What Is the Diagnosis in Patients with Type 2 Diabetes Who Have a Painful Shoulder? Results from a Prospective Cross-Sectional Study
Abstract
1. Introduction
2. Methods
2.1. Participants and Study Design
2.2. Questionnaire
2.3. Ultrasound Imaging
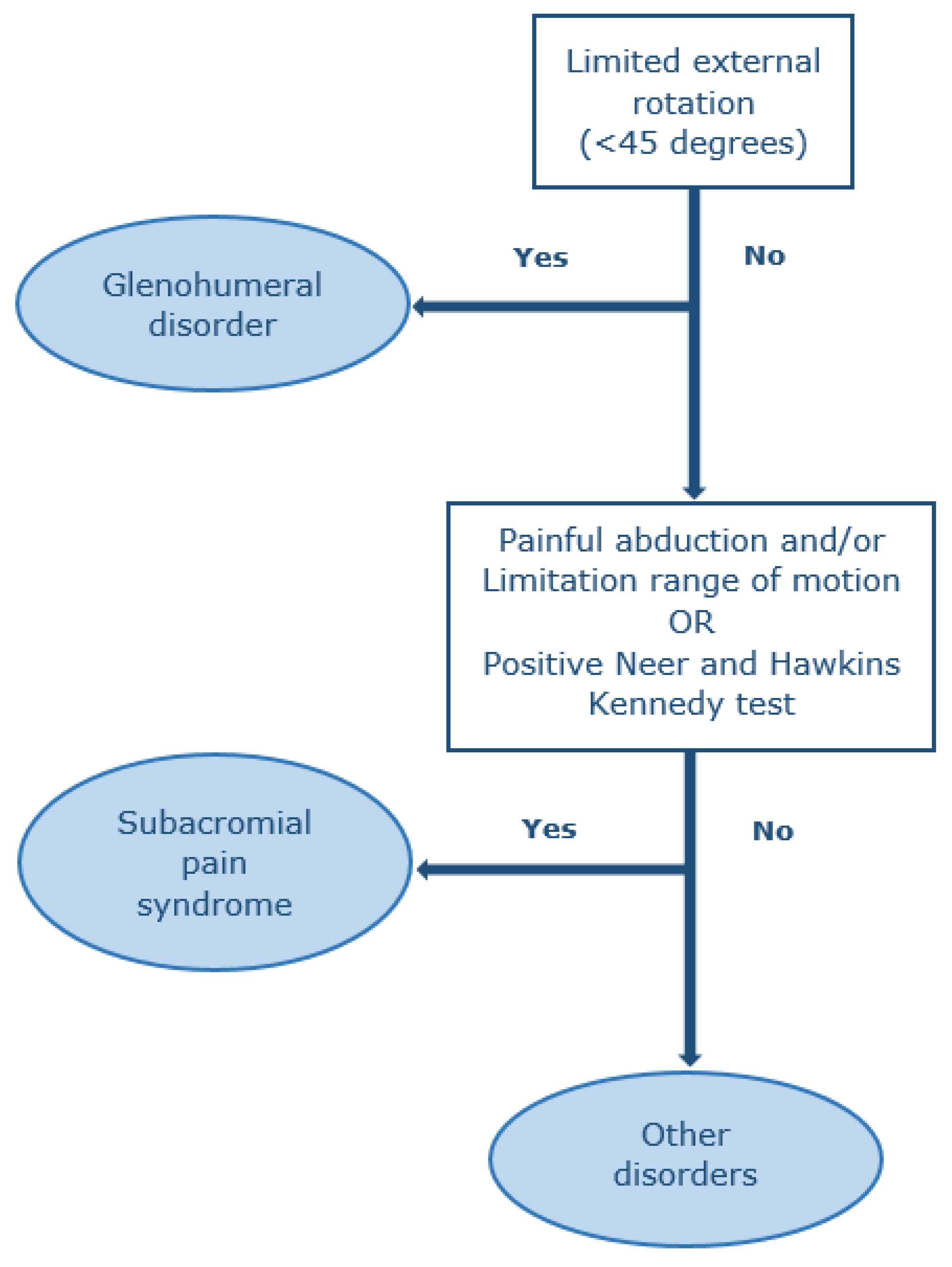
2.4. Physical Examination
2.5. Statistical Analysis
3. Results
3.1. Physical-Examination-Diagnosed Symptomatic Shoulder Disorders
3.2. Polyneuropathy
3.3. Ultrasound-Diagnosed Shoulder Disorders
4. Discussion
4.1. Diagnosis by Physical Examination and Ultrasound Imaging
4.2. Neuropathy
4.3. Strengths and Limitations
4.4. Implications for Practice and Future Research
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Smith, L.L.; Burnet, S.P.; McNeil, J.D. Musculoskeletal manifestations of diabetes mellitus. Br. J. Sports Med. 2003, 37, 30–35. [Google Scholar] [CrossRef] [PubMed]
- Cagliero, E.; Apruzzese, W.; Perlmutter, G.S.; Nathan, D.M. Musculoskeletal disorders of the hand and shoulder in patients with diabetes mellitus. Am. J. Med. 2002, 112, 487–490. [Google Scholar] [CrossRef]
- Thomas, S.J.; McDougall, C.; Brown, I.D.; Jaberoo, M.C.; Stearns, A.; Ashraf, R.; Fisher, M.; Kelly, I.G. Prevalence of symptoms and signs of shoulder problems in people with diabetes mellitus. J. Shoulder Elb. Surg. 2007, 16, 748–751. [Google Scholar] [CrossRef] [PubMed]
- Kidwai, S.S.; Wahid, L.; Siddiqi, S.A.; Khan, R.M.; Ghauri, I.; Sheikh, I. Upper limb musculoskeletal abnormalities in type 2 diabetic patients in low socioeconomic strata in Pakistan. BMC Res. Notes 2013, 6, 16. [Google Scholar] [CrossRef]
- Abate, M.; Schiavone, C.; Salini, V.; Andia, I. Management of limited joint mobility in diabetic patients. Diabetes Metab. Syndr. Obes. 2013, 6, 197–207. [Google Scholar] [CrossRef]
- Lebiedz-Odrobina, D.; Kay, J. Rheumatic manifestations of diabetes mellitus. Rheum. Dis. Clin. N. Am. 2010, 36, 681–699. [Google Scholar] [CrossRef]
- Bhat, T.A.; Dhar, S.A.; Dar, T.A.; Naikoo, M.A.; Naqqash, M.A.; Bhat, A.; Butt, M.F. The Musculoskeletal Manifestations of Type 2 Diabetes Mellitus in a Kashmiri Population. Int. J. Health Sci. (Qassim) 2016, 10, 57–68. [Google Scholar] [CrossRef]
- Abate, M.; Schiavone, C.; Salini, V. Sonographic evaluation of the shoulder in asymptomatic elderly subjects with diabetes. BMC Musculoskelet. Disord. 2010, 11, 278. [Google Scholar] [CrossRef]
- Abourazzak, F.E.; Akasbi, N.; Houssaini, G.S.; Bazouti, S.; Bensbaa, S.; Hachimi, H.; Ajdi, F.; Harzy, T. Articular and abarticular manifestations in type 2 diabetes mellitus. Eur. J. Rheumatol. 2014, 1, 132–134. [Google Scholar] [CrossRef]
- Arkkila, P.E.; Gautier, J.F. Musculoskeletal disorders in diabetes mellitus: An update. Best Pract. Res. Clin. Rheumatol. 2003, 17, 945–970. [Google Scholar] [CrossRef]
- Boulton, A.J. Diabetic neuropathy: Classification, measurement and treatment. Curr. Opin. Endocrinol. Diabetes Obes. 2007, 14, 141–145. [Google Scholar] [CrossRef] [PubMed]
- Dyck, P.J.; Overland, C.J.; Low, P.A.; Litchy, W.J.; Davies, J.L.; Dyck, P.J.; O’Brien, P.C.; Albers, J.W.; Andersen, H.; Bolton, C.F.; et al. Signs and symptoms versus nerve conduction studies to diagnose diabetic sensorimotor polyneuropathy: Cl vs. NPhys trial. Muscle Nerve 2010, 42, 157–164. [Google Scholar] [CrossRef] [PubMed]
- Tesfaye, S.; Boulton, A.J.; Dyck, P.J.; Freeman, R.; Horowitz, M.; Kempler, P.; Lauria, G.; Malik, R.A.; Spallone, V.; Vinik, A.; et al. Diabetic neuropathies: Update on definitions, diagnostic criteria, estimation of severity, and treatments. Diabetes Care 2010, 33, 2285–2293. [Google Scholar] [CrossRef] [PubMed]
- Association, A.D. Standards of Medical Care in Diabetesd2017. J. Clin. Appl. Res. Educ. 2017, 40, 142. [Google Scholar]
- Yagihashi, S.; Mizukami, H.; Sugimoto, K. Mechanism of diabetic neuropathy: Where are we now and where to go? J. Diabetes Investig. 2011, 2, 18–32. [Google Scholar] [CrossRef] [PubMed]
- Pica, E.C.; Verma, K.K. Bilateral brachial plexopathy as an initial presentation in a newly-diagnosed, uncontrolled case of diabetes mellitus. Singap. Med. J. 2008, 49, e29–e32. [Google Scholar]
- Ogawa, K.; Sasaki, H.; Kishi, Y.; Yamasaki, H.; Okamoto, K.; Yamamoto, N.; Hanabusa, T.; Nakao, T.; Nishi, M.; Nanjo, K. A suspected case of proximal diabetic neuropathy predominantly presenting with scapulohumeral muscle weakness and deep aching pain. Diabetes Res. Clin. Pract. 2001, 54, 57–64. [Google Scholar] [CrossRef]
- Wada, Y.; Yanagihara, C.; Nishimura, Y.; Oka, N. A case of diabetic amyotrophy with severe atrophy and weakness of shoulder girdle muscles showing good response to intravenous immune globulin. Diabetes Res. Clin. Pract. 2007, 75, 107–110. [Google Scholar] [CrossRef]
- Diercks, R.; Bron, C.; Dorrestijn, O.; Meskers, C.; Naber, R.; de Ruiter, T.; Willems, J.; Winters, J.; van der Woude, H.J. Guideline for diagnosis and treatment of subacromial pain syndrome: A multidisciplinary review by the Dutch Orthopaedic Association. Acta Orthop. 2014, 85, 314–322. [Google Scholar] [CrossRef]
- Nederlands Huisartsen Genootschap. NHG-Standaard Schouderklachten (Dutch College of General Practitioners Guidelines of Shoulder Complaints). Available online: https://www.nhg.org/standaarden/samenvatting/schouderklachten (accessed on 20 March 2020).
- Littlewood, C.; May, S.; Walters, S. Epidemiology of Rotator Cuff Tendinopathy: A Systematic Review. Shoulder Elb. 2013, 5, 256–265. [Google Scholar] [CrossRef]
- Littlewood, C.; Bateman, M.; Connor, C.; Gibson, J.; Horsley, I.; Jaggi, A.; Jones, V.; Meakins, A.; Scott, M. Physiotherapists’ recommendations for examination and treatment of rotator cuff related shoulder pain: A consensus exercise. Physiother. Pract. Res. 2019, 40, 87–94. [Google Scholar] [CrossRef]
- Van der Windt, D.A.; Koes, B.W.; de Jong, B.A.; Bouter, L.M. Shoulder disorders in general practice: Incidence, patient characteristics, and management. Ann. Rheum. Dis. 1995, 54, 959–964. [Google Scholar] [CrossRef]
- CKS. Shoulder Pain Guideline. Available online: http://www.cks.nhs.uk/shoulder_pain (accessed on 20 March 2020).
- New Zealand Guidelines Group. The Diagnosis and Management of Soft Tissue Shoulder Injuries and Related Disorders 2020. Available online: https://cdn.ymaws.com/www.alaskachiropracticsociety.com/resource/resmgr/imported/shoulder.pdf (accessed on 20 March 2020).
- Boer, B.C.; Boetje, J.; Stevens, M.; van den Akker-Scheek, I.; van Raay, J. Adaptation, validity and reliability of the modified painDETECT questionnaire for patients with subacromial pain syndrome. PLoS ONE 2019, 14, e0211880. [Google Scholar] [CrossRef] [PubMed]
- Molsted, S.; Tribler, J.; Snorgaard, O. Musculoskeletal pain in patients with type 2 diabetes. Diabetes Res. Clin. Pract. 2012, 96, 135–140. [Google Scholar] [CrossRef] [PubMed]
- Miksch, A.; Hermann, K.; Rolz, A.; Joos, S.; Szecsenyi, J.; Ose, D.; Rosemann, T. Additional impact of concomitant hypertension and osteoarthritis on quality of life among patients with type 2 diabetes in primary care in Germany—A cross-sectional survey. Health Qual. Life Outcomes 2009, 7, 19. [Google Scholar] [CrossRef] [PubMed]
- ESSR. Ultrasound Shoulder Protocol of the ESSR. 2016. Available online: https://essr.org/content-essr/uploads/2016/10/shoulder.pdf (accessed on 20 March 2020).
- Lee, J.C.; Sykes, C.; Saifuddin, A.; Connell, D. Adhesive capsulitis: Sonographic changes in the rotator cuff interval with arthroscopic correlation. Skelet. Radiol. 2005, 34, 522–527. [Google Scholar] [CrossRef]
- Tandon, A.; Dewan, S.; Bhatt, S.; Jain, A.K.; Kumari, R. Sonography in diagnosis of adhesive capsulitis of the shoulder: A case-control study. J. Ultrasound 2017, 20, 227–236. [Google Scholar] [CrossRef]
- Ottenheijm, R.P.; Joore, M.A.; Walenkamp, G.H.; Weijers, R.E.; Winkens, B.; Cals, J.W.; de Bie, R.A.; Dinant, G.J. The Maastricht Ultrasound Shoulder pain trial (MUST): Ultrasound imaging as a diagnostic triage tool to improve management of patients with non-chronic shoulder pain in primary care. BMC Musculoskelet. Disord. 2011, 12, 154. [Google Scholar] [CrossRef]
- Geraets, J.J.; de Jongh, A.C.; Boeke, A.J.; Buis, P.A.; Spinnewijn, W.E.; Geijer, R.M.; Goudswaard, A.N. Summary of the practice guideline for shoulder complaints from the Dutch College of General Practitioners. Ned. Tijdschr. Geneeskd. 2009, 153, A164. [Google Scholar]
- Hegedus, E.J.; Goode, A.P.; Cook, C.E.; Michener, L.; Myer, C.A.; Myer, D.M.; Wright, A.A. Which physical examination tests provide clinicians with the most value when examining the shoulder? Update of a systematic review with meta-analysis of individual tests. Br. J. Sports Med. 2012, 46, 964–978. [Google Scholar] [CrossRef]
- Gismervik, S.; Drogset, J.O.; Granviken, F.; Rø, M.; Leivseth, G. Physical examination tests of the shoulder: A systematic review and meta-analysis of diagnostic test performance. BMC Musculoskelet. Disord. 2017, 18, 41. [Google Scholar] [CrossRef] [PubMed]
- Van Seventer, R.; Vos, C.; Giezeman, M.; Meerding, W.J.; Arnould, B.; Regnault, A.; van Eerd, M.; Martin, C.; Huygen, F. Validation of the Dutch version of the DN4 diagnostic questionnaire for neuropathic pain. Pain Pract. Off. J. World Inst. Pain 2013, 13, 390–398. [Google Scholar] [CrossRef] [PubMed]
- Van Seventer, R.; Vos, C.; Meerding, W.; Mear, I.; Le Gal, M.; Bouhassira, D.; Huygen, F.J. Linguistic validation of the DN4 for use in international studies. Eur. J. Pain 2010, 14, 58–63. [Google Scholar] [CrossRef] [PubMed]
- Spallone, V.; Morganti, R.; D’Amato, C.; Greco, C.; Cacciotti, L.; Marfia, G.A. Validation of DN4 as a screening tool for neuropathic pain in painful diabetic polyneuropathy. Diabet. Med. 2012, 29, 578–585. [Google Scholar] [CrossRef] [PubMed]
- Bouhassira, D.; Attal, N.; Alchaar, H.; Boureau, F.; Brochet, B.; Bruxelle, J.; Cunin, G.; Fermanian, J.; Ginies, P.; Grun-Overdyking, A.; et al. Comparison of pain syndromes associated with nervous or somatic lesions and development of a new neuropathic pain diagnostic questionnaire (DN4). Pain 2005, 114, 29–36. [Google Scholar] [CrossRef]
- England, J.D.; Gronseth, G.S.; Franklin, G.; Miller, R.G.; Asbury, A.K.; Carter, G.T.; Cohen, J.A.; Fisher, M.A.; Howard, J.F.; Kinsella, L.J.; et al. Distal symmetric polyneuropathy: A definition for clinical research: Report of the American Academy of Neurology, the American Association of Electrodiagnostic Medicine, and the American Academy of Physical Medicine and Rehabilitation. Neurology 2005, 64, 199–207. [Google Scholar] [CrossRef]
- Alport, A.R.; Sander, H.W. Clinical approach to peripheral neuropathy: Anatomic localization and diagnostic testing. Continuum 2012, 18, 13–38. [Google Scholar] [CrossRef]
- Yang, Z.; Zhang, Y.; Chen, R.; Huang, Y.; Ji, L.; Sun, F.; Hong, T.; Zhan, S. Simple tests to screen for diabetic peripheral neuropathy. Cochrane Database Syst. Rev. 2014. [Google Scholar] [CrossRef]
- Feng, Y.; Schlösser, F.J.; Sumpio, B.E. The Semmes Weinstein monofilament examination as a screening tool for diabetic peripheral neuropathy. J. Vasc. Surg. 2009, 50, 675–682. [Google Scholar] [CrossRef]
- Smieja, M.; Hunt, D.L.; Edelman, D.; Etchells, E.; Cornuz, J.; Simel, D.L. Clinical examination for the detection of protective sensation in the feet of diabetic patients. International Cooperative Group for Clinical Examination Research. J. Gen. Intern. Med. 1999, 14, 418–424. [Google Scholar] [CrossRef]
- Kästenbauer, T.; Sauseng, S.; Brath, H.; Abrahamian, H.; Irsigler, K. The value of the Rydel-Seiffer tuning fork as a predictor of diabetic polyneuropathy compared with a neurothesiometer. Diabet. Med. 2004, 21, 563–567. [Google Scholar] [CrossRef] [PubMed]
- Liniger, C.; Albeanu, A.; Bloise, D.; Assal, J.P. The tuning fork revisited. Diabet. Med. 1990, 7, 859–864. [Google Scholar] [CrossRef] [PubMed]
- Fisher, L.; Kurtz, A.; Shipley, M. Association between cheiroarthropathy and frozen shoulder in patients with insulin-dependent diabetes mellitus. Br. J. Rheumatol. 1986, 25, 141–146. [Google Scholar] [CrossRef] [PubMed]
- Hill, N.E.; Roscoe, D.; Stacey, M.J.; Chew, S. Cheiroarthropathy and tendinopathy in diabetes. Diabet. Med. 2019, 36, 939–947. [Google Scholar] [CrossRef] [PubMed]
- Gokcen, N.; Cetinkaya Altuntas, S.; Coskun Benlidayi, I.; Sert, M.; Nazlican, E.; Sarpel, T. An overlooked rheumatologic manifestation of diabetes: Diabetic cheiroarthropathy. Clin. Rheumatol. 2019, 38, 927–932. [Google Scholar] [CrossRef]
- Lewis, J. Rotator cuff related shoulder pain: Assessment, management and uncertainties. Manual Ther. 2016, 23, 57–68. [Google Scholar] [CrossRef]
- Ottenheijm, R.P.; Cals, J.W.; Weijers, R.; Vanderdood, K.; de Bie, R.A.; Dinant, G.J. Ultrasound imaging for tailored treatment of patients with acute shoulder pain. Ann. Fam. Med. 2015, 13, 53–55. [Google Scholar] [CrossRef][Green Version]
- Cadogan, A.; Laslett, M.; Hing, W.A.; McNair, P.J.; Coates, M.H. A prospective study of shoulder pain in primary care: Prevalence of imaged pathology and response to guided diagnostic blocks. BMC Musculoskelet. Disord. 2011, 12, 119. [Google Scholar] [CrossRef]
- Ottenheijm, R.P.; van’t Klooster, I.G.; Starmans, L.M.; Vanderdood, K.; de Bie, R.A.; Dinant, G.J.; Cals, J.W. Ultrasound-diagnosed disorders in shoulder patients in daily general practice: A retrospective observational study. BMC Fam. Pract. 2014, 15, 115. [Google Scholar] [CrossRef][Green Version]
- Oliva, F.; Via, A.G.; Maffulli, N. Physiopathology of intratendinous calcific deposition. BMC Med. 2012, 10, 95. [Google Scholar] [CrossRef]
- Cook, J.L.; Purdam, C.R. Is tendon pathology a continuum? A pathology model to explain the clinical presentation of load-induced tendinopathy. Br. J. Sports Med. 2009, 43, 409–416. [Google Scholar] [CrossRef] [PubMed]
- Girish, G.; Lobo, L.G.; Jacobson, J.A.; Morag, Y.; Miller, B.; Jamadar, D.A. Ultrasound of the shoulder: Asymptomatic findings in men. AJR Am. J. Roentgenol. 2011, 197, W713–W719. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, R.L.; Moutzouros, V.; Bey, M.J. Asymptomatic Rotator Cuff Tears. JBJS Rev. 2019, 7, e9. [Google Scholar] [CrossRef] [PubMed]
- Arkkila, P.E.; Kantola, I.M.; Viikari, J.S.; Ronnemaa, T. Shoulder capsulitis in type I and II diabetic patients: Association with diabetic complications and related diseases. Ann. Rheum Dis. 1996, 55, 907–914. [Google Scholar] [CrossRef] [PubMed]
- Noël, E.; Thomas, T.; Schaeverbeke, T.; Thomas, P.; Bonjean, M.; Revel, M. Frozen shoulder. Joint Bone Spine 2000, 67, 393–400. [Google Scholar] [PubMed]
- Naunton, J.; Harrison, C.; Britt, H.; Haines, T.; Malliaras, P. General practice management of rotator cuff related shoulder pain: A reliance on ultrasound and injection guided care. PLoS ONE 2020, 15, e0227688. [Google Scholar] [CrossRef]
- Ottenheijm, R.P.; Hesselmans, N.J.; Kemper, A.; Moser, A.; de Bie, R.A.; Dinant, G.J.; Cals, J.W. GPs’ perspectives on the diagnostic work-up in patients with shoulder pain: A qualitative study. J. Eval. Clin. Pract. 2014, 20, 239–245. [Google Scholar] [CrossRef]
| Total Number of Patients with Shoulder Pain n = 66 | Symptomatic Shoulders, n = 93 | |||
|---|---|---|---|---|
| GH | SAPS | Other Disorder | ||
| n = 17 (18.2%, 95% CI: 11.3–27.9) # | n = 58 (66.6%, 95% CI: 51.6–72.0) # | n = 18 (16.1%, 95% CI: 12.1–29.1) # | ||
| Age | ||||
| mean ± SD | 63.0 ± 6.9 | 58.8 ± 5.8 | 61.5 ± 7.0 | 61.5 ± 7.6 |
| IQR range (years) | 38–70 | 48–70 | 38–70 | 52–70 |
| Female sex | 19 (28.8) | 5 (31.3) | 11 (25.0) | 3 (42.9) |
| BMI (kg/m2) | ||||
| mean ± SD | 28.5 ± 4.2 | 28.1 ± 3.7 | 28.4 ± 4.5 | 29.7 ± 3.5 |
| range | 16–41.5 | 23.9–35.9 | 16–41.5 | 25.4–34.0 |
| Bilateral shoulder pain | 27 (40.9) | 8 (50) | 15 (34.1) | 4 (57.1) |
| Dominant shoulder affected | 26 (39.3) | 13 (81.3) | 36 (81.8) | 7 (100) |
| NRS | ||||
| mean ± SD | 5.3 ± 2.1 | 6.25 ± 1.9 | 5.2 ± 2.06 | 4.0 ± 2.6 |
| range | 1–9 | 3–9 | 1–8 | 1–8 |
| Pain onset | ||||
| sudden | 13 (19.7) | 2 (11.7) | 13 (20.9) | 2 a (11.1) |
| gradual | 51 (79.7) | 15 (88.3) | 44 (70.9) | 10 (12.5) |
| Neck pain | 9 (13.6) | 2 (12.5) | 5 (11.4) | 2 (11.1) |
| Duration of diabetes | ||||
| mean ± SD | 9.0 ± 5.5 | 8.2 ± 4.3 a | 9.4 ± 5.8 b | 8.2 ± 5.8 |
| range (years) | 1–27 | 1–14 | 1–27 | 2–19 |
| HbA1C (mmol/mol) | ||||
| mean ± SD | 55.7 ± 7.7 | 55.6 ± 6.2 a | 55.8 ± 8.03 b | 53.6 ± 10.1 c |
| range | 43–75 | 44–64 | 43–75 | 43–70 |
| Rheumatoid arthritis a | 8 (12.5) | 3 (18.8) | 4 a (10.3) | 1 (14.3) |
| Osteoarthritis a | 33 (51.6) | 9 (56.3) | 22 a (52.4) | 3 (33.3) |
| Stiff hand syndrome (%) | 28 (42.4) | 9 (56.3) | 17 (38.6) | 2 (28.6) |
| Neuropathic shoulder pain by DN4 (score ≥ 5) | 2 (3.0) | 0 | 1 (1.6) | 1 (5.2) |
| Polyneuropathy | ||||
| Clinical | 19 (28.8) | 5 (29.4) | 11 (19.2) | 3 (15.7) |
| Subclinical | 37 (56.1) | 11 (64.7) | 22 (38.5) | 4 (21.1) |
| Ultrasound-Diagnosed Disorders | All 66 Patients with 132 Shoulders | |||||
|---|---|---|---|---|---|---|
| Symptomatic Shoulders (n = 93) | Asymptomatic Shoulders (n = 39) | |||||
| n | % | 95% CI | n | % | 95% CI | |
| Subacromial pain disorders | 84 | 90.3 | 81.9–95.2 | 30 | 76.9 | 60.2–88.2 |
| Subacromial bursitis | 13 | 14.0 | 7.9–23.1 | 3 | 7.7 | 2.0–21.9 |
| Rotator cuff disorder | 84 | 90.3 | 81.9–95.2 | 30 | 76.9 | 60.2–88.2 |
| LHBT disorder | 10 | 10.7 | 5.5–19.3 | 3 | 7.6 | 2.01–21.9 |
| Dynamic impingement | 14 | 15.1 | 8.7–24.3 | 3 | 7.6 | 2.0–21.9 |
| Glenohumeral disorders | 8 | 8.6 | 4.1–16.7 | 0 | 0 | 0 |
| Adhesive capsulitis | 4 | 4.3 | 1.3–11.2 | 0 | 0 | 0 |
| GH effusion only | 4 | 4.3 | 1.3–11.2 | 0 | 0 | 0 |
| Other disorders | ||||||
| Acromioclavicular OA | 55 | 59.1 | 48.4–69.1 | 17 | 43.5 | 28.1–60.2 |
| No disorders | 0 | 0 | 0 | 0 | 0 | 0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alabdali, L.A.S.; Jaeken, J.; van Alfen, N.; Dinant, G.-J.; Borghans, R.A.P.; Ottenheijm, R.P.G. What Is the Diagnosis in Patients with Type 2 Diabetes Who Have a Painful Shoulder? Results from a Prospective Cross-Sectional Study. J. Clin. Med. 2020, 9, 4097. https://doi.org/10.3390/jcm9124097
Alabdali LAS, Jaeken J, van Alfen N, Dinant G-J, Borghans RAP, Ottenheijm RPG. What Is the Diagnosis in Patients with Type 2 Diabetes Who Have a Painful Shoulder? Results from a Prospective Cross-Sectional Study. Journal of Clinical Medicine. 2020; 9(12):4097. https://doi.org/10.3390/jcm9124097
Chicago/Turabian StyleAlabdali, Login Ahmed S., Jasmien Jaeken, Nens van Alfen, Geert-Jan Dinant, Rob A. P. Borghans, and Ramon P. G. Ottenheijm. 2020. "What Is the Diagnosis in Patients with Type 2 Diabetes Who Have a Painful Shoulder? Results from a Prospective Cross-Sectional Study" Journal of Clinical Medicine 9, no. 12: 4097. https://doi.org/10.3390/jcm9124097
APA StyleAlabdali, L. A. S., Jaeken, J., van Alfen, N., Dinant, G.-J., Borghans, R. A. P., & Ottenheijm, R. P. G. (2020). What Is the Diagnosis in Patients with Type 2 Diabetes Who Have a Painful Shoulder? Results from a Prospective Cross-Sectional Study. Journal of Clinical Medicine, 9(12), 4097. https://doi.org/10.3390/jcm9124097





