Intraductal Papillary Neoplasm of Bile Duct: Updated Clinicopathological Characteristics and Molecular and Genetic Alterations
Abstract
1. Introduction
2. Clinical Features, Epidemiology and Imaging and Endoscopic Findings of IPNBs
2.1. Clinical Features, Epidemiology and Risks, Related Diseases and Complication
2.1.1. Clinical Features
2.1.2. Epidemiology and Risks
2.1.3. Related Diseases Outside the Hepatobiliary System
2.1.4. Complication
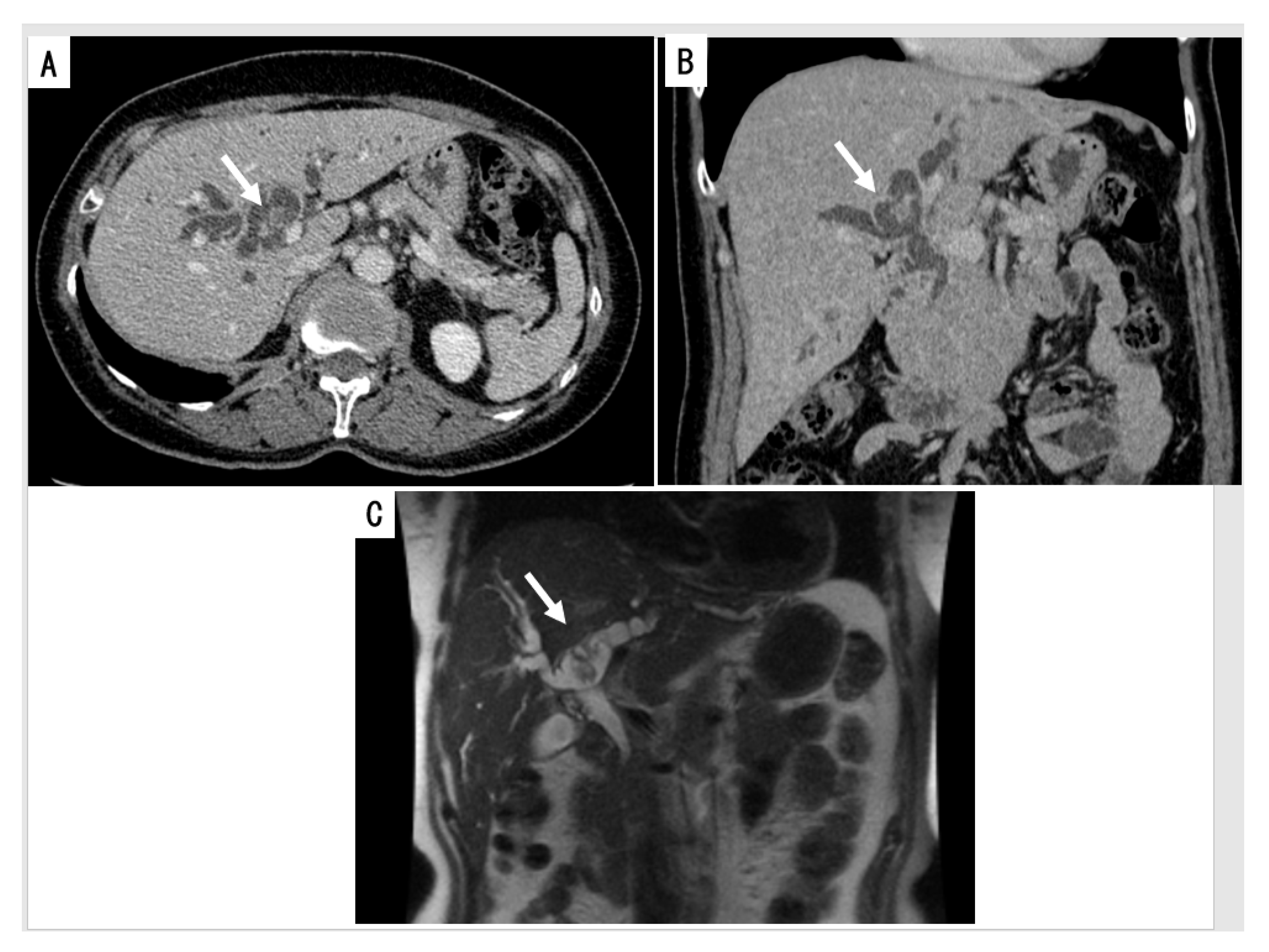
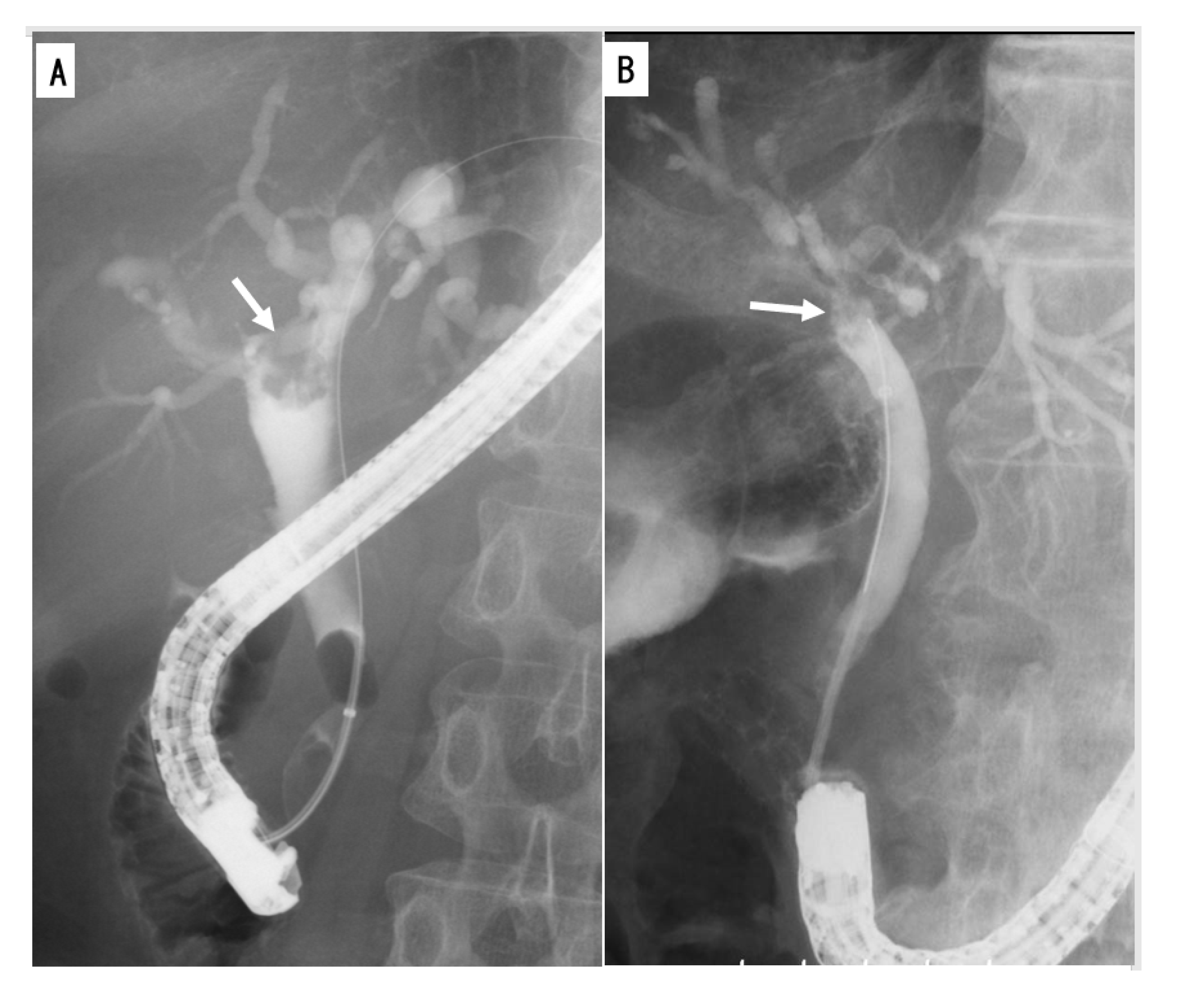
2.2. Imaging and Endoscopy
2.2.1. Cross Sectional Imaging
2.2.2. Cholangiography
2.2.3. Intraductal Ultrasonography (IDUS)
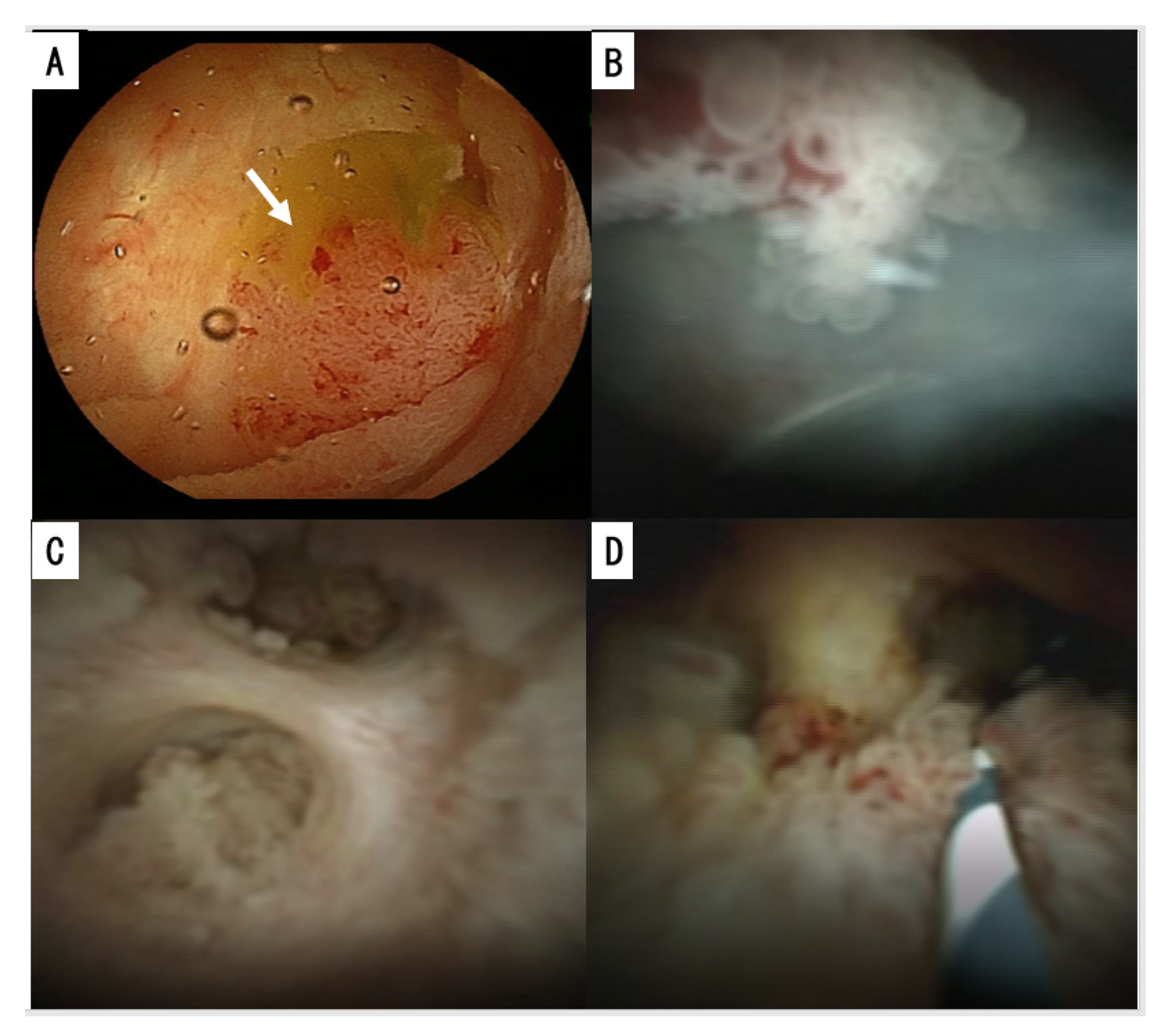
2.2.4. Cholangioscopy and Duodenoscopy
3. Pathologies of IPNBs
3.1. Location along the Biliary Tree
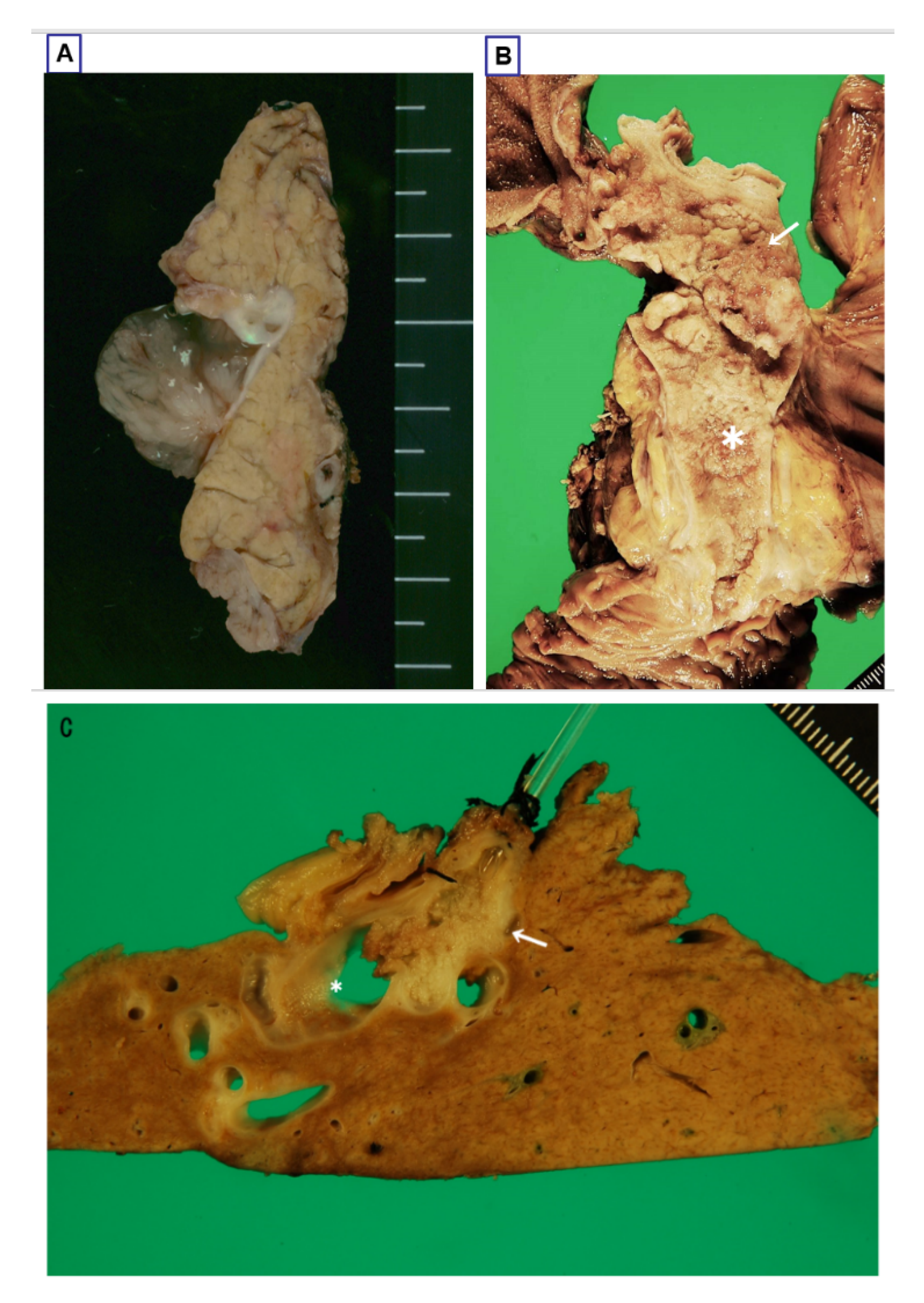
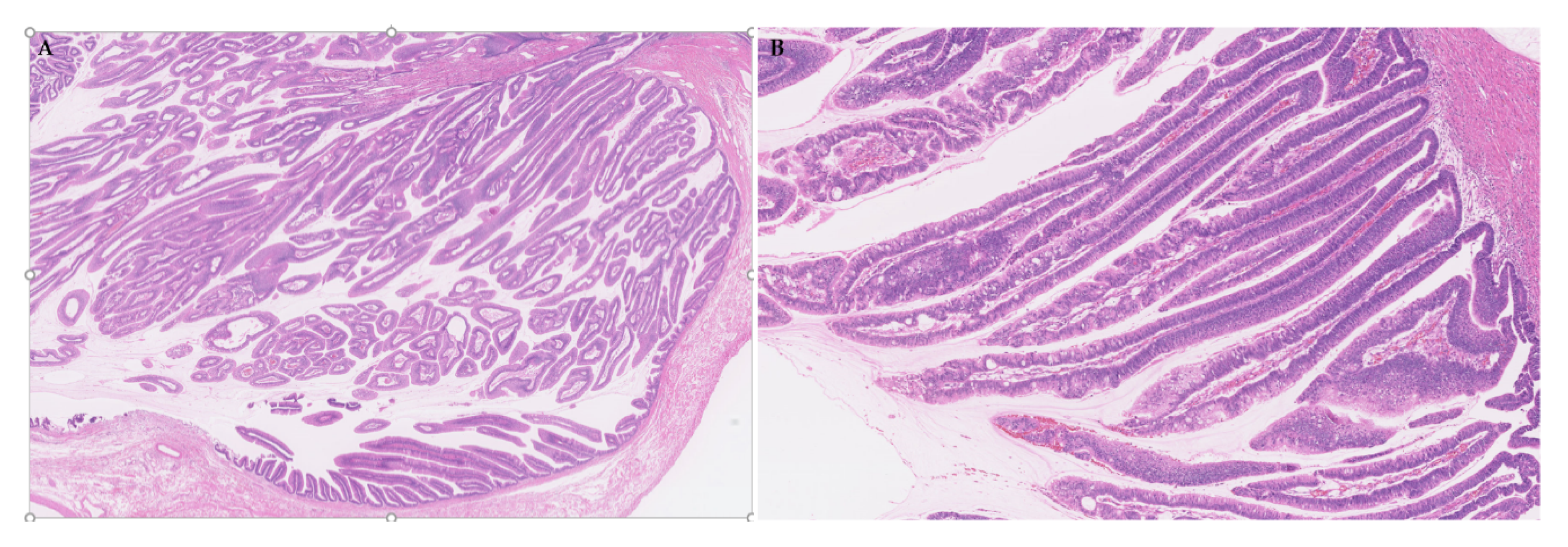
3.2. Gross Features
3.2.1. Intraductal Tumors
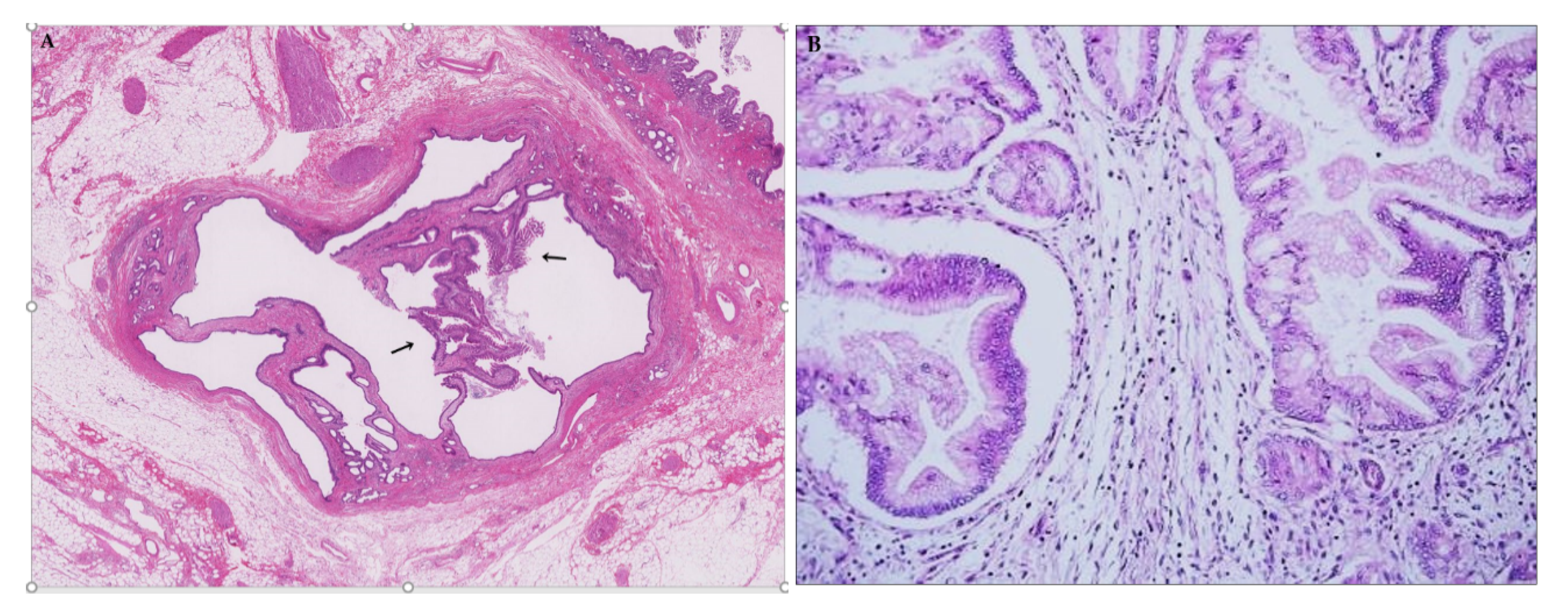
3.2.2. Mucin Hypersecretion
3.2.3. Bile Duct Dilatation
3.2.4. Classification Based on the Radio-Pathological Appearance
3.3. Histologies
3.3.1. General Features
3.3.2. Four Subtypes
3.3.3. Two Tiered Grading: Low- and High-Grade Dysplasia
3.3.4. Invasion and Metastasis and Recurrence
Invasion
Lymph Node Metastasis
Recurrence
3.4. Variants
3.4.1. Bile Duct Dilatation with Microscopic IPNB (Superficial Spreading IPNB)
3.4.2. IPNB Arising in Peribiliary Glands and Other Parts of the Liver
4. A Novel Subclassification of IPNB Based on Cytoarchitectural Alterations
4.1. Morphological Features Characterizing Type 1 and 2
4.1.1. Type 1
4.1.2. Type 2
4.2. Characteristic Findings of Types 1 and 2 in Recent Clinical Studies
5. Genetic Changes of IPNBs
5.1. General Survey
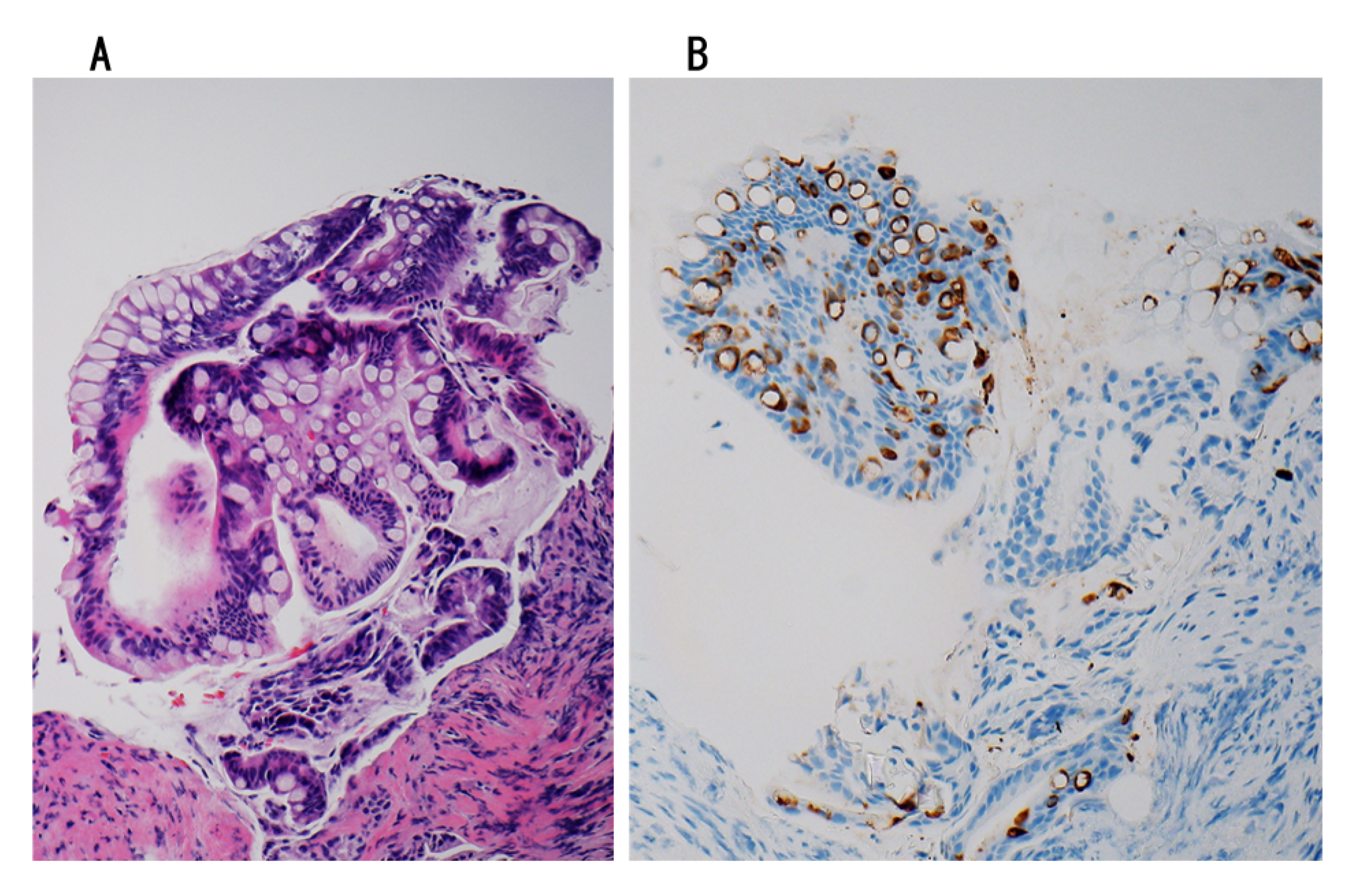
5.2. Four Subtypes
5.2.1. IPNB with Intestinal Differentiation
5.2.2. IPNB with Non-Intestinal Differentiation
5.2.3. IPNB with Oncocytic Differentiation
5.3. Type 1 and 2 Subclassification
5.4. Similarities and Dissimilarities to IPMN
6. Molecular Alterations and Signal Pathways in Development and Progression of IPNBs
6.1. Different Backgrounds and Risks
6.2. Low- and High-Grade Dysplasia
6.3. Stromal Invasion and Occurrence of Complicated Lesions
6.4. Targettable Genes and Proteins in IPNB
7. The Diagnosis, Treatment and Prognosis, Including the Post-Operative Outcomes, of IPNBs
7.1. Preoperative Diagnosis
7.2. Treatment
7.2.1. Surgical Resection
7.2.2. Non-Surgical Treatment
7.3. Post-Operative Outcomes and Influencing Factors
7.3.1. Gross Features
7.3.2. Anatomical Location
7.3.3. Invasion
7.3.4. Subtypes
7.3.5. Subclassification: Type 1 or 2
7.3.6. Surgical Margin
7.3.7. Metastasis
7.3.8. Others
7.4. Staging (TNM)
8. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- WHO Classification of Tumours Editorial Board. WHO Classification of Tumours, Digestive System Tumours, 5th ed.; International Agency for Reearch on Cancer: Lyon, France, 2019; pp. 1–635. [Google Scholar]
- Nakanuma, Y.; Sudo, Y. Biliary tumors with pancreatic counterparts. Semin. Diagn. Pathol. 2017, 34, 167–175. [Google Scholar] [CrossRef]
- Nakanuma, Y.; Basturk, O.; Esposito, I.; Limstra, D.S.; Komuta, M.; Zen, Y. Intraductal papillary neoplasm of the bile ducts. In The WHO Classification of Tumours Editoral Board, WHO Classification of Tumours of Digestive System, 5th ed.; IARC: Lyon, France, 2019; pp. 279–282. [Google Scholar]
- Basturk, O.; Aishima, S.; Esposito, I. Biliaray intraepithelial neoplasia. In The WHO Classification of Tumours Editoral Board, WHO Classification of Tumours of Digestive System, 5th ed.; International Agency for Research on Cancer: Lyon, France, 2019; pp. 273–275. [Google Scholar]
- Hucl, T. Precursors to Cholangiocarcinoma. Gastroenterol. Res. Pract. 2019. [Google Scholar] [CrossRef] [PubMed]
- Aishima, S.; Kubo, Y.; Tanaka, Y.; Oda, Y. Histological features of precancerous and early cancerous lesions of biliary tract carcinoma. J. Hepatobiliary Pancreat Sci. 2014, 21, 448–452. [Google Scholar] [CrossRef]
- Yoon, K.C.; Yu, Y.D.; Kang, W.H.; Kim, D.S.; Kim, J.Y. Prevalence and clinical significance of biliary intraepithelial neoplasia (BilIN) in cholangiocarcinoma. Am. Surg. 2019, 85, 511–517. [Google Scholar] [CrossRef]
- Chen, T.C.; Nakanuma, Y.; Zen, Y.; Zen, Y.; Chen, M.F.; Jan, Y.Y.; Yeh, T.S.; Chiu, C.T.; Kuo, T.T.; Kamiya, J.; et al. Intraductal papillary neoplasia of the liver associated with hepatolithiasis. Hepatology 2001, 34, 651–658. [Google Scholar] [CrossRef]
- Schlitter, A.M.; Born, D.; Bettstetter, M.; Specht, K.; Kim-Fuchs, C.; Riener, M.O.; Jeliazkova, P.; Sipos, B.; Siveke, J.T.; Terris, B.; et al. Intraductal papillary neoplasm of the bile duct: Stepwise progression to carcinoma involves common molecular pathways. Mod. Pathol. 2014, 27, 73–86. [Google Scholar] [CrossRef]
- Loeffler, M.A.; Hu, J.; Kirchner, M.; Wei, X.; Xiao, Y.; Albrecht, T.; De La Torre, C.; Sticht, C.; Banales, J.M.; Vogel, M.N.; et al. miRNA profiling of biliary intraepithelial neoplasia reveals stepwise tumorigenesis in distal cholangiocarcinoma via the miR-451a/ATF2 axis. J. Pathol. 2020, 252, 239–251. [Google Scholar] [CrossRef]
- Sato, Y.; Sasaki, M.; Harada, K.; Aishima, S.; Fukusato, T.; Ojima, H.; Kanai, Y.; Kage, M.; Nakanuma, Y.; Tsubouchi, H. Hepatolithiasis Subdivision of Intractable Hepatobiliary Diseases Study Group of Japan (Chairman, Hirohito Tsubouchi). Pathological diagnosis of flat epithelial lesions of the biliary tract with emphasis on biliary intraepithelial neoplasia. J. Gastroenterol. 2014, 49, 64–72. [Google Scholar] [CrossRef]
- Nakanuma, Y.; Uchida, T.; Sato, Y.; Uesaka, K. An S100P-positive biliary epithelial field is a preinvasive intraepithelial neoplasm in nodular-sclerosing cholangiocarcinoma. Hum. Pathol. 2017, 60, 46–57. [Google Scholar] [CrossRef]
- Kubo, Y.; Aishima, S.; Tanaka, Y.; Shindo., K.; Mizuuchi, Y.; Abe, K.; Shirabe, K.; Maehara, Y.; Honda, H.; Oda, Y. Different expression of glucose transporters in the progression of intrahepatic cholangiocarcinoma. Hum. Pathol. 2014, 45, 1610–1617. [Google Scholar] [CrossRef]
- Zen, Y.; Adsay, N.V.; Bardadin, K.; Colombari, R.; Ferrell, L.; Haga, H.; Hong, S.M.; Hytiroglou, P.; Klöppel, G.; Lauwers, G.Y.; et al. Biliary intraepithelial neoplasia: An international interobserver agreement study and proposal for diagnostic criteria. Mod. Pathol. 2007, 20, 701–709. [Google Scholar] [CrossRef]
- Kubota, K.; Jang, J.Y.; Nakanuma, Y.; Jang, K.T.; Haruyama, Y.; Fukushima, N.; Furukawa, T.; Hong, S.M.; Sakuraoka, Y.; Kim, H.; et al. Clinicopathological characteristics of intraductal papillary neoplasm of the bile duct: A Japan-Korea collaborative study. J. Hepatobiliary Pancreat Sci. 2020. [Google Scholar] [CrossRef]
- Yang, J.; Wang, W.; Yan, L. The clinicopathological features of intraductal papillary neoplasms of the bile duct in a Chinese population. Digest Liver Dis. 2012, 44, 251–256. [Google Scholar] [CrossRef]
- Höhn, P.; Braumann, C.; Munding, J.; Tannapfel, A.; Uhl, W.; Künzli, B.M. Outcome determining factors of intraductal papillary neoplasm of the biliary tract (IPNB)—A single center survey and analysis of current literature. J. Gastrointest Cancer 2019, 50, 160–169. [Google Scholar] [CrossRef]
- Paik, K.Y.; Heo, J.S.; Choi, S.H.; Choi, D.W. Intraductal papillary neoplasm of the bile ducts: The clinical features and surgical outcome of 25 cases. J. Surg. Oncol. 2008, 97, 508–512. [Google Scholar] [CrossRef]
- Basturk, O.; Nakanuma, Y.; Aishima, S.; Esposito, I.; Klimstra, D.S.; Komuta, M.; Zen, Y. Mucinous cystic neoplasm of the liver and biliary system. In The WHO Classification of Tumours Editoral Board, WHO Classification of Tumours of Digestive System, 5th ed.; IARC: Lyon, France, 2019; pp. 250–253. [Google Scholar]
- Quigley, B.; Reid, M.D.; Pehlivanoglu, B.; Squires, M.H., 3rd; Maithel, S.; Xue, Y.; Hyejeong, C.; Akkas, G.; Muraki, T.; Kooby, D.A.; et al. Hepatobiliary mucinous cystic neoplasms with ovarian type stroma (so-called “hepatobiliary cystadenoma/cystadenocarcinoma”): Clinicopathologic analysis of 36 cases illustrates rarity of carcinomatous change. Am. J. Surg. Pathol. 2018, 42, 95–102. [Google Scholar] [CrossRef]
- Nakanuma, Y. A novel approach to biliary tract pathology based on similarities to pancreatic counterparts: Is the biliary tract an incomplete pancreas? Pathol. Int. 2010, 60, 419–429. [Google Scholar] [CrossRef]
- Zen, Y.; Fujii, T.; Itatsu, K.; Nakamura, K.; Minato, H.; Kasashima, S.; Kurumaya, H.; Katayanagi, K.; Kawashima, A.; Masuda, S.; et al. Biliary papillary tumors share pathological features with intraductal papillary mucinous neoplasm of the pancreas. Hepatology 2006, 44, 1333–1343. [Google Scholar] [CrossRef]
- Rocha, F.G.; Lee, H.; Katabi, N.; DeMatteo, R.P.; Fong, Y.; D’Angelica, M.I.; Allen, P.J.; Klimstra, D.S.; Jarnagin, W.R. Intraductal papillary neoplasm of the bile duct: A biliary equivalent to intraductal papillary mucinous neoplasm of the pancreas? Hepatology 2012, 56, 1352–1360. [Google Scholar] [CrossRef]
- Kloek, J.J.; van der Gaag, N.A.; Erdogan, D.; Rauws, E.A.; Busch, O.R.; Gouma, D.J.; ten Kate, F.J.; van Gulik, T.M. A comparative study of intraductal papillary neoplasia of the biliary tract and pancreas. Hum. Pathol. 2011, 42, 824–832. [Google Scholar] [CrossRef] [PubMed]
- Fukumura, Y.; Nakanuma, Y.; Kakuda, Y.; Takase, M.; Yao, T. Clinicopathological features of intraductal papillary neoplasms of the bile duct: A comparison with intraductal papillary mucinous neoplasm of the pancreas with reference to subtypes. Virchows Arch. 2017, 471, 65–76. [Google Scholar] [CrossRef] [PubMed]
- Minagawam, N.; Sato, N.; Mori, Y.; Tamura, T.; Higure, A.; Yamaguchi, K. A comparison between intraductal papillary neoplasms of the biliary tract (BT-IPMNs) and intraductal papillary mucinous neoplasms of the pancreas (P-IPMNs) reveals distinct clinical manifestations and outcomes. Eur. J. Surg. Oncol. 2013, 39, 554–558. [Google Scholar] [CrossRef] [PubMed]
- Matsubara, T.; Kozaka, K.; Matsui, O.; Nakanuma, Y.; Uesaka, K.; Inoue, D.; Yoneda, N.; Yoshida, K.; Kitao, A.; Yokka, A.; et al. Peribiliary glands: Development, dysfunction, related conditions and imaging findings. Abdom. Radiol. 2020, 45, 416–436. [Google Scholar] [CrossRef] [PubMed]
- Aslam, A.; Wasnik, A.P.; Shi, J.; Sahai, V.; Mendiratta-Lala, M. Intraductal papillary neoplasm of the bile duct (IPNB): CT and MRI appearance with radiology-pathology correlation. Clin. Imaging 2020, 66, 10–17. [Google Scholar] [CrossRef]
- Katabathina, V.S.; Flaherty, E.M.; Dasyam, A.K.; Menias, C.O.; Riddle, N.D.; Lath, N.; Kozaka, K.; Matsui, O.; Nakanuma, Y.; Prasad, S.R. “Biliary diseases with pancreatic counterparts”: Cross-sectional imaging findings. Radiographics 2016, 36, 374–392. [Google Scholar] [CrossRef]
- Nakanuma, Y.; Kakuda, Y.; Uesaka, K.; Miyata, T.; Yamamoto, Y.; Fukumura, Y.; Sato, Y.; Sasaki, M.; Harada, K.; Takase, M. Characterization of intraductal papillary neoplasm of bile duct with respect to histopathologic similarities to pancreatic intraductal papillary mucinous neoplasm. Hum. Pathol. 2016, 51, 103–113. [Google Scholar] [CrossRef]
- Ohtsuka, M.; Kimura, F.; Shimizu, H.; Yoshidome, H.; Kato, A.; Yoshitomi, H.; Furukawa, K.; Takeuchi, D.; Takayashiki, T.; Suda, K.; et al. Similarities and differences between intraductal papillary tumors of the bile duct with and without macroscopically visible mucin secretion. Am. J. Surg. Pathol. 2011, 35, 512–521. [Google Scholar] [CrossRef]
- Nakanuma, Y.; Kakuda, Y.; Uesaka, K. Characterization of intraductal papillary neoplasm of the bile duct withrespect to the histopathologic similarities to pancreatic intraductal papillary mucinous neoplasm. Gut Liver. 2019, 13, 617–627. [Google Scholar] [CrossRef]
- Nakanuma, Y.; Uesaka, K.; Terada, T.; Fukumura, Y.; Sugino, T.; Kakuda, Y.; Ikeda, H.; Harada, K.; Sato, Y.; Shimizu, S.; et al. Gastric subtype of intraductal papillary neoplasm of the bile duct: The pathologic spectrum. J. Hepatobiliary Pancreat Sci. 2020, 27, 402–413. [Google Scholar] [CrossRef]
- Matthaei, H.; Wu, J.; Dal Molin, M.; Debeljak, M.; Lingohr, P.; Katabi, N.; Klimstra, D.S.; Adsay, N.V.; Eshleman, J.R.; Schulick, R.D.; et al. GNAS codon 201 mutations are uncommon in intraductal papillary neoplasms of the bile duct. HPB 2012, 14, 677–683. [Google Scholar] [CrossRef]
- Gordon-Weeks, A.N.; Jones, K.; Harriss, E.; Smith, A.; Silva, M. Systematic review and meta-analysis of current experience in treating IPNB: Clinical and pathological correlates. Ann. Surg. 2016, 263, 656–663. [Google Scholar] [CrossRef] [PubMed]
- Marín-Serrano, E.; Barbado Cano, A. Intraductal papillary neoplasm of the bile duct: A recurring disease. Rev. Esp. Enferm. Dig. 2019, 111, 890–891. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.R.; Lee, K.B.; Kwon, W.; Kim, E.; Kim, S.W.; Jang, J.Y. Comparison of the clinicopathologic characteristics of intraductal papillary neoplasm of the bile duct according to morphological and anatomical classifications. J. Korean Med. Sci. 2018, 33, e266. [Google Scholar] [CrossRef] [PubMed]
- Fujikura, K.; Fukumoto, T.; Ajiki, T.; Otani, K.; Kanzawa, M.; Akita, M.L.; Kido, M.; Ku, Y.; Itoh, T.; Zen, Y. Comparative clinicopathological study of biliary intraductal papillary neoplasms and papillary cholangiocarcinomas. Histopathology 2016, 69, 950–961. [Google Scholar] [CrossRef]
- Kubota, K.; Nakanuma, Y.; Kondo, F.; Hachiya, H.; Miyazaki, M.; Nagino, M.; Yamamoto, M.; Isayama, H.; Tabata, M.; Kinoshita, H.; et al. Clinicopathological features and prognosis of mucin-producing bile duct tumor and mucinous cystic tumor of the liver: A multi-institutional study by the Japan Biliary Association. J. Hepatobiliary Pancreat Sci. 2014, 21, 176–185. [Google Scholar] [CrossRef]
- Luvira, V.; Pugkhem, A.; Bhudhisawasdi, V.; Pairojkul, C.; Sathitkarnmanee, E.; Luvira, V.; Kamsa-Ard, S. Long-term outcome of surgical resection for intraductal papillary neoplasm of the bile duct. J. Gastroenterol. Hepatol. 2017, 32, 527–533. [Google Scholar] [CrossRef]
- Luvira, V.; Somsap, K.; Pugkhem, A.; Eurboonyanun, C.; Luvira, V.; Bhudhisawasdi, V.; Pairojkul, C.; Kamsa Ard, S. Morphological classification of intraductal papillary neoplasm of the bile duct with survival correlation. Asian Pac. J. Cancer Prev. 2017, 18, 207–213. [Google Scholar] [CrossRef]
- Wan, X.S.; Xu, Y.Y.; Qian, J.Y.; Yang, X.B.; Wang, A.Q.; He, L.; Zhao, H.T.; Sang, X.T. Intraductal papillary neoplasm of the bile duct. World J. Gastroenterol. 2013, 19, 8598–8604. [Google Scholar] [CrossRef]
- Siripongsakun, S.; Sapthanakorn, W.; Mekraksakit, P.; Vichitpunt, S.; Chonyuen, S.; Seetasarn, J.; Bhumiwat, S.; Sricharunrat, T.; Srittanapong, S. Premalignant lesions of cholangiocarcinoma: Characteristics on ultrasonography and MRI. Abdom. Radiol. 2019, 44, 2133–2146. [Google Scholar] [CrossRef]
- Jung, G.; Park, K.M.; Lee, S.S.; Yu, E.; Hong, S.M.; Kim, J. Long-term clinical outcome of the surgically resected intraductal papillary neoplasm of the bile duct. J. Hepatol. 2012, 57, 787–793. [Google Scholar] [CrossRef]
- Zen, Y.; Jang, K.T.; Ahn, S.; Kim, D.H.; Choi, D.W.; Choi, S.H.; Heo, J.S.; Yeh, M.M. Intraductal papillary neoplasms and mucinous cystic neoplasms of the hepatobiliary system: Demographic differences between Asian and Western populations, and comparison with pancreatic counterparts. Histopathology 2014, 65, 164–173. [Google Scholar] [CrossRef]
- Lim, J.H.; Jang, K.T.; Choi, D. Biliary intraductal papillary-mucinous neoplasm manifesting only as dilatation of the hepatic lobar or segmental bile ducts: Imaging features in six patients. AJR Am. J. Roentgenol. 2008, 191, 78–82. [Google Scholar] [CrossRef]
- Barton, J.G.; Barrett, D.A.; Maricevich, M.A.; Schnelldorfer, T.; Wood, C.M.; Smyrk, T.C.; Baron, T.H.; Sarr, M.G.; Donohue, J.H.; Farnell, M.B.; et al. Intraductal papillary mucinous neoplasm of the biliary tract: A real disease? HPB 2009, 11, 684–691. [Google Scholar] [CrossRef]
- Ren, X.; Zhu, C.L.; Qin, X.F.; Jiang, H.; Xia, T.; Qu, Y.P. Co-occurrence of IPMN and malignant IPNB complicated by a pancreatobiliary fistula: A case report and review of the literature. World J. Clin. Cases 2019, 7, 102–108. [Google Scholar] [CrossRef]
- Park, H.J.; Kim, S.Y.; Kim, H.J.; Lee, S.S.; Hong, G.S.; Byun, J.H.; Hong, S.M.; Lee, M.G. Intraductal papillary neoplasm of the bile duct: Clinical, imaging, and pathologic features. Am. J. Roentgenol. 2018, 211, 67–75. [Google Scholar] [CrossRef]
- Hachiya, H.; Kita, J.; Shiraki, T.; Iso, Y.; Shimoda, M.; Kubßota, K. Intraductal papillary neoplasm of the bile duct developing in a patient with primary sclerosing cholangitis: A case report. World J. Gastroenterol. 2014, 20, 15925–15930. [Google Scholar] [CrossRef]
- Kim, B.S.; Joo, S.H.; Lim, S.J.; Joo, K.R. Intrahepatic biliary intraductal papillary mucinous neoplasm with gallbladder agenesis: Case report. Surg. Laparosc. Endosc. Percutan. Tech. 2013, 23, 61–64. [Google Scholar] [CrossRef]
- Nakanuma, Y.; Kakuda, Y. Pathologic classification of cholangiocarcinoma: New concepts. Best Pract. Res. Clin. Gastroenterol. 2015, 29, 277–293. [Google Scholar] [CrossRef]
- Kubo, S.; Nakanuma, Y.; Takemura, S.; Sakata, C.; Urata, Y.; Nozawa, A.; Nishioka, T.; Kinoshita, M.; Hamano, G.; Terajima, H.; et al. Case series of 17 patients with cholangiocarcinoma among young adult workers of a printing company in Japan. J. Hepatobiliary Pancreat Sci. 2014, 21, 479–488. [Google Scholar] [CrossRef]
- Sato, Y.; Kubo, S.; Takemura, S.; Sugawara, Y.; Tanaka, S.; Fujikawa, M.; Arimoto, A.; Harada, K.; Sasaki, M.; Nakanuma, Y.I. Different carcinogenic process in cholangiocarcinoma cases epidemically developing among workers of a printing company in Japan. Int. J. Clin. Exp. Pathol. 2014, 7, 4745–4754. [Google Scholar]
- Kubo, S.; Takemura, S.; Tanaka, S.; Shinkawa, H.; Kinoshita, M.; Hamano, G.; Ito, T.; Koda, M.; Aota, T.; Yamamoto, T.; et al. Outcomes after resection of occupational cholangiocarcinoma. J. Hepatobiliary Pancreat Sci. 2016, 23, 556–564. [Google Scholar] [CrossRef] [PubMed]
- Date, K.; Ohtsuka, T.; Fujimoto, T.; Gotoh, Y.; Nakashima, Y.; Kimura, H.; Matsunaga, T.; Mori, Y.; Mochidome, N.; Miyazaki, T.; et al. GNAS and KRAS mutational analyses of intraductal papillary neoplasms of the pancreas and bile duct developing in the same individual: A case report. Pancreatology 2015, 15, 713–716. [Google Scholar] [CrossRef] [PubMed]
- Ishida, M.; Seki, K.; Honda, K.; Kimura, T.; Katayama, K.; Hirose, K.; Dojo, M.; Azuma, T.; Imamura, Y.; Hutchins, R.R.; et al. Intraductal mucinous tumors occurring simultaneously in the liver and pancreas. J. Gastroenterol. 2002, 37, 1073–1078. [Google Scholar] [CrossRef] [PubMed]
- Riall, T.S.; Stager, V.M.; Nealon, W.H.; Townsend, C.M., Jr.; Kuo, Y.F.; Goodwin, J.S.; Freeman, J.L. Incidence of additional primary cancers in patients with invasive intraductal papillary mucinous neoplasms and sporadic pancreatic adenocarcinomas. J. Am. Coll. Surg. 2007, 204, 803–813. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, M.; Kobayashi, K.; Mizumoto, K.; Yamaguchi, K. Clinical aspects of intraductal papillary mucinous neoplasm of the pancreas. J. Gastroenterol. 2005, 40, 669–675. [Google Scholar] [CrossRef]
- Brennan, G.T.; Lee, J.G. Metachronous intraductal papillary neoplasm of the bile duct and intraductal papillary mucinous neoplasm of the pancreas in a patient diagnosed with mucinous adenocarcinoma. ACG Case Rep. J. 2019, 6, e00023. [Google Scholar] [CrossRef]
- Lee, J.M.; Lee, J.M.; Hyun, J.J.; Choi, H.S.; Kim, E.S.; Keum, B.; Jeen, Y.T.; Chun, H.J.; Lee, H.S.; Kim, C.D.; et al. Intraductal papillary bile duct adenocarcinoma and gastrointestinal stromal tumor in a case of neurofibromatosis type. World J. Gastroenterol. 2018, 24, 537–542. [Google Scholar] [CrossRef]
- Xu, J.; Sato, Y.; Harada, K.; Yoneda, N.; Ueda, T.; Kawashima, A.; Ooi, A.; Nakanuma, Y. Intraductal papillary neoplasm of the bile duct in liver cirrhosis with hepatocellular carcinoma. World J. Gastroenterol. 2011, 17, 1923–1926. [Google Scholar] [CrossRef]
- Terasaki, F.; Sugiura, T.; Uesaka, K. Intraductal papillary neoplasm of the bile duct accompanied by hepatogastric fistula. J. Hepatobiliary Pancreat Sci. 2020, 27, 352–353. [Google Scholar] [CrossRef]
- Takahashi, N.; Taniguchi, T.; Adachi, M. A case of needle tract seeding of an intraductal papillary neoplasm of the bile duct (IPNB) after percutaneous biopsy. Eur. J. Dermatol. 2014, 24, 128–130. [Google Scholar] [CrossRef]
- Yoon, H.J.; Kim, Y.K.; Jang, K.T.; Lee, K.T.; Lee, J.K.; Choi, D.W.; Lim, J.H. Intraductal papillary neoplasm of the bile ducts: Description of MRI and added value of diffusion-weighted MRI. Abdom. Imaging 2013, 38, 1082–1090. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Zhao, F.; Pan, J.; Liin, G.; Chen, B.; Fu, W. Cystic intraductal papillary neoplasms with infiltrating carcinoma of the intrahepatic bile duct: A case report. Medicine 2020, 99, e18758. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Kim, M.J.; Kim, S.; Choi, D.; Jang, K.T.; Park, Y.N. Intraductal papillary neoplasm of the bile duct: Assessment of invasive carcinoma and long-term outcomes using MRI. J. Hepatol. 2019, 70, 692–699. [Google Scholar] [CrossRef] [PubMed]
- Tsuyuguchi, T.; Sakai, Y.; Sugiyama, H.; Miyakawa, K.; Ishihara, T.; Ohtsuka, M.; Miyazaki, M.; Yokosuka, O. Endoscopic diagnosis of intraductal papillary mucinous neoplasm of the bile duct. Hepatobiliary Pancreat Sci. 2010, 17, 230–235. [Google Scholar] [CrossRef]
- Yeh, T.S.; Tseng, J.H.; Chiu, C.T.; Liu, N.J.; Chen, T.C.; Jan, Y.Y.; Chen, M.F. Cholangiographic spectrum of intraductal papillary mucinous neoplasm of the bile ducts. Ann. Surg. 2006, 244, 248–253. [Google Scholar] [CrossRef] [PubMed]
- Hyodo, T.; Kumano, S.; Kushihata, F.; Okada, M.; Hirata, M.; Tsuda, T.; Takada, Y.; Mochizuki, T.; Murakami, T. CT and MR cholangiography: Advantages and pitfalls in perioperative evaluation of biliary tree. Br. J. Radiol. 2012, 85, 887–896. [Google Scholar] [CrossRef]
- Yeh, T.S.; Tseng, J.H.; Chen, T.C.; Liu, N.J.; Chiu, C.T.; Jan, Y.Y.; Chen, M.F. Characterization of intrahepatic cholangiocarcinoma of the intraductal growth-type and its precursor lesions. Hepatology 2005, 42, 657–764. [Google Scholar] [CrossRef]
- Choi, E.R.; Chung, Y.H.; Lee, J.K.; Lee, K.T.; Lee, K.H.; Choi, D.W.; Choi, S.H.; Heo, J.S.; Jang, K.T.; Park, S.M.; et al. Preoperative evaluation of the longitudinal extent of borderline resectable hilar cholangiocarcinoma by intraductal ultrasonography. J. Gastroenterol. Hepatol. 2011, 26, 1804–1810. [Google Scholar] [CrossRef]
- Takanami, K.; Yamada, T.; Tsuda, M.; Takase, K.; Ishida, K.; Nakamura, Y.; Kanno, A.; Shimosegawa, T.; Unno, M.; Takahashi, S. Intraductal papillary mucininous neoplasm of the bile ducts: Multimodality assessment with pathologic correlation. Abdom. Imaging 2011, 36, 447–456. [Google Scholar] [CrossRef]
- Ito, Y.; Shibutani, S.; Egawa, T.; Hayashi, S.; Nagashima, A.; Kitagawa, Y. Utility of intraductal ultrasonography as a diagnostic tool in patients with early distal cholangiocarcinoma. Hepatogastroenterology 2015, 62, 782–786. [Google Scholar]
- Itoi, T.; Sofuni, A.; Itokawa, F.; Tsuchiya, T.; Kurihara, T.; Ishii, K.; Tsuji, S.; Moriyasu, F.; Gotoda, T. Peroral cholangioscopic diagnosis of biliary-tract diseases by using narrow-band imaging (with videos). Gastrointest Endosc. 2007, 66, 730–736. [Google Scholar] [CrossRef]
- Igarashi, Y.; Okano, N.; Ito, K.; Suzuki, T.; Mimura, T. Effectiveness of peroral cholangioscopy and narrow band imaging for endoscopically diagnosing the bile duct cancer. Dig. Endosc. 2009, 21 (Suppl. 1), S101–S102. [Google Scholar] [CrossRef]
- Itoi, T.; Sofuni, A.; Itokawa, F.; Tsuchiya, T.; Kurihara, T. Evaluation of peroral videocholangioscopy using narrow-band imaging for diagnosis of intraductal papillary neoplasm of the bile duct. Dig. Dig. Endosc. 2009, 21 (Suppl. 1), S103–S107. [Google Scholar] [CrossRef]
- Hajer, J.; Havlůj, L.; Whitley, A.; Oliverius, M.; Gürlich, R. The role of single-operator cholangioscopy (SpyGlass) in the intraoperative diagnosis of intraductal borders of cholangiocarcinoma proliferation - pilot study. Cas Lek Cesk. 2019, 58, 68–72. [Google Scholar]
- Bill, J.G.; Chaterjee, D.; Mullady, D.K. Using peroral cholangioscopy to diagnose an intraductal papillary neoplasm of the bile duct. VideoGIE 2017, 3, 55–57. [Google Scholar] [CrossRef]
- Kung, J.W.; Parks, R.W.; Ireland, H.M.; Kendall, T.J.; Church, N.I. Intraductal papillary neoplasm of the bile duct: The role of single-operator cholangioscopy. VideoGIE 2017, 3, 55–57. [Google Scholar] [CrossRef][Green Version]
- Park, M.S.; Kim, T.K.; Kim, K.W.; Park, S.W.; Lee, J.K.; Kim, J.S.; Lee, J.H.; Kim, K.A.; Kim, A.Y.; Kim, P.N.; et al. Differentiation of extrahepatic bile duct cholangiocarcinoma from benign stricture: Findings at MRCP versus ERCP. Radiology 2004, 233, 234–240. [Google Scholar] [CrossRef]
- Patel, A.; Sonnenday, C.J.; Schulman, A.R. Recurrent extrahepatic cholangiocarcinoma after bile duct resection for intraductal papillary mucinous neoplasm of the bile duct. VideoGIE 2019, 4, 519–521. [Google Scholar] [CrossRef]
- Nanashima, A.; Imamura, N.; Hiyoshi, M.; Hamada, T.; Yano, K.; Wada, T.; Kawakami, H.; Ban, T.; Kubota, Y.; Sato, Y.; et al. Planned limited resection of the extrahepatic bile duct in a case of intraductal papillary neoplasm of the bile duct based on preoperative examinations. Clin. J. Gastroenterol. 2020, 13, 233–239. [Google Scholar] [CrossRef]
- D’souza, M.A.; Isaksson, B.; Löhr, M.; Enochsson, L.; Swahn, F.; Lundell, L.; Arnelo, U. The clinicopathological spectrum and management of intraductal papillary mucinous neoplasm of the bile duct (IPMN-B). Scand J. Gastroenterol. 2013, 48, 473–479. [Google Scholar] [CrossRef]
- Tsou, Y.K.; Liu, N.J.; Wu, R.C.; Lee, C.S.; Tang, J.H.; Hung, C.F.; Jan, Y.Y. Endoscopic retrograde cholangiography in the diagnosis and treatment of mucobilia. Scand J. Gastroenterol. 2008, 43, 1137–1144. [Google Scholar] [CrossRef] [PubMed]
- Zen, Y.; Fujii, T.; Itatsu, K.; Nakamura, K.; Konishi, F.; Masuda, S.; Mitsui, T.; Asada, Y.; Miura, S.; Miyayama, S.; et al. Biliary cystic tumors with bile duct communication: A cystic variant of intraductal papillary neoplasm of the bile duct. Mod. Pathol. 2006, 19, 1243–1254. [Google Scholar] [CrossRef] [PubMed]
- Yokode, M.; Yamashita, Y.; Zen, Y. Biliary intraductal papillary neoplasm with metachronous multiple tumors - true multicentric tumors or intrabiliary dissemination: A case report and review of the literature. Mol. Clin. Oncol. 2017, 6, 315–320. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, D.; Hayashi, H.; Kitamura, F.; Uemura, N.; Miyata, T.; Okabe, H.; Imai, K.; Yamasita, Y.; Kubo, S.; Baba, H. Multiple cholangiocarcinomas in the intrahepatic and extrahepatic biliary tree due to dichloromethane exposure: A case report. Surg Case Rep. 2020, 6, 79. [Google Scholar] [CrossRef] [PubMed]
- Hokuto, D.; Nomi, T.; Yasuda, S.; Yoshikawa, T.; Ishioka, K.; Yamada, T.; Akahori, T.; Nakagawa, K.; Nagai, M.; Nakamura, K.; et al. Long-term observation and treatment of a widespread intraductal papillary neoplasm of the bile duct extending from the intrapancreatic bile duct to the bilateral intrahepatic bile duct: A case report. Int. J. Surg. Case Rep. 2017, 38, 166–171. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Lim, J.H.; Jang, K.T.; Kim, M.J.; Lee, J.; Lee, J.Y.; Choi, D.; Lim, H.K.; Choi, D.W.; Lee, J.K.; et al. Morphology of intraductal papillary neoplasm of the bile ducts: Radiologic-pathologic correlation. Abdom Imaging 2011, 36, 438–446. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, A.; Suzuki, H.; Kubo, N.; Araki, K.; Kobayashi, T.; Sasaki, S.; Wada, W.; Arai, H.; Sakamoto, K.; Sakurai, S.; et al. An oncocytic variant of intraductal papillary neoplasm of the bile duct that formed a giant hepatic cyst. Rare Tumors 2013, 5, e30. [Google Scholar] [CrossRef]
- Tsujimae, M.; Sakai, A.; Masuda, A.; Inomata, N.; Masuda, S.; Gonda, M.; Abe, S.; Yamakawa, K.; Ashina, S.; Kakihara, M.; et al. A case in which an intraductal papillary neoplasm of the bile duct was surgically resected 12 years after the initial diagnosis. Intern. Med. 2020. [Google Scholar] [CrossRef]
- Lim, J.H.; Zen, Y.; Jang, K.T.; Kim, Y.K.; Nakanuma, Y. Cyst-forming intraductal papillary neoplasm of the bile ducts: Description of imaging and pathologic aspects. AJR Am. J. Roentgenol. 2011, 197, 1111–1120. [Google Scholar] [CrossRef]
- Nakanuma, Y.; Jang, K.T.; Fukushima, N.; Furukawa, T.; Hong, S.M.; Kim, H.; Lee, K.B.; Zen, Y.; Jang, J.Y.; Kubota, K. A statement by the Japan-Korea expert pathologists for future clinicopathological and molecular analyses toward consensus building of intraductal papillary neoplasm of the bile duct through several opinions at the present stage. J. Hepatobiliary Pancreat Sci. 2018, 25, 181–187. [Google Scholar] [CrossRef]
- Nakanuma, Y.; Miyata, T.; Uchida, T.; Uesaka, K. Intraductal papillary neoplasm of bile duct is associated with a unique intraepithelial spreading pattern. Int. J. Clin. Exp. Pathol. 2016, 9, 11129–11138. [Google Scholar]
- Ohtsuka, M.; Shimizu, H.; Kato, A.; Yoshitomi, H.; Furukawa, K.; Tsuyuguchi, T.; Sakai, Y.; Yokosuka, O.; Miyazaki, M. Intraductal papillary neoplasms of the bile duct. Int. J. Hepatol. 2014, 2014, 459091. [Google Scholar] [CrossRef]
- Kim, K.M.; Lee, J.K.; Shin, J.U.; Lee, K.H.; Lee, K.T.; Sung, J.Y.; Jang, K.T.; Heo, J.S.; Choi, S.H.; Choi, D.W.; et al. Clinicopathologic features of intraductal papillary neoplasm of the bile duct according to histologic subtype. Am. J. Gastroenterol. 2012, 107, 118–125. [Google Scholar] [CrossRef]
- Kakisaka, T.; Kamiyama, T.; Yokoo, H.; Nakanishi, K.; Wakayama, K.; Tsuruga, Y.; Kamachi, H.; Mitsuhashi, T.; Taketomi, A. An intraductal papillary neoplasm of the bile duct mimicking a hemorrhagic hepatic cyst: A case report. World J. Surg. Oncol. 2013, 11, 111. [Google Scholar] [CrossRef]
- Kunovsky, L.; Kala, Z.; Svaton, R.; Moravcik, P.; Mazanec, J.; Husty, J.; Prochazka, V. Mucinous cystic neoplasm of the liver or intraductal papillary mucinous neoplasm of the bile duct? A Case Report and a Review of Literature. Ann. Hepatol. 2018, 17, 519–524. [Google Scholar] [CrossRef]
- Won, J.H.; Choi, S.Y.; Lee, H.K.; Yi, B.H.; Lee, M.H.; Jung, M.J. Accessory gallbladder in an intrahepatic location mimicking a cystic tumor of the liver: A case report. Medicine 2016, 95, e5293. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.R.; Jang, K.T.; Jang, J.Y.; Lee, K.; Kim, J.H.; Kim, H.; Kim, S.W.; Kwon, W.; Choi, D.W.; Heo, J.; et al. Clinicopathologic analysis of intraductal papillary neoplasm of bile duct: Korean multicenter cohort study. HPB 2020, 22, 1139–1148. [Google Scholar] [CrossRef]
- Matono, R.; Ninomiya, M.; Morita, K.; Tomino, T.; Oshiro, Y.; Yokota, T.; Nishizaki, T. Branch-type intraductal papillary neoplasm of the bile duct treated with laparoscopic anatomical resection: A case report. Surg. Case Rep. 2020, 6, 103. [Google Scholar] [CrossRef]
- Fujita, M.; Wakui, N.; Yamauchi, Y.; Takeda, Y.; Sato, T.; Ueki, N.; Otsuka, T.; Oba, N.; Nishinakagawa, S.; Minagawa, M.; et al. A case of branch duct type intraductal papillary neoplasm of the bile duct treated by open surgery after 11 years of follow-up. Mol. Clin. Oncol. 2013, 1, 965–969. [Google Scholar] [CrossRef][Green Version]
- Kato, H.; Tabata, M.; Azumi, Y.; Osawa, I.; Kishiwada, M.; Hamada, T.; Mizuno, S.; Usui, M.; Sakurai, H.; Isaji, S. Proposal for a morphological classification of intraductal papillary neoplasm of the bile duct (IPN-B). J. Hepatobiliary Pancreat Sci. 2013, 20, 165–1672. [Google Scholar] [CrossRef]
- Tanaka, M.; Fukushima, N.; Noda, N.; Shibahara, J.; Kokudo, N.; Fukayama, M. Intraductal oncocytic papillary neoplasm of the bile duct: Clinicopathologic and immunohistochemical characteristics of 6 cases. Hum. Pathol. 2009, 40, 1543–1552. [Google Scholar] [CrossRef]
- Nakanuma, Y.; Uesaka, K.; Miyayama, S.; Yamaguchi, H.; Ohtsuka, M. Intraductal neoplasms of the bile duct. A new challenge to biliary tract tumor pathology. Histol. Histopathol. 2017, 32, 1001–1015. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.J.; Hwang, S.; Lee, Y.J.; Kim, K.H.; Park, K.M.; Ahn, C.S.; Moon, D.B.; Ha, T.Y.; Song, G.W.; Jung, D.H.; et al. clinicopathological features and long-term outcomes of intraductal papillary neoplasms of the intrahepatic bile duct. J. Gastrointest Surg. 2016, 20, 1368–1375. [Google Scholar] [CrossRef]
- Uemura, S.; Higuchi, R.; Yazawa, T.; Izumo, W.; Matsunaga, Y.; Shiihara, M.; Ota, T.; Furukawa, T.; Yamamoto, M. Prognostic factors for surgically resected intraductal papillary neoplasm of the bile duct: A retrospective cohort study. Ann. Surg Oncol. 2020. [Google Scholar] [CrossRef]
- Onoe, S.; Shimoyama, Y.; Ebata, T.; Yokoyama, Y.; Igami, T.; Sugawara, G.; Nakamura, S.; Nagino, M. Prognostic delineation of papillary cholangiocarcinoma based on the invasive proportion: A single-institution study with 184 patients. Surgery 2014, 155, 280–291. [Google Scholar] [CrossRef]
- Onoe, S.; Shimoyama, Y.; Ebata, T.; Yokoyama, Y.; Igami, T.; Sugawara, G.; Mizuno, T.; Nakamura, S.; Nagino, M. Clinicopathological significance of mucin production in patients with papillary cholangiocarcinoma. World J. Surg. 2015, 39, 1177–1184. [Google Scholar] [CrossRef]
- Aoki, Y.; Mizuma, M.; Hata, T.; Aoki, T.; Omori, Y.; Ono, Y.; Mizukami, Y.; Unno, M.; Furukawa, T. Intraductal papillary neoplasms of the bile duct are consisted of two distinct types specifically, with clinicopathological features and molecular phenotypes. J. Pathol. 2020, 251, 38–48. [Google Scholar] [CrossRef]
- You, Y.; Choi, S.H.; Choi, D.W.; Heo, J.S.; Han, I.W.; Jang, K.T.; Han, S. Recurrence after resection for intraductal papillary neoplasm of bile duct (IPNB) according to tumor location. J. Gastrointest Surg. 2020, 24, 804–812. [Google Scholar] [CrossRef]
- Nanashima, A.; Sumida, Y.; Tamaru, N.; Nakanuma, Y.; Abo, T.; Tanaka, K.; Sawai, T.; Yasutake, T.; Nagayasu, T.; Hayashi, T.; et al. Intraductal papillary neoplasm of the bile duct extending superficially from the intrahepatic to extrahepatic bile duct. J. Gastroenterol. 2006, 41, 495–499. [Google Scholar] [CrossRef]
- Kang, M.J.; Jang, J.Y.; Lee, K.B.; Han, I.W.; Kim, S.W. Impact of macroscopic morphology, multifocality, and mucin secretion on survival outcome of intraductal papillary neoplasm of the bile duct. J. Gastrointest Surg. 2013, 17, 931–938. [Google Scholar] [CrossRef]
- Nakanuma, Y.; Sato, Y. Cystic and papillary neoplasm involving peribiliary glands: A biliary counterpart of branch-type intraductal papillary mucinous [corrected] neoplasm? Hepatology 2012, 55, 2040–2041. [Google Scholar] [CrossRef]
- Nakanishi, Y.; Zen, Y.; Hirano, S.; Tanaka, E.; Takahashi, O.; Yonemori, A.; Doumen, H.; Kawakami, H.; Itoh, T.; Nakanuma, Y.; et al. Intraductal oncocytic papillary neoplasm of the bile duct: The first case of peribiliary gland origin. J. Hepatobiliary Pancreat Surg. 2009, 16, 869–1873. [Google Scholar] [CrossRef]
- Miyata, T.; Uesaka, K.; Nakanuma, Y. Cystic and Papillary Neoplasm at the Hepatic hilum possibly originating in the peribiliary glands. Case Rep. Pathol. 2016, 2016, 9130754. [Google Scholar] [CrossRef]
- Pedica, F.; Heaton, N.; Quaglia, A. Peribiliary glands pathology in a large series of end-stage alcohol-related liver disease. Virchows Arch. 2020. [Google Scholar] [CrossRef] [PubMed]
- Sato, Y.; Harada, K.; Sasaki, M.; Nakanuma, Y. Cystic and micropapillary epithelial changes of peribiliary glands might represent a precursor lesion of biliary epithelial neoplasms. Virchows Arch. 2014, 464, 157–163. [Google Scholar] [CrossRef]
- Uchida, T.; Yamamoto, Y.; Ito, T.; Okamura, Y.; Sugiura, T.; Uesaka, K.; Nakanuma, Y. Cystic micropapillary neoplasm of peribiliary glands with concomitant perihilar cholangiocarcinoma. World J. Gastroenterol. 2016, 22, 2391–2397. [Google Scholar] [CrossRef]
- Terada, T.Y.; Ohta, G. Glandular elements around the intrahepatic bile ducts in man; their morphology and distribution in normal livers. Liver 1987, 7, 1–8. [Google Scholar] [CrossRef]
- Zen, Y.; Hubscher, S.G.; Nakanuma, Y. Bile duct diseases. In MacSween’s Pathology of the Liver; Elsevier: Amsterdam, The Netherlands, 2018; pp. 515–593. [Google Scholar]
- Onishi, I.; Kitagawa, H.; Harada, K.; Maruzen, S.; Sakai, S.; Makino, I.; Hayashi, H.; Nakagawara, H.; Tajima, H.; Takamura, H.; et al. Intraductal papillary neoplasm of the bile duct accompanying biliary mixed adenoneuroendocrine carcinoma. World J. Gastroenterol. 2013, 19, 3161–3164. [Google Scholar] [CrossRef]
- Albores-Saavedra, J.; Murakata, L.; Krueger, J.E.; Henson, D.E. Noninvasive and minimally invasive papillary carcinoma of the extrahepatic bile ducts. Cancer 2000, 89, 508–515. [Google Scholar] [CrossRef]
- Hoang, M.P.; Murakata, L.A.; Katabi, N.; Henson, D.E.; Albores-Saavedra, J. Invasive papillary carcinomas of the extrahepatic bile ducts: A clinicopathological and immunohistochemical study of 13 cases. Mod. Pathol. 2002, 15, 1251–1258. [Google Scholar] [CrossRef]
- Albores-Saavedra, J.; Adsay, N.V.; Crawford, J.M.; Klimstra, D.S. Carcinoma of the gallbladder and extrahepatic bile duct. In The WHO Classification of Tumours of the Digestive System, 4th ed.; Bosman, F.T., Carneiro, F., Hruban, R.H., Theise, N.D., Eds.; IARC: Lyon, France, 2010; pp. 266–273. [Google Scholar]
- Suh, K.S.; Roh, H.R.; Koh, H.R.; Lee, K.U.; Park, Y.H.; Kim, S.W. Clinicopathologic features of the intraductal growth type of peripheral cholangiocarcinoma. Hepatology 2000, 31, 12–17. [Google Scholar] [CrossRef]
- Okamoto, A.; Tsuruta, K.; Matsumoto, G.; Takahashi, T.; Kamisawa, T.; Egawa, N.; Funata, N. Papillary carcinoma of the extrahepatic bile duct: Characteristic features and implications in surgical treatment. J. Am. Coll Surg. 2003, 196, 394–401. [Google Scholar] [CrossRef]
- Liu, Y.; Zhong, X.; Yan, L.; Zheng, J.; Liu, Z.; Liang, C. Diagnostic performance of CT and MRI in distinguishing intraductal papillary neoplasm of the bile duct from cholangiocarcinoma with intraductal papillary growth. Eur. Radiol. 2015, 25, 1967–1974. [Google Scholar] [CrossRef]
- Yang, C.Y.; Huang, W.J.; Tsai, J.H.; Cheng, A.; Chen, C.C.; Hsu, H.P.; Jeng, Y.M. Targeted next-generation sequencing identifies distinct clinicopathologic and molecular entities of intraductal papillary neoplasms of the bile duct. Mod. Pathol. 2019, 32, 1637–1645. [Google Scholar] [CrossRef]
- Sasaki, M.; Matsubara, T.; Nitta, T.; Sato, Y.; Nakanuma, Y. GNAS and KRAS mutations are common in intraductal papillary neoplasms of the bile duct. PLoS ONE 2013. [Google Scholar] [CrossRef]
- Tsai, J.H.; Liau, J.Y.; Yuan, C.T.; Cheng, M.L.; Yuan, R.H.; Jeng, Y.M. RNF43 mutation frequently occurs with GNAS mutation and mucin hypersecretion in intraductal papillary neoplasms of the bile duct. Histopathology 2017, 70, 756–765. [Google Scholar] [CrossRef]
- Tsai, J.H.; Yuan, R.H.; Chen, Y.L.; Liau, J.Y.; Jeng, Y.M. GNAS Is frequently mutated in a specific subgroup of intraductal papillary neoplasms of the bile duct. Am. J. Surg. Pathol. 2013, 37, 1862–1870. [Google Scholar] [CrossRef]
- Xian, Z.H.; Qin, C.; Cong, W.M. KRAS mutation and immunohistochemical profile in intraductal papillary neoplasm of the intrahepatic bile ducts. Pathol. Res. Pract. 2018, 214, 105–111. [Google Scholar] [CrossRef]
- Nakanuma, Y.; Kakuda, Y.; Fukumura, Y.; Sugino, T.; Uesaka, K.; Serizawa, M.; Terada, T.; Ohnishi, Y. The pathologic and genetic characteristics of the intestinal subtype of intraductal papillary neoplasms of the bile duct. Am. J. Surg. Pathol. 2019, 43, 1212–1220. [Google Scholar] [CrossRef]
- Wu, J.; Matthaei, H.; Maitra, A.; Dal Molin, M.; Wood, L.D.; Eshleman, J.R.; Goggins, M.; Canto, M.I.; Schulick, R.D.; Edil, B.H.; et al. Recurrent GNAS mutations define an unexpected pathway for pancreatic cyst development. Sci. Transl. Med. 2011, 3, 92ra66. [Google Scholar] [CrossRef]
- Fujikura, K.; Fukumoto, T.; Ajiki, T.; Otani, K.; Kanzawa, M.; Akita, M.; Kido, M.; Ku, Y.; Itoh, T.; Zen, Y. Recurrent mutations in APC and CTNNB1 and activated Wnt/β-catenin signaling in intraductal papillary neoplasms of the bile duct: A whole exome sequencing study. Am. J. Surg. Pathol. 2018, 42, 1674–1685. [Google Scholar] [CrossRef]
- Singhi, A.D.; Wood, L.D.; Parks, E.; Torbenson, M.S.; Felsenstein, M.; Hruban, R.H.; Nikiforova, M.N.; Wald, A.I.; Kaya, C.; Nikiforov, Y.E.; et al. Recurrent rearrangements in PRKACA and PRKACB in intraductal oncocytic papillary neoplasms of the pancreas and bile duct. Gastroenterology 2020, 158, 573–582. [Google Scholar] [CrossRef]
- Nakahodo, J.; Fukumura, Y.; Saito, T.; Hirabayashi, K.; Doi, R.; Hayashi, T.; Yao, T. Upregulation of follistatin and low apoptotic activity in intraductal oncocytic papillary neoplasm of the pancreatobiliary system. Sci. Rep. 2020, 10, 8179. [Google Scholar] [CrossRef]
- Basturk, O.; Chung, S.M.; Hruban, R.H.; Adsay, N.V.; Askan, G.; Iacobuzio-Donahue, C.; Balci, S.; Zee, S.Y.; Memis, B.; Shia, J.; et al. Distinct pathways of pathogenesis of intraductal oncocytic papillary neoplasms and intraductal papillary mucinous neoplasms of the pancreas. Virchows Arch. 2016, 469, 523–532. [Google Scholar] [CrossRef]
- Basturk, O.; Esposito, I.; Fukushima, N.; Furukawa, T.; Hong, S.M.; Kloppel, G.; Maitra, A.; Zamboni, G. Pancreatic intraductal oncocytic papillary neoplasm. In The WHO Clasification of Tumours Editorial Board. The WHO Classificastion of Digestive System Tumour, 5th ed.; International Agency for Research on Cancer: Lyon, France, 2019; pp. 315–316. [Google Scholar]
- Zen, Y.; Sasaki, M.; Fujii, T.; Chen, T.C.; Chen, M.F.; Yeh, T.S.; Jan, Y.Y.; Huang, S.F.; Nimura, Y.; Nakanuma, Y. Different expression patterns of mucin core proteins and cytokeratins during intrahepatic cholangiocarcinogenesis from biliary intraepithelial neoplasia and intraductal papillary neoplasm of the bile duct—An immunohistochemical study of 110 cases of hepatolithiasis. J. Hepatol. 2006, 44, 350–358. [Google Scholar] [CrossRef]
- Jang, K.T.; Hong, S.M.; Lee, K.T.; Lee, J.G.; Choi, S.H.; Heo, J.S.; Choi, D.W.; Choi, D.; Lim, J.H. Intraductal papillary neoplasm of the bile duct associated with Clonorchis sinensis infection. Virchows Arch. 2008, 453, 589–598. [Google Scholar] [CrossRef]
- Itatsu, K.; Zen, Y.; Ohira, S.; Ishikawa, A.; Sato, Y.; Harada, K.; Ikeda, H.; Sasaki, M.; Nimura, Y.; Nakanuma, Y. Immunohistochemical analysis of the progression of flat and papillary preneoplastic lesions in intrahepatic cholangiocarcinogenesis in hepatolithiasis. Liver Int. 2007, 27, 1174–1184. [Google Scholar] [CrossRef]
- Ong, C.K.; Subimerb, C.; Pairojkul, C.; Wongkham, S.; Cutcutache, I.; Yu, W.; McPherson, J.R.; Allen, G.E.; Ng, C.C.; Wong, B.H.; et al. Exome sequencing of liver fluke-associated cholangiocarcinoma. Nat. Genet. 2012, 44, 690–693. [Google Scholar] [CrossRef]
- Mimaki, S.; Totsuka, Y.; Suzuki, Y.; Nakai, C.; Goto, M.; Kojima, M.; Arakawa, H.; Takemura, S.; Tanaka, S.; Marubashi, S.; et al. Hypermutation and unique mutational signatures of occupational cholangiocarcinoma in printing workers exposed to haloalkanes. Carcinogenesis 2016, 37, 817–826. [Google Scholar] [CrossRef]
- Sato, Y.; Kinoshita, M.; Takemura, S.; Tanaka, S.; Hamano, G.; Nakamori, S.; Fujikawa, M.; Sugawara, Y.; Yamamoto, T.; Arimoto, A.; et al. The PD-1/PD-L1 axis may be aberrantly activated in occupational cholangiocarcinoma. Pathol. Int. 2017, 67, 163–170. [Google Scholar] [CrossRef]
- Sato, Y.; Tanaka, S.; Kinoshita, M.; Takemura, S.; Shinkawa, H.; Kokudo, T.; Hasegawa, K.; Tanaka, H.; Yoshimoto, H.; Mori, A.; et al. Immunosuppressive tumor microenvironment in occupational cholangiocarcinoma: Supportive evidence for the efficacy of immune checkpoint inhibitor therapy. J. Hepatobiliary Pancreat Sci. 2020, 27, 860–869. [Google Scholar] [CrossRef]
- Sasaki, M.; Matsubara, T.; Yoneda, N.; Nomoto, K.; Tsuneyama, K.; Sato, Y.; Nakanuma, Y. Overexpression of enhancer of zeste homolog 2 and MUC1 may be related to malignant behaviour in intraductal papillary neoplasm of the bile duct. Histopathology 2013, 62, 446–457. [Google Scholar] [CrossRef]
- Sasaki, M.; Nitta, T.; Sato, Y.; Nakanuma, Y. Autophagy may occur at an early stage of cholangiocarcinogenesis via biliary intraepithelial neoplasia. Hum. Pathol. 2015, 46, 202–209. [Google Scholar] [CrossRef]
- Nakanishi, Y.; Zen, Y.; Kondo, S.; Itoh, T.; Itatsu, K.; Nakanuma, Y. Expression of cell cycle-related molecules in biliary premalignant lesions: Biliary intraepithelial neoplasia and biliary intraductal papillary neoplasm. Hum. Pathol. 2008, 39, 1153–1161. [Google Scholar] [CrossRef]
- Sasaki, M.; Yamaguchi, J.; Itatsu, K.; Ikeda, H.; Nakanuma, Y. Over-expression of polycomb group protein EZH2 relates to decreased expression of p16 INK4a in cholangiocarcinogenesis in hepatolithiasis. J. Pathol. 2008, 215, 175–183. [Google Scholar] [CrossRef]
- Naito, Y.; Kusano, H.; Nakashima, O.; Sadashima, E.; Hattori, S.; Taira, T.; Kawahara, A.; Okabe, Y.; Shimamatsu, K.; Taguchi, J.; et al. Intraductal neoplasm of the intrahepatic bile duct: Clinicopathological study of 24 cases. World J. Gastroenterol. 2012, 18, 3673–3680. [Google Scholar] [CrossRef]
- Wu, X.; Li, B.; Zheng, C.; Chang, X.; Zhang, T.; He, X.; Zhao, Y. Intraductal papillary neoplasm of the bile duct: A single-center retrospective study. J. Int. Med. Res. 2018, 46, 4258–4268. [Google Scholar] [CrossRef]
- Yeh, C.N.; Jan, Y.Y.; Yeh, T.S.; Hwang, T.L.; Chen, M.F. Hepatic resection of the intraductal papillary type of peripheral cholangiocarcinoma. Ann. Surg. Oncol. 2004, 11, 606–611. [Google Scholar] [CrossRef]
- Miyazaki, M.; Kimura, F.; Shimizu, H.; Yoshidome, H.; Otsuka, M.; Kato, A.; Hideyuki, Y.; Nozawa, S.; Furukawa, K.; Mituhashi, N.; et al. Surgical strategy for mucin-producing bile duct tumor. J. Hepatobiliary Pancreat Sci. 2010, 17, 236–240. [Google Scholar] [CrossRef]
- Gunven, P.; Gorsetman, J.; Ohlsen, H.; Ruden, B.I.; Lundell, G.; Skoog, L. Six-year recurrence free survival after intraluminal iridium-192 therapy of human bilobar biliary papillomatosis. A case report. Cancer 2000, 89, 69–73. [Google Scholar] [CrossRef]
- Brauer, B.C.; Fukami, N.; Chen, Y.K. Direct cholangioscopy with narrow-band imaging, chromoendoscopy, and argon plasma coagulation of intraductal papillary mucinous neoplasm of the bile duct. Gastrointest Endos. 2008, 67, 574–576. [Google Scholar] [CrossRef]
- Cha, B.; Park, J.S.; Jeong, S.; Lee, D.H.; Kim, J.M. Direct cholangioscopy with argon plasma coagulation of an intraductal papillary mucinous neoplasm of the bile duct. Korean J. Intern. Med. 2019, 34, 940–941. [Google Scholar] [CrossRef]
- Tan, Y.; Milikowski, C.; Toribio, Y.; Singer, A.; Rojas, C.P.; Garcia-Buitrago, M.T. Intraductal papillary neoplasm of the bile ducts: A case report and literature review. World J. Gastroenterol. 2015, 21, 12498–12504. [Google Scholar] [CrossRef]
- Arai, J.; Kato, J.; Toda, N.; Kurokawa, K.; Shibata, C.; Kurosaki, S.; Funato, K.; Kondo, M.; Takagi, K.; Kojima, K.; et al. Long-term survival after palliative argon plasma coagulation for intraductal papillary mucinous neoplasm of the bile duct. Clin. J. Gastroenterol. 2020. [Google Scholar] [CrossRef]
- Pérez Saborido, B.; Bailón Cuadrado, M.; Rodríguez López, M.; Asensio Díaz, E.; Madrigal Rubiales, B.; Barrera Rebollo, A. Intraductal papillary neoplasia of the bile duct with malignancy: A differentiated entity of cholangiocarcinoma with a better prognosis. A review of three new cases. Rev. Esp. Enferm. Dig. 2017, 109, 592–595. [Google Scholar] [CrossRef]
- Harada, F.; Matsuyama, R.; Mori, R.; Kumamoto, T.; Morioka, D.; Taguri, M.; Yamanaka, S.; Endo, I. Outcomes of surgery for 2010 WHO classification-based intraductal papillary neoplasm of the bile duct: Case-control study of a single Japanese institution’s experience with special attention to mucin expression patterns. Eur. J. Surg. Oncol. 2019, 45, 761–768. [Google Scholar] [CrossRef]
- Hasumi, A.; Matsui, H.; Sugioka, A.; Uyama, I.; Komori, Y.J.; Aoki, H. Precancerous conditions of biliary tract cancer in patients with pancreaticobiliary maljunction: Reappraisal of nationwide survey in Japan. J. Hepatobiliary Pancreat Surg. 2000, 7, 551–555. [Google Scholar] [CrossRef]
- Choi, S.C.; Lee, J.K.; Jung, J.H.; Lee, J.S.; Lee, K.H.; Lee, K.T.; Rhee, J.C.; Jang, K.T.; Choi, S.H.; Heo, J.S.; et al. The clinicopathological features of biliary intraductal papillary neoplasms according to the location of tumors. J. Gastroenterol. Hepatol. 2010, 25, 725–730. [Google Scholar] [CrossRef]
- Matsumoto, T.; Kubota, K.; ßHachiya, H.; Sakuraoka, Y.; Shiraki, T.; Shimizu, T.; Mori, S.; Iso, Y.; Kato, M.; Yamagishi, H.; et al. Impact of tumor location on postoperative outcome of intraductal papillary neoplasm of the bile duct. World J. Surg. 2019, 43, 1313–1322. [Google Scholar] [CrossRef]
- Sobin, L.; Gospodarowicz, M.; Witterkind, C. TNM: Classification of Malignant Tumours, 7th ed.; UICC, Wiley-Blackwell: West Sussex, UK, 2009. [Google Scholar]

| WHO Proposed Term | WHO Accepted Terms | WHO Unrecommended Terms | |
|---|---|---|---|
| IPNB (intraductal papillary neoplasm of bile duct) | Biliary papilloma and papillomatosis | Biliary adenoma | |
| Intestinal adenoma | |||
| Papillary (villous) adenoma | |||
| Tubulopapillary (tubule-villous) adenoma | |||
| Non-invasive papillary neoplasm (carcinoma) | |||
| Papillary carcinoma | |||
| Mucin-secreting biliary tumor | |||
| Clinicopathological Features | Localized Papillary Type | Conglomerated Type | Multifocal Type | |
|---|---|---|---|---|
| Narrow Ranged | Wide Ranged | |||
| Number of cases | 17 | 19 | 4 | |
| 12 | 7 | |||
| Intra/Extra/Both | 4/13/0 | 8/8/3 | 0/4/0 | |
| 8/3/1 | 0/5/2 | |||
| Type 1: Type 2 | 2:15 | 5:14 | 1:3 | |
| 3:9 | 2:5 | |||
| I/G/O/PB | 10/1/1/5 | 9/4/3/3 | 2/2/0/0 | |
| 4/3/2/3 | 5/1/1/0 | |||
| Stromal invasion | 4 | 13 | 2 | |
| 8 | 5 | |||
| Four Subtypes | Definitions | Immunohistochemistry |
|---|---|---|
| Intestinal subtype |
|
|
| Gastric subtype |
|
|
| Pancreatobiliary subtype |
|
|
| Onocytic subtype |
|
|
| Pathologic | Features | Type 1 IPNB | Type 2 IPNB |
|---|---|---|---|
| Structures | Regular villous, papillary or | Irregular and complicated villous, | |
| tubular structures | papillary or tubular structures | ||
| Homogeneous appearance | Heterogeneous appearance | ||
| Grade of neoplasm intraepithelial | Low-grade dysplasia | High-grade dysplasia with no or | |
| High-grade dysplasia with | minimal foci of low-grade dysplasia | ||
| foci of low-grade dysplasia | High-grade dysplasia | ||
| Location at the biliary tree | Usually intrahepatic bile duct | Intrahepatic and extrahepatic bile duct | |
| Mucin overproduction | Frequent | Infrequent | |
| Stromal invasion | Infrequent | Common | |
| Subtypes | Intestinal and oncocytic subtype | Pancreatobiliary and intestinal subtype | |
| Similarities to prototypic subtypes of IPMN | Similar (depending on subtype) | Different variably (depending on subtype) | |
| Complicated lesions such as solid or cribriform pattern, coagulative necrosis, cystic changes | Almost absent | Frequent | |
| Bizarre cellular and nuclear changes | Absent | Infrequent | |
| Fibrovascular stalks | Thin to slightly widened (depending on subtype) | Thin to widened (depending on subtype) | |
| Clinicolaboratory Features | Type 1 | Type 2 |
|---|---|---|
| Prevalence in IPNB | 30–75% | 25–70% |
| Clinical features | ||
| * Age range | 65–67 years | 69–72 years |
| * Sex | Slightly male predominant | Slightly male predominant |
| * Jaundice, fever, abdominal pain | 20%, 10%, 17% | 39%, 18% 24% |
| * Background: hepatolithiasis | 11% | 6% |
| cholecystolithiasis | 16% | 8% |
| choledocholithiasis | 9% | 4% |
| * Elevations of AST, ALT, ALP, | relatively lower | relatively higher |
| γ-GTP, and T. Bililubin | relatively lower | relatively higher |
| * Level of CEA and CA19-9 | ||
| Gross features | ||
| * Location: | ||
| intrahepatic | 58–68% | 14–27% |
| hilar, extrahepatic | 32–35% | 48–64% |
| mixed | 7% | 22% |
| * Tumor size | 2–205 mm | 2–220 mm |
| * Communication between | ||
| cyst and bile duct | 45% | 50% |
| * Mucobilia | 29–86% | 12–21% |
| Histological features | ||
| * Four subtypes (I:G:PB:O) | 18–48%:23–32%:12–23.5%:6–32% | 0–39%:51–86%:6.7–1.4%%:0–3% |
| * Similar to prototypic IPMN | Similar | Variably different |
| * Low-: high-grade dysplasia | 4.5–7.9%:32–68% | 0–0.6%:5.8–29% |
| * Stromal invasion | 27–50% | 71–94% |
| Lymph node metastasis | 0.5–5.8% | 21.4–14.7% |
| Post-operative outcome | ||
| * 5 year cumulative survival rate | 75.20% | 50.90% |
| * 5 year cumulative disease- free | 64.10% | 35.30% |
| year |
| Yang et al. (Taiwanese, 37 Cases) [130] | Aoki et al. (Japanese, 35 Cases) [111] |
|---|---|
| KRAS (49%) | TP53 (34.3%) |
| GNAS (32%) | KRAS (24%) |
| RNF (24%) | STK11 (25.7%) |
| APC (24%) | CTNNB1 (17.1%) |
| TP53 (24%) | APC (14.3%) |
| CTNNB1 (11%) | SMAD4 (14.3%) |
| GNAS (11.4%) | |
| PBRM1 (11.4%) | |
| ELF3 (8.6%) | |
| KMT2C (8.6%) | |
| NF1 (8.6%) | |
| PIK3CA (8.6%) | |
| ARID1A (5.7%) | |
| ARID2 (5.7%) | |
| BAP1 (5.7%) | |
| BRAF (5.7%) | |
| EPHA6 (5.7%) | |
| ERBB2 (5.7%) | |
| KMT2D (5.7%) | |
| RNF43 (5.7%) |
| Cancer Related Protein | Type 1 (22 Cases) | Type 2 (14 Cases) |
|---|---|---|
| MUC1 * | 11 | 14 |
| P53 * | 2 | 9 |
| SMAD4 | 2 | 6 |
| Mutated Genes | Type 1 (21 Cases) | Type 2 (14 Cases) |
|---|---|---|
| KRAS * | 10 | 1 |
| GNAS * | 4 | 0 |
| RNF43 | 2 | 0 |
| TP53 * | 3 | 9 |
| SMAD4 * | 0 | 5 |
| ARID1A | 0 | 2 |
| ERBB2 | 0 | 2 |
| Factors | Worse Prognosis | ||
|---|---|---|---|
| Clinical features | Lymph node metastasis, older age, jaundice, elevation of serum CA19-9 and CEA | ||
| Pathological factors of tumor | Multiplicity, perineural invasion, pancreatobiliary subtype, mucin | ||
| hypersecretion, low and high grade dysplasia, tumor expression of CK20 | |||
| in tumor tissue, MUC1 expression in tumor, | |||
| Location | Extrahepatic location | ||
| Subtypes | Pancreatobiliary subtype | ||
| Subclassification | Type 2 | ||
| Staging | Stromal invasion | ||
| UICC staging, periductal invasion | |||
| Surgical margin | R1, R1/R2, ductal margin with high grade dysplasia (‘carcinoma in situ’), | ||
| ductal margin with low-grade dysplasia | |||
| Favorable prognosis | |||
| Pathologic factors of tumor | Cystic IPNB with micropapillary lesion, intrahepatic location, no invasion, | ||
| low-grade dysplasia, MUC6 expression in tumor tissue | |||
| Subclassification | Type 1 | ||
| Surgical margin | Negative surgical margin | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nakanuma, Y.; Uesaka, K.; Kakuda, Y.; Sugino, T.; Kubota, K.; Furukawa, T.; Fukumura, Y.; Isayama, H.; Terada, T. Intraductal Papillary Neoplasm of Bile Duct: Updated Clinicopathological Characteristics and Molecular and Genetic Alterations. J. Clin. Med. 2020, 9, 3991. https://doi.org/10.3390/jcm9123991
Nakanuma Y, Uesaka K, Kakuda Y, Sugino T, Kubota K, Furukawa T, Fukumura Y, Isayama H, Terada T. Intraductal Papillary Neoplasm of Bile Duct: Updated Clinicopathological Characteristics and Molecular and Genetic Alterations. Journal of Clinical Medicine. 2020; 9(12):3991. https://doi.org/10.3390/jcm9123991
Chicago/Turabian StyleNakanuma, Yasuni, Katsuhiko Uesaka, Yuko Kakuda, Takashi Sugino, Keiichi Kubota, Toru Furukawa, Yuki Fukumura, Hiroyuki Isayama, and Takuro Terada. 2020. "Intraductal Papillary Neoplasm of Bile Duct: Updated Clinicopathological Characteristics and Molecular and Genetic Alterations" Journal of Clinical Medicine 9, no. 12: 3991. https://doi.org/10.3390/jcm9123991
APA StyleNakanuma, Y., Uesaka, K., Kakuda, Y., Sugino, T., Kubota, K., Furukawa, T., Fukumura, Y., Isayama, H., & Terada, T. (2020). Intraductal Papillary Neoplasm of Bile Duct: Updated Clinicopathological Characteristics and Molecular and Genetic Alterations. Journal of Clinical Medicine, 9(12), 3991. https://doi.org/10.3390/jcm9123991







