Kim, Hye-Joo Yang, Yeon Joo Lee, Jong Sun Park, Ho Il Yoon, Hong Bin Kim, Jae-Joon Yim, Jae-Ho Lee, Choon-Taek Lee, and et al. 2020. "Pilot Study of Aerosolised Plus Intravenous Vancomycin in Mechanically Ventilated Patients with Methicillin-Resistant Staphylococcus Aureus Pneumonia" Journal of Clinical Medicine 9, no. 2: 476. https://doi.org/10.3390/jcm9020476
APA StyleCho, J. Y., Kim, H.-S., Yang, H.-J., Lee, Y. J., Park, J. S., Yoon, H. I., Kim, H. B., Yim, J.-J., Lee, J.-H., Lee, C.-T., & Cho, Y.-J. (2020). Pilot Study of Aerosolised Plus Intravenous Vancomycin in Mechanically Ventilated Patients with Methicillin-Resistant Staphylococcus Aureus Pneumonia. Journal of Clinical Medicine, 9(2), 476. https://doi.org/10.3390/jcm9020476





