Magnetic-Activated Cell Sorting (MACS): A Useful Sperm-Selection Technique in Cases of High Levels of Sperm DNA Fragmentation
Abstract
1. Introduction
2. Experiment Section
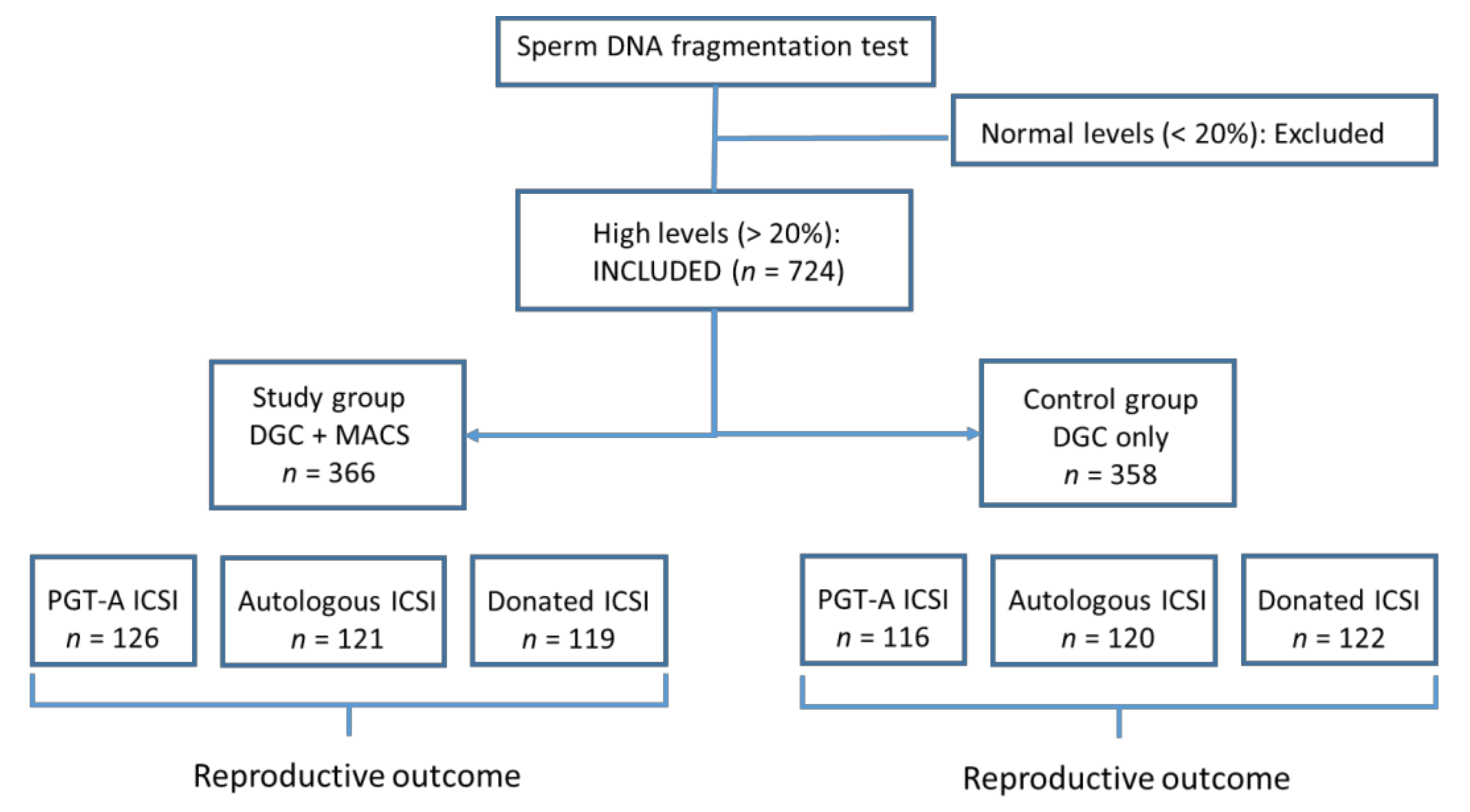
2.1. Study Population and Design
2.2. Sperm DNA Fragmentation (SDF) Assessment
2.3. Male Evaluation and Conventional Sperm Selection
2.4. MACS Sperm-Selection Technique
2.5. Cycle Procedure and Outcome
2.6. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Van Der Steeg, J.; Steures, P.; Eijkemans, M.J.C.; Habbema, J.D.F.; Hompes, P.G.A.; Kremer, J.A.M.; Van Der Leeuw-Harmsen, L.; Bossuyt, P.M.M.; Repping, S.; Silber, S.J.; et al. Role of semen analysis in subfertile couples. Fertil. Steril. 2011, 95, 1013–1019. [Google Scholar] [CrossRef]
- Santi, D.; Spaggiari, G.; Simoni, M. Sperm DNA fragmentation index as a promising predictive tool for male infertility diagnosis and treatment management—Meta-analyses. Reprod. Biomed. Online 2018, 37, 315–326. [Google Scholar] [CrossRef]
- Borini, A.; Tarozzi, N.; Bizzaro, D.; Bonu, M.; Fava, L.; Flamigni, C.; Coticchio, G. Sperm DNA fragmentation: Paternal effect on early post-implantation embryo development in ART. Hum. Reprod. 2006, 21, 2876–2881. [Google Scholar] [CrossRef]
- Tesarik, J.; Greco, E.; Mendoza, C. Late, but not early, paternal effect on human embryo development is related to sperm DNA fragmentation. Hum. Reprod. 2004, 19, 611–615. [Google Scholar] [CrossRef]
- Simon, L.; Murphy, K.; Shamsi, M.; Liu, L.; Emery, B.; Aston, K.; Hotaling, J.; Carrell, D.T. Paternal influence of sperm DNA integrity on early embryonic development. Hum. Reprod. 2014, 29, 2402–2412. [Google Scholar] [CrossRef]
- Barroso, G.; Valdespin, C.; Vega, E.; Kershenovich, R.; Avila, R.; Avendaño, C.; Oehninger, S. Developmental sperm contributions: Fertilization and beyond. Fertil. Steril. 2009, 92, 835–848. [Google Scholar] [CrossRef]
- Cissen, M.; Van Wely, M.; Scholten, I.; Mansell, S.; De Bruin, J.P.; Mol, B.W.; Braat, D.; Repping, S.; Hamer, G. Measuring Sperm DNA Fragmentation and Clinical Outcomes of Medically Assisted Reproduction: A Systematic Review and Meta-Analysis. PLoS ONE 2016, 11, e0165125. [Google Scholar] [CrossRef]
- Aitken, R.J.; Bronson, R.; Smith, T.B.; De Iuliis, G.N. The source and significance of DNA damage in human spermatozoa; a commentary on diagnostic strategies and straw man fallacies. Mol. Hum. Reprod. 2013, 19, 475–485. [Google Scholar] [CrossRef]
- Ribas-Maynou, J.; Gawecka, J.; Benet, J.; Ward, W.S. Double-stranded DNA breaks hidden in the neutral Comet assay suggest a role of the sperm nuclear matrix in DNA integrity maintenance. Mol. Hum. Reprod. 2013, 20, 330–340. [Google Scholar] [CrossRef]
- Shukla, K.K.; Mahdi, A.A.; Rajender, S. Apoptosis, spermatogenesis and male infertility. Front. Biosci. 2012, 4, 746. [Google Scholar] [CrossRef]
- Agarwal, A.; Barbarosie, C.; Ambar, R.; Finelli, R. The Impact of Single and Double-Strand DNA Breaks in Human Spermatozoa on Assisted Reproduction. Int. J. Mol. Sci. 2020, 21, 3882. [Google Scholar] [CrossRef] [PubMed]
- Sakkas, D.; Seli, E.; Bizzaro, D.; Tarozzi, N.; Manicardi, G.-C. Abnormal spermatozoa in the ejaculate: Abortive apoptosis and faulty nuclear remodelling during spermatogenesis. Reprod. Biomed. Online 2003, 7, 428–432. [Google Scholar] [CrossRef]
- Smith, T.B.; Cortez-Retamozo, V.; Grigoryeva, L.S.; Hill, E.; Pittet, M.J.; Da Silva, N. Mononuclear phagocytes rapidlyclear apoptotic epithelial cells in the proximal epididymis. Andrology 2014, 2, 755–762. [Google Scholar] [CrossRef] [PubMed]
- Sakkas, D.; Ramalingam, M.; Garrido, N.; Barratt, C.L. Sperm selection in natural conception: What can we learn from Mother Nature to improve assisted reproduction outcomes? Hum. Reprod. 2015, 21, 711–726. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.-H.; Liu, C.-H.; Shih, Y.-T.; Tsao, H.-M.; Huang, C.-C.; Chen, H.-H.; Lee, M.-S. Magnetic-activated cell sorting for sperm preparation reduces spermatozoa with apoptotic markers and improves the acrosome reaction in couples with unexplained infertility. Hum. Reprod. 2010, 25, 839–846. [Google Scholar] [CrossRef]
- Cakar, Z.; Cetinkaya, B.; Aras, D.; Koca, B.; Ozkavukcu, S.; Kaplanoglu, I.; Cinar, O. Does combining mag-netic-activated cell sorting with density gradient or swim-up improve sperm selection? J. Assist. Reprod. Genet. 2016, 33, 1059–1065. [Google Scholar] [CrossRef]
- Grunewald, S.; Paasch, U. Sperm selection for ICSIusing annexin V. Methods Mol. Biol. 2013, 927, 257–262. [Google Scholar]
- Nasr-Esfahani, M.H.; Deemeh, M.R.; Tavalaee, M. New era in sperm selection for ICSI. Int. J. Androl. 2012, 35, 475–484. [Google Scholar] [CrossRef]
- Chi, H.-J.; Kwak, S.-J.; Kim, S.-G.; Kim, Y.-Y.; Park, J.-Y.; Yoo, C.-S.; Park, I.-H.; Sun, H.-G.; Kim, J.-W.; Lee, K.-H. Efficient isolation of sperm with high DNA integrity and stable chromatin packaging by a combination of density-gradient centrifugation and magnetic-activated cell sorting. Clin. Exp. Reprod. Med. 2016, 43, 199–206. [Google Scholar] [CrossRef]
- Romany, L.; Garrido, N.; Cobo, A.; Aparicio-Ruiz, B.; Serra, V.; Meseguer, M. Obstetric and perinatal outcome of babies born from sperm selected by MACS from a randomized controlled trial. J. Assist. Reprod. Genet. 2016, 34, 201–207. [Google Scholar] [CrossRef]
- Sedó, C.A.; Uriondo, H.; Lavolpe, M.; Noblia, F.; Papier, S.; Nodar, F. Clinical outcome using non-apoptotic sperm selection for ICSI procedures: Report of 1 year experience. Fertil. Steril. 2010, 94, S232. [Google Scholar] [CrossRef]
- Ziarati, N.; Tavalaee, M.; Bahadorani, M.; Nasr-Esfahani, M.H. Clinical outcomes of magnetic activated sperm sorting in infertile men candidate for ICSI. Hum. Fertil. 2018, 22, 118–125. [Google Scholar] [CrossRef]
- Romany, L.; Garrido, N.; Motato, Y.; Aparicio, B.; Remohí, J.; Meseguer, M. Removal of annexin V–positive sperm cells for intracytoplasmic sperm injection in ovum donation cycles does not improve reproductive outcome: A controlled and randomized trial in unselected males. Fertil. Steril. 2014, 102, 1567–1575. [Google Scholar] [CrossRef] [PubMed]
- Horta, F.; Crosby, M.; Mackenna, A.; Huidobro, C. Male factor infertility outcomes using magnetic activated cell sorting in ntra cytoplasmic sperm injection cycles. Andrology 2016, 5, 155–159. [Google Scholar]
- Sergerie, M.; Laforest, G.; Bujan, L.; Bissonnette, F.; Bleau, G. Sperm DNA fragmentation: Threshold value in male fertility. Hum. Reprod. 2005, 20, 3446–3451. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization, Department of Reproductive Health and Research. WHO Laboratory Manual for the Examination and Processing of Human Semen, 5th ed.; WHO Press: Geneva, Switzerland, 2010. [Google Scholar]
- Alecsandru, D.; Pacheco, A.; Guerrero-Mayo, A.; Fabris, A.; Aparicio, P.; Barrio, A.; Pellicer, A.; Garcia-Velasco, J.A. Ovarian stimulation does not influence the uterine immune environment in healthy infertile women. Reprod. Biomed. Online 2020, 40, 113–123. [Google Scholar] [CrossRef]
- Carrell, D.T.; Wilcox, A.L.; Lowy, L.; Peterson, C.M.; Jones, K.P.; Erickson, L.; Hatasaka, H.H. Elevated sperm chromosome aneuploidy and apoptosis in patients with unexplained recurrent pregnancy loss. Obstet. Gynecol. 2003, 101, 1229–1235. [Google Scholar]
- Benchaib, M.; Braun, V.; Lornage, J.; Hadj, S.; Salle, B.; Lejeune, H.; Guérin, J.F. Sperm DNA fragmentation decreases the pregnancy rate in an assisted reproductive technique. Hum. Reprod. 2003, 18, 1023–1028. [Google Scholar] [CrossRef]
- Robinson, L.; Gallos, I.D.; Conner, S.J.; Rajkhowa, M.; Miller, D.; Lewis, S.; Kirkman-Brown, J.; Coomarasamy, A. The effect of sperm DNA fragmentation on miscarriage rates: A systematic review and meta-analysis. Hum. Reprod. 2012, 27, 2908–2917. [Google Scholar] [CrossRef]
- Wdowiak, A.; Bakalczuk, S.; Bakalczuk, G. The effect of sperm DNA fragmentation on the dynamics of the embryonic development in intracytoplasmatic sperm injection. Reprod. Biol. 2015, 15, 94–100. [Google Scholar] [CrossRef]
- Zini, A.; Boman, J.M.; Belzile, E.; Ciampi, A. Sperm DNA damage is associated with an increased risk of pregnancy loss after IVF and ICSI: Systematic review and meta-analysis. Hum. Reprod. 2008, 23, 2663–2668. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Zhang, Q.; Wang, Y.; Li, Y. Whether sperm deoxyribonucleic acid fragmentation has an effect on pregnancy and miscarriage after in vitro fertilization/intracytoplasmic sperm injection: A systematic review and meta-analysis. Fertil. Steril. 2014, 102, 998–1005. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Zhu, L.; Jiang, H.; Chen, H.; Chen, Y.; Dai, Y.-T. Sperm DNA fragmentation index and pregnancy outcome after IVF or ICSI: A meta-analysis. J. Assist. Reprod. Genet. 2014, 32, 17–26. [Google Scholar] [CrossRef]
- Ribas-Maynou, J.; García-Peiró, A.; Encinas, A.F.; Amengual, M.J.; Prada, E.; Cortés, P.; Navarro, J.; Benet, J. Double Stranded Sperm DNA Breaks, Measured by Comet Assay, Are Associated with Unexplained Recurrent Miscarriage in Couples without a Female Factor. PLoS ONE 2012, 7, e44679. [Google Scholar] [CrossRef] [PubMed]
- Ferreyra, J.G.; García-Ferreyra, L.V.J. High Pregnancy and Implantation Rates Can Be Obtained Using Magnetic-Activated Cell Sorting (MACS) to Selection Spermatozoa in Patients with High Levels of Spermatic DNA Fragmentation. JFIV Reprod. Med. Genet. 2014, 3, 133. [Google Scholar] [CrossRef]
- Buzzi, J.; Valcarcel, A.; Lombardi, E.; Oses, R.; Rawe, V.; Young, E. Magnetic activated cell sorting (MACS) improves oocyte donation results associated to severe male factor infertility. Hum. Reprod. 2010, 25 (Suppl. 1), i118–i152. [Google Scholar]
- Esbert, M.; Pacheco, A.; Soares, S.R.; Amorós, D.; Florensa, M.; Ballesteros, A.; Meseguer, M. High sperm DNA fragmentation delays human embryo kinetics when oocytes from young and healthy donors are microinjected. Andrology 2018, 6, 697–706. [Google Scholar] [CrossRef]
- Jerre, E.; Bungum, M.; Evenson, D.; Giwercman, A. Sperm chromatin structure assay high DNA stainability sperm as a marker of early miscarriage after intracytoplasmic sperm injection. Fertil. Steril. 2019, 112, 46–53. [Google Scholar] [CrossRef]
- Sánchez-Martín, P.; Dorado-Silva, M.; Sánchez-Martín, F.; Martínez, M.; Johnston, S.D.; Gosálvez, J. Magnetic cell sorting of semen containing spermatozoa with high DNA fragmentation in ICSI cycles decreases miscarriage rate. Reprod. Biomed. Online 2017, 34, 506–512. [Google Scholar] [CrossRef]
- Dutta, S.; Majzoub, A.; Agarwal, A. Oxidative stress and sperm function: A systematic review on evaluation and management. Arab. J. Urol. 2019, 17, 87–97. [Google Scholar] [CrossRef]
| Study Group MACS (n = 366) | Control Group w/o MACS (n = 358) | p | |
|---|---|---|---|
| Female age (year) a | 372 ± 3.6 | 36.7 ± 3.5 | 0.3 |
| Male age (year) a | 40.0 ± 15.7 | 38.7 ± 15.4 | 0.28 |
| SDF (%) a | 28.9 ± 8.2 | 29.6 ± 9.1 | 0.30 |
| Total sperm count (mil) a | 89.1 ± 79.4 | 95.1 ± 87.2 | 0.15 |
| Progressive motility (%) a | 35.2 ± 18.1 | 33.3 ± 16.7 | 0.12 |
| Number of collected oocytes | 11.3 ± 7.2 | 11.9 ± 6.8 | 0.36 |
| Number of Metaphase II oocytes | 9.7 ± 5.0 | 9.5 ± 5.1 | 0.31 |
| Study Group MACS (n = 366) | Control Group w/o MACS (n = 358) | p | |
|---|---|---|---|
| Fertilization rate (%) | 75.1 | 73.3 | 0.133 |
| Pregnancy rate (%) | 60.7 | 51.5 | 0.014 |
| Miscarriage rate (%) | 14.7 | 20.6 | 0.034 |
| Livebirth rate (%) | 47.4 | 31.2 | 0.001 |
| Preimplantation Genetic Testing for Aneuploidy (PGT-A) Cycles | |||
| Study Group MACS (n = 126) | Control Group w/o MACS (n = 116) | p | |
| SDF (%) | 28.8 | 30.1 | 0.168 |
| Fertilization rate (%) | 76 | 76.8 | 0.201 |
| Pregnancy rate (%) | 60.4 | 50.6 | 0.121 |
| Miscarriage rate (%) | 15.1 | 11.4 | 0.307 |
| Live-birth rate (%) | 43.4 | 31.6 | 0.127 |
| Autologous Oocyte ICSI Cycles | |||
| Study Group MACS (n = 121) | Control Group w/o MACS (n = 120) | p | |
| SDF (%) | 30.7 ± 10.2 | 30.8 ± 10.8 | 0.958 |
| Fertilization rate (%) | 76.8 | 75.1 | 0.586 |
| Pregnancy rate (%) | 52.2 | 50.0 | 0.424 |
| Miscarriage rate (%) | 11.3 | 25.5 | 0.005 |
| Live-birth rate (%) | 40.9 | 24.6 | 0.03 |
| Oocyte-Donation Cycles | |||
| Study Group MACS (n = 121) | Control Group w/o MACS (n = 120) | p | |
| SDF (%) | 27.7 ± 6.8 | 28.0 ± 7.2 | 0.959 |
| Fertilization rate (%) | 76.85 | 76.9 | 0.750 |
| Pregnancy rate (%) | 69.6 | 53.9 | 0.013 |
| Miscarriage rate (%) | 17.9 | 22.5 | 0.247 |
| Live-birth rate (%) | 51.8 | 29.4 | 0.03 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pacheco, A.; Blanco, A.; Bronet, F.; Cruz, M.; García-Fernández, J.; García-Velasco, J.A. Magnetic-Activated Cell Sorting (MACS): A Useful Sperm-Selection Technique in Cases of High Levels of Sperm DNA Fragmentation. J. Clin. Med. 2020, 9, 3976. https://doi.org/10.3390/jcm9123976
Pacheco A, Blanco A, Bronet F, Cruz M, García-Fernández J, García-Velasco JA. Magnetic-Activated Cell Sorting (MACS): A Useful Sperm-Selection Technique in Cases of High Levels of Sperm DNA Fragmentation. Journal of Clinical Medicine. 2020; 9(12):3976. https://doi.org/10.3390/jcm9123976
Chicago/Turabian StylePacheco, Alberto, Arancha Blanco, Fernando Bronet, María Cruz, Jaime García-Fernández, and Juan Antonio García-Velasco. 2020. "Magnetic-Activated Cell Sorting (MACS): A Useful Sperm-Selection Technique in Cases of High Levels of Sperm DNA Fragmentation" Journal of Clinical Medicine 9, no. 12: 3976. https://doi.org/10.3390/jcm9123976
APA StylePacheco, A., Blanco, A., Bronet, F., Cruz, M., García-Fernández, J., & García-Velasco, J. A. (2020). Magnetic-Activated Cell Sorting (MACS): A Useful Sperm-Selection Technique in Cases of High Levels of Sperm DNA Fragmentation. Journal of Clinical Medicine, 9(12), 3976. https://doi.org/10.3390/jcm9123976





