1. Introduction
Catheter ablation is increasingly indicated in patients with atrial fibrillation (AF) [
1]. This procedure carries a transient but high thromboembolic risk dominated by strokes requiring anticoagulation. This additional anticoagulation leads to an increased risk of major bleeding, including hemopericardium (trans-septal puncture-related or not) and vascular access complications. Guidelines now recommend to perform AF catheter ablation with uninterrupted oral anticoagulation and to administer unfractionated heparin (UFH) intravenously after the trans-septal puncture. To control thrombotic and bleeding risks, and because response to UFH is highly variable, UFH effects must be closely monitored to achieve and maintain an activated clotting time (ACT) equal or greater than 300 s throughout the procedure [
2].
For patients receiving vitamin K antagonist (VKA), both UFH monitoring based on the ACT, a validated whole blood coagulation assay, and the ACT target at 300 s are supported by robust evidence [
3]. A clear relationship is demonstrated between ACT values, which reflect the level of anticoagulation resulting from both VKA and UFH, and outcomes. A narrow therapeutic window justifies careful monitoring: ACT < 250 s is associated with stroke whereas increasing values beyond ACT ≥ 300 s progressively increases the incidence of bleeding events [
4,
5]. For patients receiving non-VKA oral anticoagulant (DOAC), anticoagulation management has been extrapolated from VKA experience, without additional specific evidence. Recent open-labelled randomized controlled trials (RCTs) performed in patients with uninterrupted DOAC treatment reported a similar incidence of thromboembolism and bleeding events as for VKA [
6,
7,
8,
9]. However, until now, few studies have specifically addressed intra-procedural anticoagulation management. They have all uniformly reported that an unexplained greater amount of UFH was required to achieve a similar ACT target in patients with uninterrupted DOAC, compared to patients receiving VKA [
6,
8,
9,
10,
11,
12]. The management of these procedures cannot safely be resolved without addressing this simple question: can we transpose intra-procedural anticoagulation strategy from VKA to DOACs? To answer this question, we performed an ex-vivo study using samples from patients receiving either DOAC or VKA and untreated-patients. We assessed (1) the relationship between ACT value and the level of anticoagulation, and (2) the effect of spiked fixed UFH doses on ACT prolongation according to the anticoagulant on board.
2. Methods
The ACT Apixaban-Rivaroxaban-Dabigatran (ACTARD) study was an ex vivo study, carried out from June 2016 to February 2018 at Hôpital d’Instruction des Armées Percy, France. The study was approved by the ethical committee (Comité de Protection des Personnes Ile de France V, ref. 15053) and registered at
ClinicalTrials.gov (
NCT02839434). All patients were informed before inclusion and the physician in charge of the patient signed the non-opposition form.
2.1. Patients and Study Design
The physician in charge of the study prospectively enrolled patients receiving uninterrupted apixaban, rivaroxaban, or dabigatran at any dose approved for non-valvular AF and who required a blood sampling in their clinical management. Two other groups were considered, namely VKA-treated AF patients with therapeutic INR, and patients free from any anticoagulant treatment. Patients with co-medication such as antiplatelet agents, or parenteral anticoagulants were excluded.
Blood was collected into haemolysis tubes immediately spiked ex vivo with UFH (heparin calcium Choay
® 25 000 UI/5 mL) for a final concentration of 0.2 IU/mL corresponding to 1000 IU UFH intravenous bolus administration in vivo (
Figure S1). After 5-minute incubation at room temperature, ACT testing (UFH 0.2-ACT) was performed. Then, UFH was added in previous blood samples to achieve a final UFH concentration of 0.5 IU/mL, corresponding to a 2500 IU UFH bolus administration in vivo and ACT testing (UFH 0.5-ACT) was performed after 5 minute-incubation. Similar protocol was applied for 1.0 and 2.0 IU/mL UFH final concentration, corresponding to 5000 and 10000 IU bolus administration in vivo respectively (UFH 1-ACT and UFH 2-ACT). At the end of the sampling, a drop of blood free from UFH was immediately tested to assess baseline ACT. In addition, blood was collected into 109 mM citrate tube and was centrifuged within 2 h to obtain plasma for coagulation testing.
2.2. Coagulation Testing
Coagulation testing included measurement of DOAC concentration for patients receiving apixaban, rivaroxaban and dabigatran or INR for VKA-treated patients. DOAC concentrations were measured using specific commercial assays, dedicated calibrators and controls in accordance with manufacturer recommendations. Quantitative determination of apixaban and rivaroxaban concentrations was assessed with an anti-Xa chromogenic assay, STA-Liquid-anti-Xa® (Stago) and the concentration of dabigatran was measured using the ecarin-based chromogenic assay, STA-ECA II® (Stago). These specific dosages have been validated for the accurate measurement of drugs over a wide range of concentrations up to 500 ng/mL and over 500 ng/mL after plasma dilution. The lower limit of quantification was of 25 ng/mL for apixaban and rivaroxaban measurements and 15 ng/mL for dabigatran measurement. PT was performed according to manufacturer instructions using Neoplastin® CI Plus (Stago) and expressed in ratio (patient PT (s)/normal pool plasma PT (s)) using local normal pool plasma. International Normalized Ratio (INR) was calculated as (PT ratio)ISI using manufacturer-provided generic ISI values (ISI = 1.26).
2.3. Activated Clotting Time
Activated clotting time was measured on whole blood using the point of care Hemochron® Jr. Signature+, with “low range” cartridges (ACT-LR), in accordance with manufacturer’s instructions and performed by a trained physician. Briefly, 15 µL of blood drawn by a 23-gauge catheter were dropped in the cuvette. After mixing with the reagent composed of celite, kaolin, phospholipids, stabilizers and buffer, the sample is moved at a predetermined rate within the test channel and monitored for clot formation by LED optical detectors. When the blood clots, the flow is impeded. This reduction in flow below a predetermined value signals to the instrument that a clot has formed. An internal timer measures the elapsed time between the start of the test and the clot formation, expressed in seconds. The relationship between ACT values and UFH concentrations is linear up to 2.5 IU/mL of blood. The ACT measurement range is 60 to 400 s. If the ACT result is higher than 400 s, an “Out of range-Hi” message is indicated. In these cases, ACT values were coded as equal to 400 s, for the purpose of statistical analysis.
2.4. Data Collection
For each patient, age and weight were collected as well as the type, dose and regimen of oral anticoagulation, and the time of the last DOAC intake. Coagulation test results were reported, including apixaban, rivaroxaban or dabigatran concentration for DOAC-treated patients, or INR for VKA-treated patients. Renal function parameters including creatinine level and creatinine clearance according to the Cockcroft–Gault formula were obtained. For all the samples, baseline ACT, UFH 0.2-ACT, UFH 0.5-ACT, UFH 1-ACT, UFH 2-ACT were recorded. ACT value equal or greater than 300 s was selected as the ACT target, in accordance with international guidelines [
1,
2].
2.5. Statistical Analysis
Continuous variables are expressed as mean (standard deviation, SD). Normality of distribution was assessed using histograms and the Shapiro-Wilk test. In blood samples of patients treated by oral anticoagulants, we evaluated the correlation of ACT with DOAC concentration or INR at baseline by calculating Spearman’s rank correlation coefficients in each oral anticoagulant group (apixaban, rivaroxaban, dabigatran, and VKA). We compared the ACT values assessed at baseline between the 5 study groups (controls, apixaban-, rivaroxaban-, dabigatran, and VKA-treated patients) using one-way analysis of variance (ANOVA); post-hoc pairwise comparisons were adjusted for multiple comparisons by Holm-Bonferroni method. We assessed the effect of increasing UFH doses on ACT values in each study group separately using linear mixed models with a random intercept to account the correlation between samples obtained within the same patients; UFH dose was included as fixed effect. We also used a linear mixed model with a random intercept to compare the ACT changes from samples with UFH to samples without UFH between the 4 oral anticoagulant groups (apixaban, rivaroxaban, dabigatran, and VKA); in this model, treatment group, UFH doses and treatment group*UFH doses interaction were included as fixed effects. Post-hoc comparisons between oral anticoagulant groups at given UFH doses were done using linear contrast after multiple comparison adjustment by Holm-Bonferroni method. Finally, the rate of blood samples with an ACT ≥ 300 s were compared between oral anticoagulant groups for each UFH dose by using the Chi-Square test. Statistical testing was conducted at the two-tailed α-level of 0.05. Data were analyzed using the SAS software version 9.4 (SAS Institute, Cary, NC, USA).
4. Discussion
Our study provides new evidence that intra-procedural anticoagulation management for patients undergoing AF catheter ablation with uninterrupted oral anticoagulants cannot be transposed from VKA to all DOAC-treated patients. Our findings demonstrate that ACT monitoring of UFH cannot be applied to patients receiving FXa inhibitors, especially apixaban. Until now, very few studies have addressed the issue of intra-procedural anticoagulation during AF catheter ablation [
10,
11,
12]. They mainly concluded that DOAC-treated patients often require a greater amount of UFH to achieve the ACT target, compared to VKA-treated patients. However, in these studies, pre-procedural DOAC management was heterogeneous, basal ACT was not measured and the effects of a fixed UFH dose were not compared between anticoagulant groups. All these are strong caveats to discover the determinants of intra-procedural UFH differences according to the oral anticoagulant on board. To overcome these limits, we proposed an ex-vivo model analyzing the ACT dose-response to UFH according to the different oral anticoagulants present at baseline. This was achieved using fixed UFH doses, according to a standardized protocol, in plasma samples obtained from patients representative of “real life”, that is, taking VKA or DOAC without interruption prior to AF catheter ablation, resulting in various levels of drug concentrations.
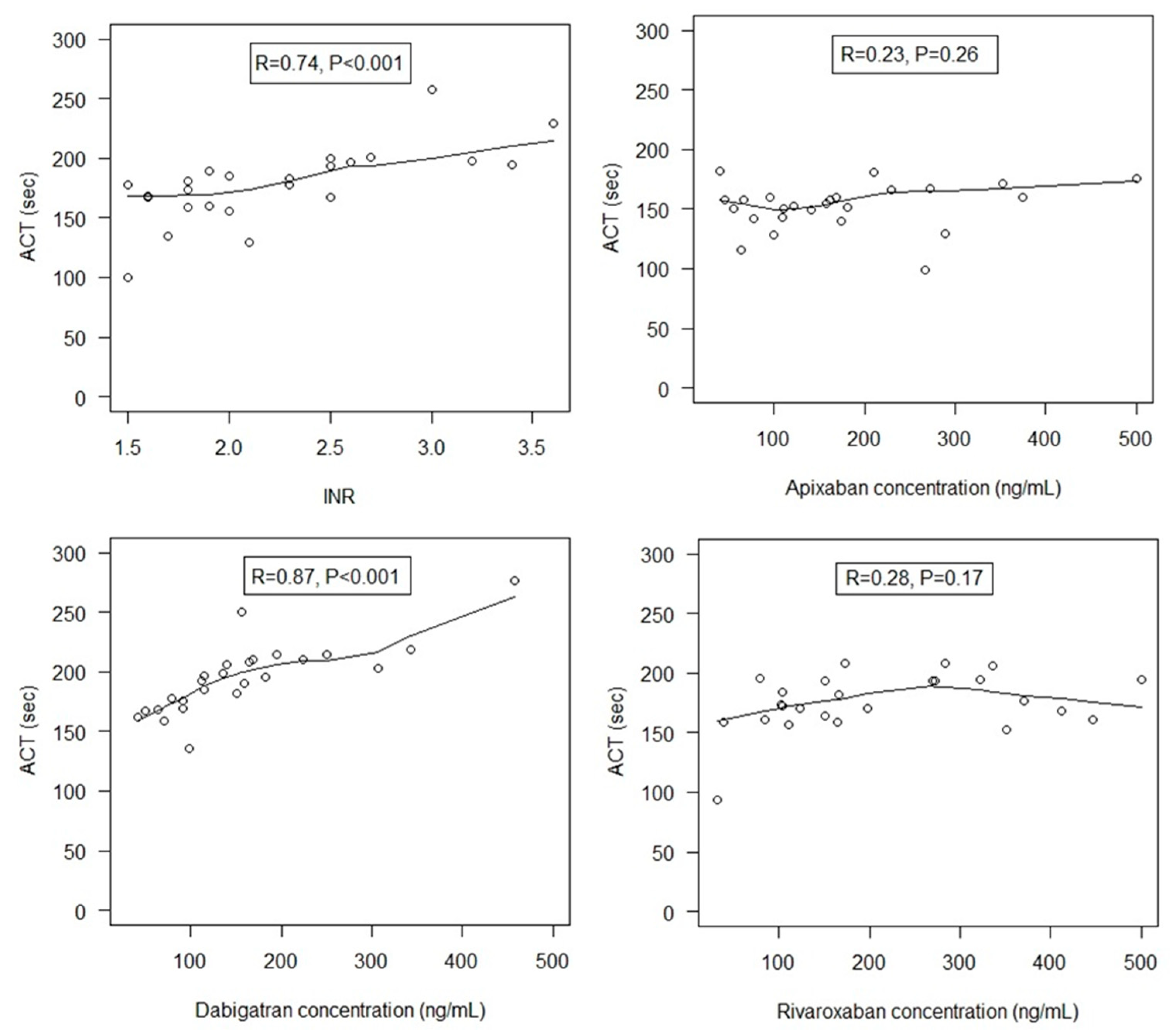
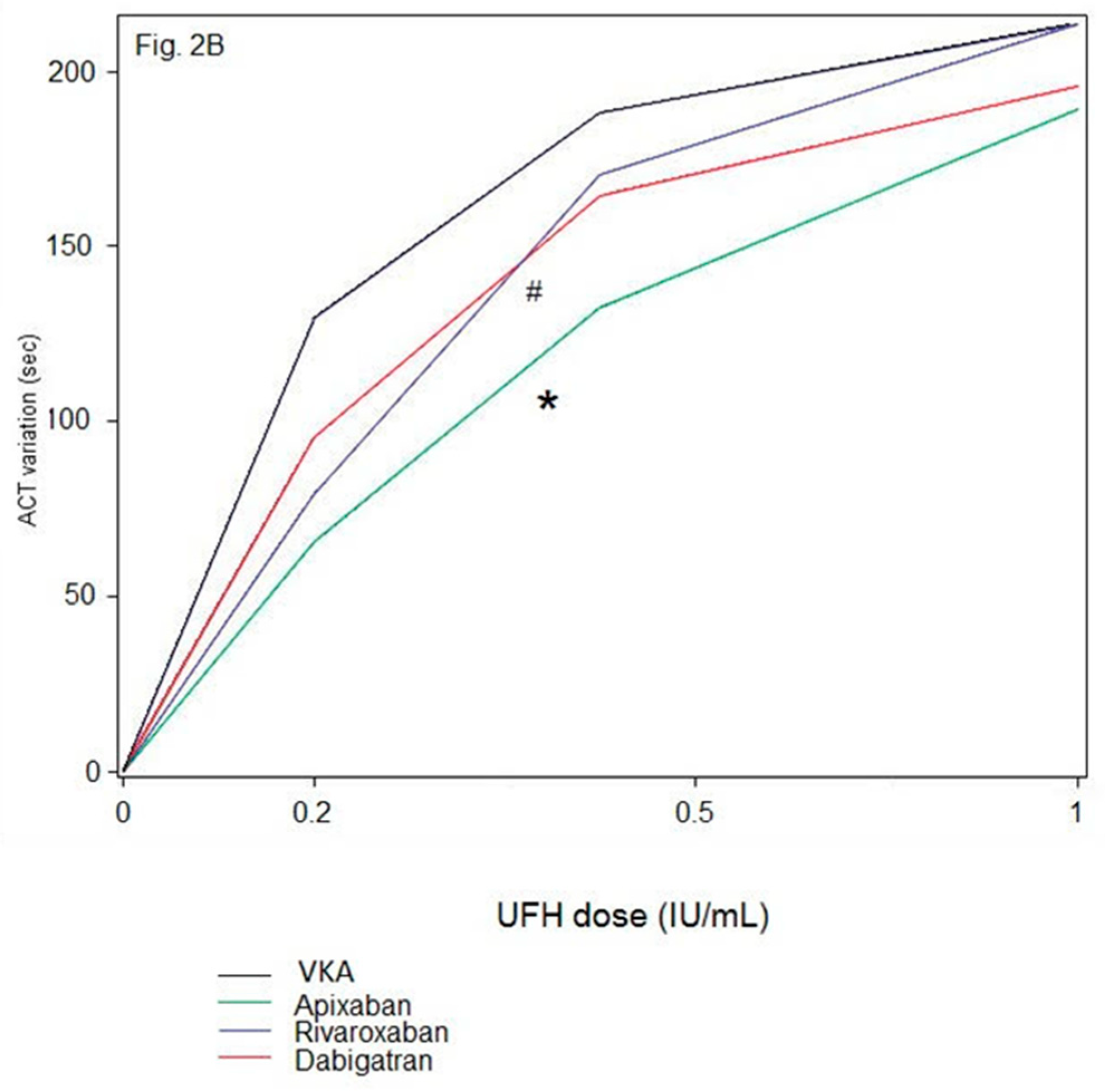
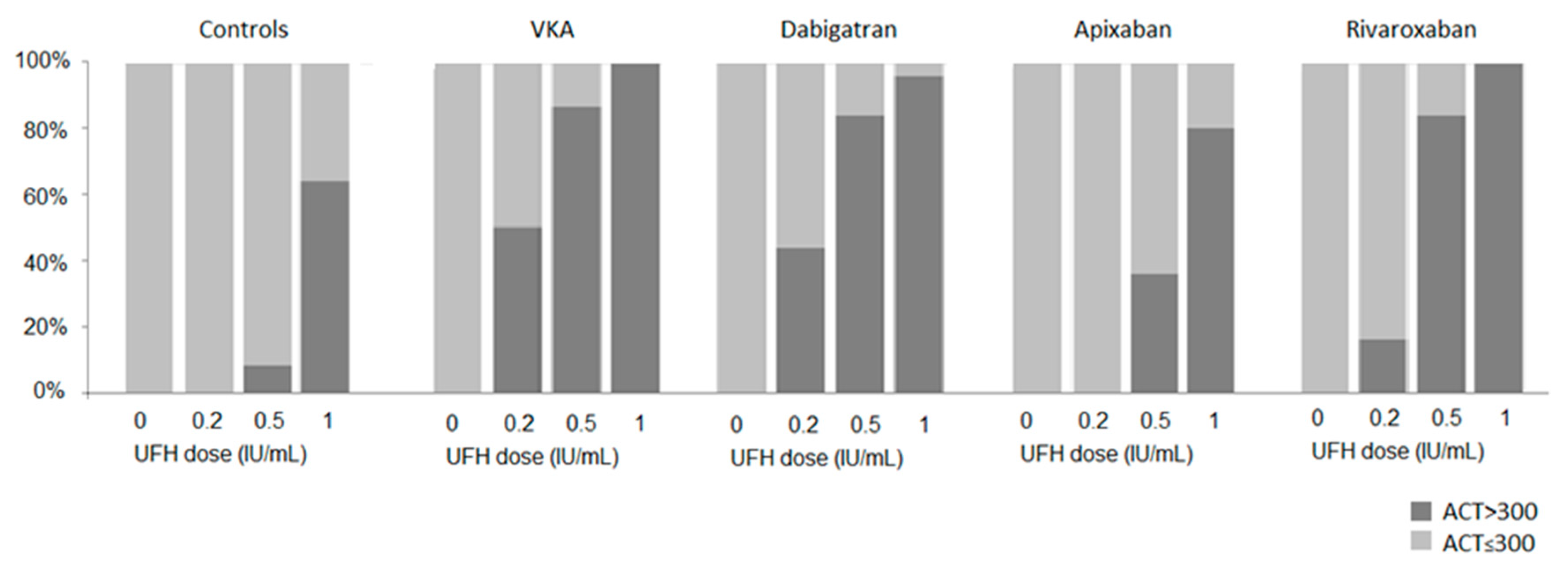
We found that UFH increased ACT values similarly in the presence of VKA and dabigatran, but ACT dose-response to UFH was different between VKA and FXa inhibitors, and between rivaroxaban and apixaban. In other words, a given UFH dose had different effects on the ACT depending on the DOAC on board, whereas intrinsically, at similar antithrombin concentrations (which was the case, data not shown), a given UFH dose has a similar inherent anticoagulant activity in vivo regardless of the oral anticoagulant on board. This result strongly challenges the relevance of ACT target at 300 s as the holy grail and raises the question of the role of the ACT assay as a universal monitoring of intra-procedural UFH in the presence of oral anticoagulants. For patients receiving dabigatran, the similar effects of UFH on ACT prolongation observed when compared to those with VKA, together with the strong correlation between ACT and dabigatran concentrations suggest not only the validity of ACT to monitor UFH but also support the same management of intra-procedural anticoagulation for these patients as for VKA-treated patients, with a similar ACT target ≥ 300 s. In contrast, for patients receiving FXa inhibitors, especially apixaban, ACT strongly underestimates UFH effects, thus leading to overdosing UFH to achieve the ACT target. Moreover, the lack of ACT sensitivity to apixaban, resulting in lower ACT values at baseline, leads to inappropriately higher requirements of UFH, and also contributes to overdosing.
Consequently, ACT values measured during AF catheter ablation do not reflect the level of anticoagulation resulting from FXa inhibitors and UFH [
13]. Running after an ACT target of 300 s is deceptive as it would lead to UFH overdosing compared to VKA-treated patients and inevitably increase the risk of peri-procedural bleeding complications.
Our results provide the rationale for the differences observed in the recent RCTs. In accordance with our results, in the RE-CIRCUIT trial similar UFH doses and ACT were achieved in dabigatran and VKA-treated patients (12,402 ± 10,721 vs. 11,910 ± 8359 IU, and 330 ± 81 vs. 342 ± 74 s, respectively) [
14]. And more UFH was required when the time from the last dabigatran dose to septal puncture increased, since ACT strongly correlates with dabigatran concentration. In contrast, in the VENTURE-AF, the AXAFA-AFNET 5 and more recently, the ELIMINATE-AF trials, a significantly higher dose of UFH was required to achieve ACT > 300 s among rivaroxaban, apixaban and edoxaban-treated patients respectively compared to VKA-treated patients [
6,
8,
9]. Together these results combined with our data, demonstrate that the effect of UFH on ACT prolongation is dramatically altered by the anticoagulant on board. One could argue that more UFH is administered because more UFH is needed. However, this hypothesis is supported neither from a mechanistic point of view nor from consistent available data on the efficacy of FXa inhibitors as compared to VKA in various clinical settings including stroke prevention in AF. Moreover, we provide here the demonstration that UFH doses are increased because UFH (with an intrinsic anticoagulant effect) prolongs the ACT to a lesser extent in the presence of FXa inhibitors compared to dabigatran or VKA, despite a fixe ACT target at 300 s.
As a result, physicians should be aware that the paradigm validated in patients receiving VKA cannot be applied to those receiving FXa inhibitors, especially apixaban. The development of an alternative comprehensive monitoring device, enabling UFH monitoring in the presence of FXa inhibitors is the way forward. In the meantime, it would be reasonable to change our view on the ACT target and no longer consider ACT ≥ 300 s as an indiscriminately therapeutic target, but rather as a strong signal for supra-therapeutic anticoagulation. Going forward, one option would be to administer a fixed UFH dose corresponding to the average dose given to VKA-treated patients and measure the ACT to check for UFH overdosing rather than to monitor it.
It may be challenging to reconcile our findings with clinical outcomes since none of the open RCTs have sufficient sample size to demonstrate an increase in bleeding complications. In particular, the AXAFA-AFNET 5 trial was underpowered to reliably detect differences in the components of the composite primary outcome, including all-cause death, stroke and major bleeding events [
8]. However, the ELIMINATE-AF trial supports our findings about the association between UFH overdosing and bleeding events in the presence of FXa inhibitors [
9]. Indeed, the 24% more UFH administered in the edoxaban arm translated into a trend towards higher peri-procedural clinically relevant non-major bleeding events during the ablation and within the first 48 h after compared to the VKA arm.