Increased Cancer Incidence Following up to 15 Years after Cardiac Catheterization in Infants under One Year between 1980 and 1998—A Single Center Observational Study
Abstract
:1. Introduction
1.1. Background
1.2. Objective
2. Methods
2.1. Study Design
2.2. Participants
2.3. Data Sources
2.4. Quantitative Variables-Calculation of Organ and Effective Doses
2.5. Statistics
3. Results
3.1. Participants
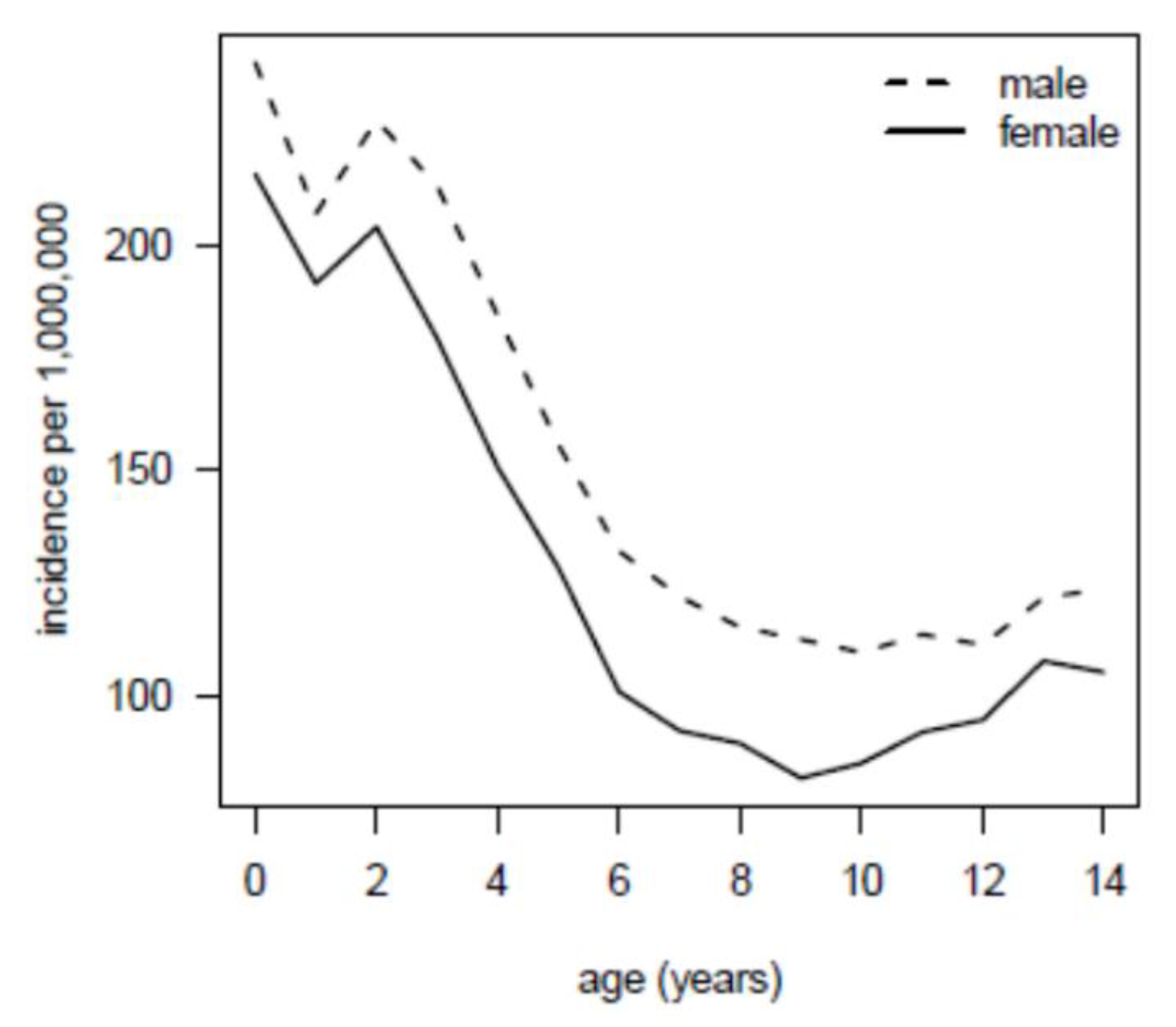
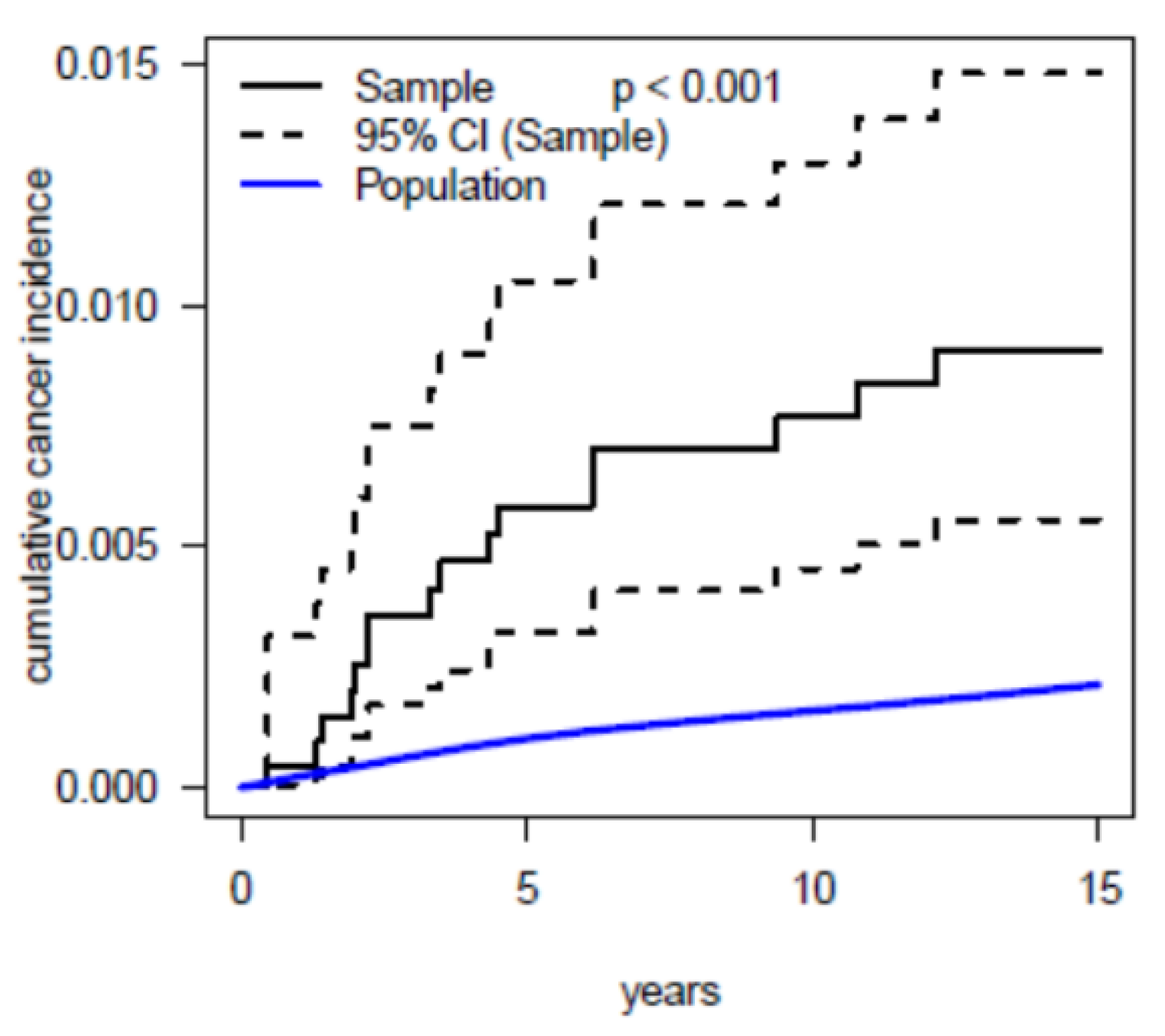
3.2. Main Results
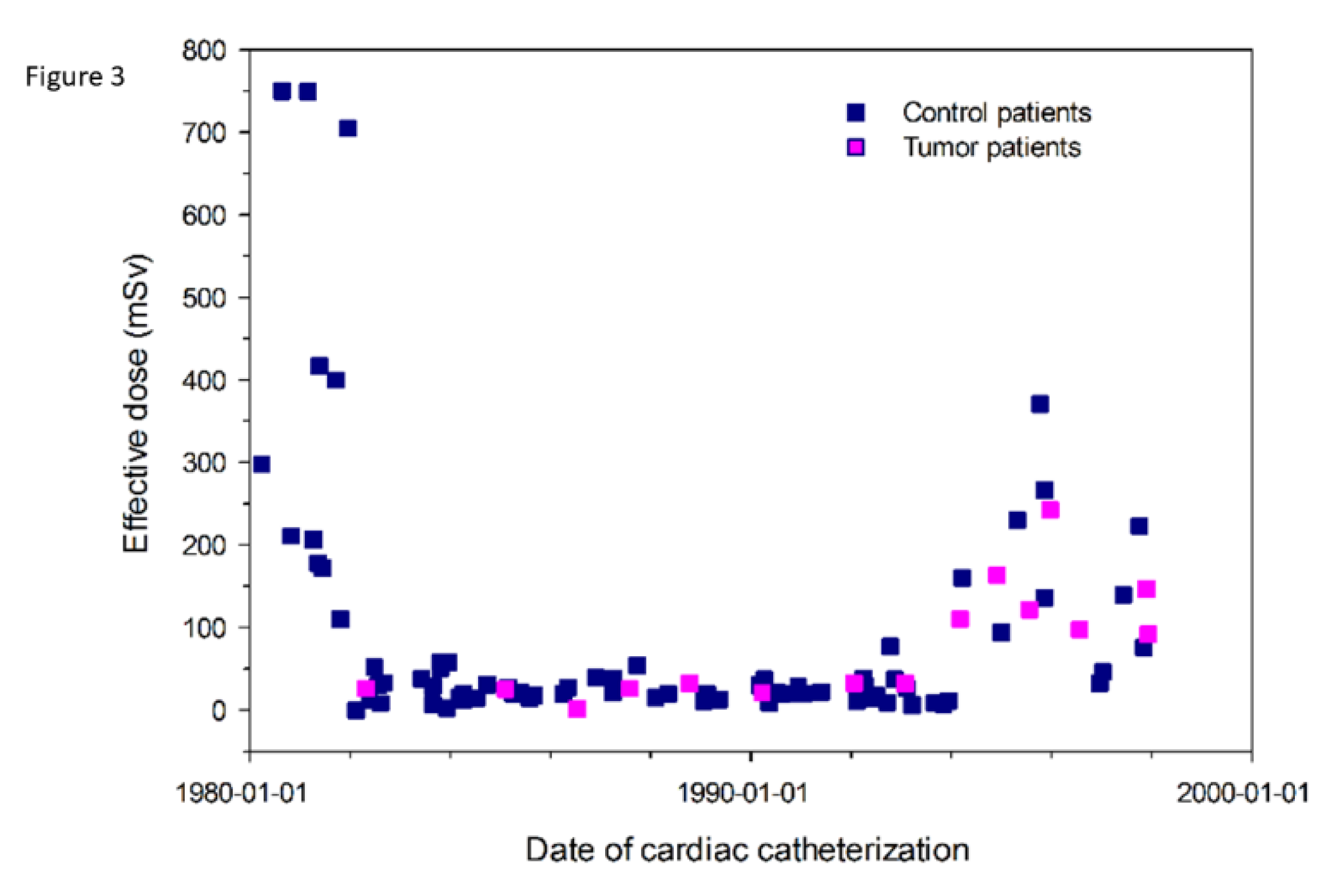
3.3. Radiation Burden
4. Discussion
4.1. Limitations
4.2. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Meinertz, T. Deutscher Herzbericht 2017: 29. In Bericht/Sektorenübergreifende Versorgungsanalyse zur Kardiologie, Herzchirurgie und Kinderherzmedizin in Deutschland; Deutsche Herzstiftung: Frankfurt, Germany, 2018. [Google Scholar]
- Bacher, K.; Bogaert, E.; Lapere, R.; De Wolf, D.; Thierens, H. Patient-specific dose and radiation risk estimation in pediatric cardiac catheterization. Circulation 2005, 111, 83–89. [Google Scholar] [CrossRef] [Green Version]
- El Sayed, M.H.; Roushdy, A.M.; El Farghaly, H.; El Sherbini, A. Radiation exposure in children during the current era of pediatric cardiac intervention. Pediatr. Cardiol. 2012, 33, 27–35. [Google Scholar] [CrossRef]
- Li, L.B.; Kai, M.; Kusama, T. Radiation exposure to patients during paediatric cardiac catheterisation. Radiat. Prot. Dosim. 2001, 94, 323–327. [Google Scholar] [CrossRef] [PubMed]
- Onnasch, D.G.; Schroder, F.K.; Fischer, G.; Kramer, H.H. Diagnostic reference levels and effective dose in paediatric cardiac catheterization. Br. J. Radiol. 2007, 80, 177–185. [Google Scholar] [CrossRef] [PubMed]
- Rassow, J.; Schmaltz, A.A.; Hentrich, F.; Streffer, C. Effective doses to patients from paediatric cardiac catheterization. Br. J. Radiol. 2000, 73, 172–183. [Google Scholar] [CrossRef] [PubMed]
- Smith, B.G.; Tibby, S.M.; Qureshi, S.A.; Rosenthal, E.; Krasemann, T. Quantification of temporal, procedural, and hardware-related factors influencing radiation exposure during pediatric cardiac catheterization. Catheter. Cardiovasc. Interv. Off. J. Soc. Card. Angiogr. Interv. 2012, 80, 931–936. [Google Scholar] [CrossRef]
- Tsapaki, V.; Kottou, S.; Korniotis, S.; Nikolaki, N.; Rammos, S.; Apostolopoulou, S.C. Radiation doses in paediatric interventional cardiology procedures. Radiat. Prot. Dosim. 2008, 132, 390–394. [Google Scholar] [CrossRef]
- Baysson, H.; Rehel, J.L.; Boudjemline, Y.; Petit, J.; Girodon, B.; Aubert, B.; Laurier, D.; Bonnet, D.; Bernier, M.O. Risk of cancer associated with cardiac catheterization procedures during childhood: A cohort study in France. BMC Public Health 2013, 13, 266. [Google Scholar] [CrossRef] [Green Version]
- Little, M.P. Risks associated with ionizing radiation. Br. Med. Bull. 2003, 68, 259–275. [Google Scholar] [CrossRef]
- UNSCEAR. The united nations scientific committee on the effects of atomic radiation. Health Phys. 2000, 79, 314. [Google Scholar]
- Preston, D.L.; Cullings, H.; Suyama, A.; Funamoto, S.; Nishi, N.; Soda, M.; Mabuchi, K.; Kodama, K.; Kasagi, F.; Shore, R.E. Solid cancer incidence in atomic bomb survivors exposed in utero or as young children. J. Natl Cancer Inst. 2008, 100, 428–436. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- 1990 recommendations of the international commission on radiological protection. Ann. ICRP 1991, 21, 1–201.
- Mathews, J.D.; Forsythe, A.V.; Brady, Z.; Butler, M.W.; Goergen, S.K.; Byrnes, G.B.; Giles, G.G.; Wallace, A.B.; Anderson, P.R.; Guiver, T.A. Cancer risk in 680,000 people exposed to computed tomography scans in childhood or adolescence: Data linkage study of 11 million Australians. BMJ 2013, 346, f2360. [Google Scholar] [CrossRef] [Green Version]
- Pearce, M.S.; Salotti, J.A.; Little, M.P.; McHugh, K.; Lee, C.; Kim, K.P.; Howe, N.L.; Ronckers, C.M.; Rajaraman, P.; Sir Craft, A.W.; et al. Radiation exposure from CT scans in childhood and subsequent risk of leukaemia and brain tumours: A retrospective cohort study. Lancet 2012, 380, 499–505. [Google Scholar] [CrossRef] [Green Version]
- McLaughlin, J.R.; Kreiger, N.; Sloan, M.P.; Benson, L.N.; Hilditch, S.; Clarke, E.A. An historical cohort study of cardiac catheterization during childhood and the risk of cancer. Int. J. Epidemiol. 1993, 22, 584–591. [Google Scholar] [CrossRef]
- Modan, B.; Keinan, L.; Blumstein, T.; Sadetzki, S. Cancer following cardiac catheterization in childhood. Int. J. Epidemiol. 2000, 29, 424–428. [Google Scholar] [CrossRef]
- Hammer, G.P.; Seidenbusch, M.C.; Schneider, K.; Regulla, D.F.; Zeeb, H.; Spix, C.; Blettner, M. A cohort study of childhood cancer incidence after postnatal diagnostic X-ray exposure. Radiat. Res. 2009, 171, 504–512. [Google Scholar] [CrossRef] [Green Version]
- Krille, L.; Dreger, S.; Schindel, R.; Albrecht, T.; Asmussen, M.; Barkhausen, J.; Berthold, J.D.; Chavan, A.; Claussen, C.; Forsting, M.; et al. Risk of cancer incidence before the age of 15 years after exposure to ionising radiation from computed tomography: Results from a German cohort study. Radiat. Environ. Biophys. 2015, 54, 1–12. [Google Scholar] [CrossRef]
- Schnell, R.; Bachteler, T.; Reiher, J. MTB: Ein Record Linkage-Programm für die empirische Sozialforschung. ZA Infrmation 2005, 56, 93–103. [Google Scholar]
- Steliarova-Foucher, E.; Stiller, C.; Lacour, B.; Kaatsch, P. International classification of childhood cancer, third edition. Cancer. 2005, 103, 1457–1467. [Google Scholar] [CrossRef]
- Servomaa, A.; Tapiovaara, M. Organ dose calculation in medical X-ray examinations by the program PCXMC. Radiat. Prot. Dosim. 1998, 80, 213–219. [Google Scholar] [CrossRef]
- Tapiovaara, M.; Lakkisto, M.; Servomaa, A. PCXMC. A PC-based Monte Carlo program for calculating patient doses in medical X-ray examinations; Säteilyturvakeskus (STUK), Report STUK A-139; Finnish Centre for Radiation and Nuclear Safety: Helsinki, Finland, 1997; p. 57.
- Cristy, M. Mathematical Phantoms Representing Children of Various Ages for Use in Estimates of Internal Dose; Oak Ridge National Lab: Oak Ridge, TN, USA, 1980. [Google Scholar]
- Protection ICoR. Report of the Task Group on Reference Man: Anatomical, Physiological and Metabolic Characteristics; ICRP Publication: Ottawa, ON, Canada, 1975. [Google Scholar]
- Seidenbusch, M.C.; Regulla, D.; Schneider, K. [Radiation exposure of children in pediatric radiology--part 2: The PAEDOS algorithm for computer-assisted dose reconstruction in pediatric radiology and results for X-ray examinations of the skull]. RoFo 2008, 180, 522–539. [Google Scholar] [CrossRef]
- Birch, R.; Marshall, M. Computation of bremsstrahlung X-ray spectra and comparison with spectra measured with a Ge(Li) detector. Phys. Med. Biol. 1979, 24, 505–517. [Google Scholar] [CrossRef]
- Seidenbusch, M.; Schneider, K. Conversion coefficients for determining organ doses in paediatric spine radiography. Pediatri. Radiol. 2014, 44, 434–456. [Google Scholar] [CrossRef]
- Einstein, A.J.; Knuuti, J. Cardiac imaging: Does radiation matter? Eur. Heart J. 2012, 33, 573–578. [Google Scholar] [CrossRef] [Green Version]
- Breslow, N.E. The Design and Analysis of Cohort Studies (IARC Scientific Publication No. 82) Statistical Methods in Cancer Research; IARC Scientific Publication: Lyon, France, 1987; Volume II. [Google Scholar]
- Sahai, H.K.A. Statistics in Epidemiology: Methods, Techniques, and Applications; CRC Press Inc.: Boca Raton, FL, USA, 1996. [Google Scholar]
- Hasle, H.; Clemmensen, I.H.; Mikkelsen, M. Risks of leukaemia and solid tumours in individuals with Down’s syndrome. Lancet 2000, 355, 165–169. [Google Scholar] [CrossRef]
- Hothorn TaL, B. On the exact distribution of maximally selected rank statistics. Comput. Stat. Data Anal. 2003, 43, 121–137. [Google Scholar] [CrossRef]
- Mandalenakis, Z.; Karazisi, C.; Skoglund, K.; Rosengren, A.; Lappas, G.; Eriksson, P.; Dellborg, M. Risk of cancer among children and young adults with congenital heart disease compared with healthy controls. JAMA Netw. Open 2019, 2, e196762. [Google Scholar] [CrossRef]
- Fong, C.T.; Brodeur, G.M. Down’s syndrome and leukemia: Epidemiology, genetics, cytogenetics and mechanisms of leukemogenesis. Cancer Genet. Cytogenet. 1987, 28, 55–76. [Google Scholar] [CrossRef]
- Freeman, S.B.; Taft, L.F.; Dooley, K.J.; Allran, K.; Sherman, S.L.; Hassold, T.J.; Khoury, M.J.; Saker, D.M. Population-based study of congenital heart defects in down syndrome. Am. J. Med. Genet. 1998, 80, 213–217. [Google Scholar] [CrossRef]
- Okuda, T.; Cai, Z.; Yang, S.; Lenny, N.; Lyu, C.J.; van Deursen, J.M.; Harada, H.; Downing, J.R. Expression of a knocked-in AML1-ETO leukemia gene inhibits the establishment of normal definitive hematopoiesis and directly generates dysplastic hematopoietic progenitors. Blood 1998, 91, 3134–3143. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brioude, F.; Kalish, J.M.; Mussa, A.; Foster, A.C.; Bliek, J.; Ferrero, G.B.; Boonen, S.E.; Cole, T.; Baker, R.; Bertoletti, M.; et al. Expert consensus document: Clinical and molecular diagnosis, screening and management of Beckwith-Wiedemann syndrome: An international consensus statement. Nat. Rev. Endocrinol. 2018, 14, 229–249. [Google Scholar] [CrossRef] [PubMed]
- Harbron, R.W.; Chapple, C.L.; O’Sullivan, J.J.; Best, K.E.; Berrington de Gonzalez, A.; Pearce, M.S. Survival adjusted cancer risks attributable to radiation exposure from cardiac catheterisations in children. Heart 2017, 103, 341–346. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harbron, R.W.; Pearce, M.S.; Salotti, J.A.; McHugh, K.; McLaren, C.; Abernethy, L.; Reed, S.; O’Sullivan, J.; Chapple, C.L. Radiation doses from fluoroscopically guided cardiac catheterization procedures in children and young adults in the United Kingdom: A multicentre study. Br. J. Radiol. 2015, 88, 20140852. [Google Scholar] [CrossRef] [PubMed]
- Einstein, A.J. Effects of radiation exposure from cardiac imaging: How good are the data? J. Am. Coll. Cardiol. 2012, 59, 553–565. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Einstein, A.J. Beyond the bombs: Cancer risks of low-dose medical radiation. Lancet 2012, 380, 455–457. [Google Scholar] [CrossRef] [Green Version]
- Hijazi, Z.M.; Kenny, D. Landmark lecture on interventional cardiology: Interventional cardiac catheterisation for CHD: The past, present, and the future. Cardiol. Young. 2017, 27, 1974–1985. [Google Scholar] [CrossRef]
- Hill, K.D.; Frush, D.P.; Han, B.K.; Abbott, B.G.; Armstrong, A.K.; DeKemp, R.A.; Glatz, A.C.; Greenberg, S.B.; Herbert, A.S.; Justino, H.; et al. Radiation safety in children with congenital and acquired heart Disease: A scientific position statement on multimodality dose optimization from the image gently alliance. JACC Cardiovasc. Imaging 2017, 10, 797–818. [Google Scholar] [CrossRef]
| Patient No | Sex | Type of Cancer | Age at Diagnosis | Genetic Disorder | Cardiac Diagnosis |
|---|---|---|---|---|---|
| 1 | m | Embryonal sarcoma of the lungs, metastasized | 1.6 | none | VSD, CoA |
| 2 | f | Acute myeloid leukemia | 2.5 | Trisomy 21 | AVSD |
| 3 | f | Liposarcoma | 12.2 | none | VSD, CoA, subvalvar AS |
| 4 | m | Thyroid carcinoma | 6.3 | none | DILV |
| 5 | m | Lymphoid leukemia | 4.5 | none | VSD, PH |
| 6 | f | Neuroblastoma and ganglioneuroblastoma | 3.7 | none | TAPVC |
| 7 | f | Lymphoid leukemias | 3.0 | none | DILV, VSD, PS |
| 8 | f | Lymphoid leukemias | 5.2 | none | VSD, PH |
| 9 | m | intracerebral Tumor (histology unknown) | 0.1 | none | ASD II |
| 10 | f | Lymphoid leukemia | 11.3 | none | VSD, PH |
| 11 | m | Acute myeloid leukemia | 1.9 | Trisomy 21 | ASD II |
| 12 | m | Acute myeloid leukemia | 2.0 | Trisomy 21 | AVSD |
| 13 | f | Neuroblastoma and ganglioneuroblastoma | 9.5 | Beckwith Wiedemann Syndrome | Mitral stenosis, PH |
| 14 | m | Intracranial and intraspinal embryonal tumors | 3.5 | none | DILV, TGA |
| 15 | f | Myelodysplastic syndrome and other myeloproliferative diseases | 1.9 | Trisomy 21 | VSD, PDA |
| 16 | f | Astrocytomas | 6.2 | Tuberous Sclerosis | TA, VSD, ASD |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stern, H.; Seidenbusch, M.; Hapfelmeier, A.; Meierhofer, C.; Naumann, S.; Schmid, I.; Spix, C.; Ewert, P. Increased Cancer Incidence Following up to 15 Years after Cardiac Catheterization in Infants under One Year between 1980 and 1998—A Single Center Observational Study. J. Clin. Med. 2020, 9, 315. https://doi.org/10.3390/jcm9020315
Stern H, Seidenbusch M, Hapfelmeier A, Meierhofer C, Naumann S, Schmid I, Spix C, Ewert P. Increased Cancer Incidence Following up to 15 Years after Cardiac Catheterization in Infants under One Year between 1980 and 1998—A Single Center Observational Study. Journal of Clinical Medicine. 2020; 9(2):315. https://doi.org/10.3390/jcm9020315
Chicago/Turabian StyleStern, Heiko, Michael Seidenbusch, Alexander Hapfelmeier, Christian Meierhofer, Susanne Naumann, Irene Schmid, Claudia Spix, and Peter Ewert. 2020. "Increased Cancer Incidence Following up to 15 Years after Cardiac Catheterization in Infants under One Year between 1980 and 1998—A Single Center Observational Study" Journal of Clinical Medicine 9, no. 2: 315. https://doi.org/10.3390/jcm9020315
APA StyleStern, H., Seidenbusch, M., Hapfelmeier, A., Meierhofer, C., Naumann, S., Schmid, I., Spix, C., & Ewert, P. (2020). Increased Cancer Incidence Following up to 15 Years after Cardiac Catheterization in Infants under One Year between 1980 and 1998—A Single Center Observational Study. Journal of Clinical Medicine, 9(2), 315. https://doi.org/10.3390/jcm9020315





