Levels of Selected Matrix Metalloproteinases—MMP-1, MMP-2 and Fibronectin in the Saliva of Patients Planned for Endodontic Treatment or Surgical Extraction
Abstract
1. Introduction
2. Fibronectin
3. Material and Methods
3.1. Patients
3.2. Methods
3.3. Statistical Analysis
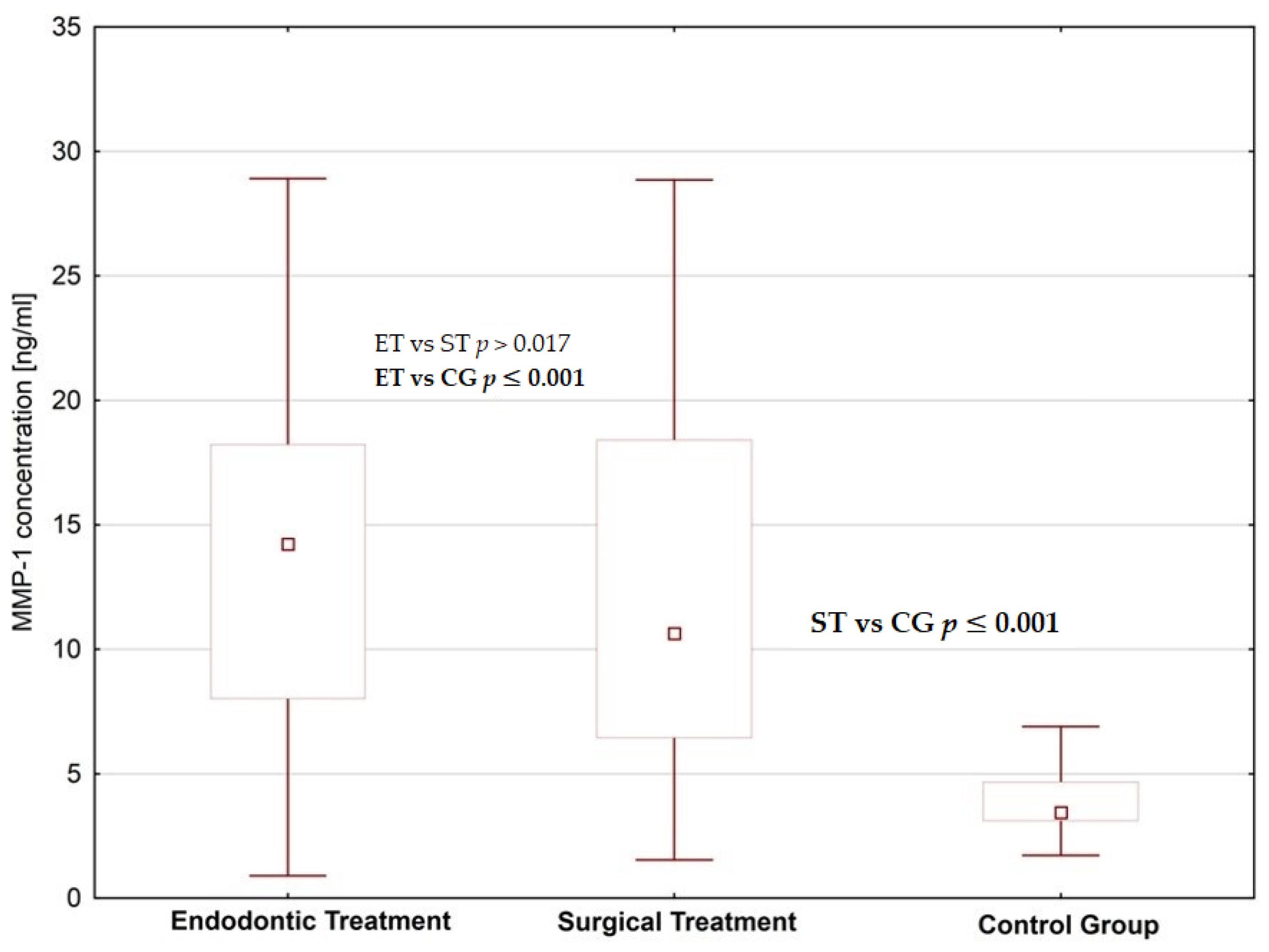
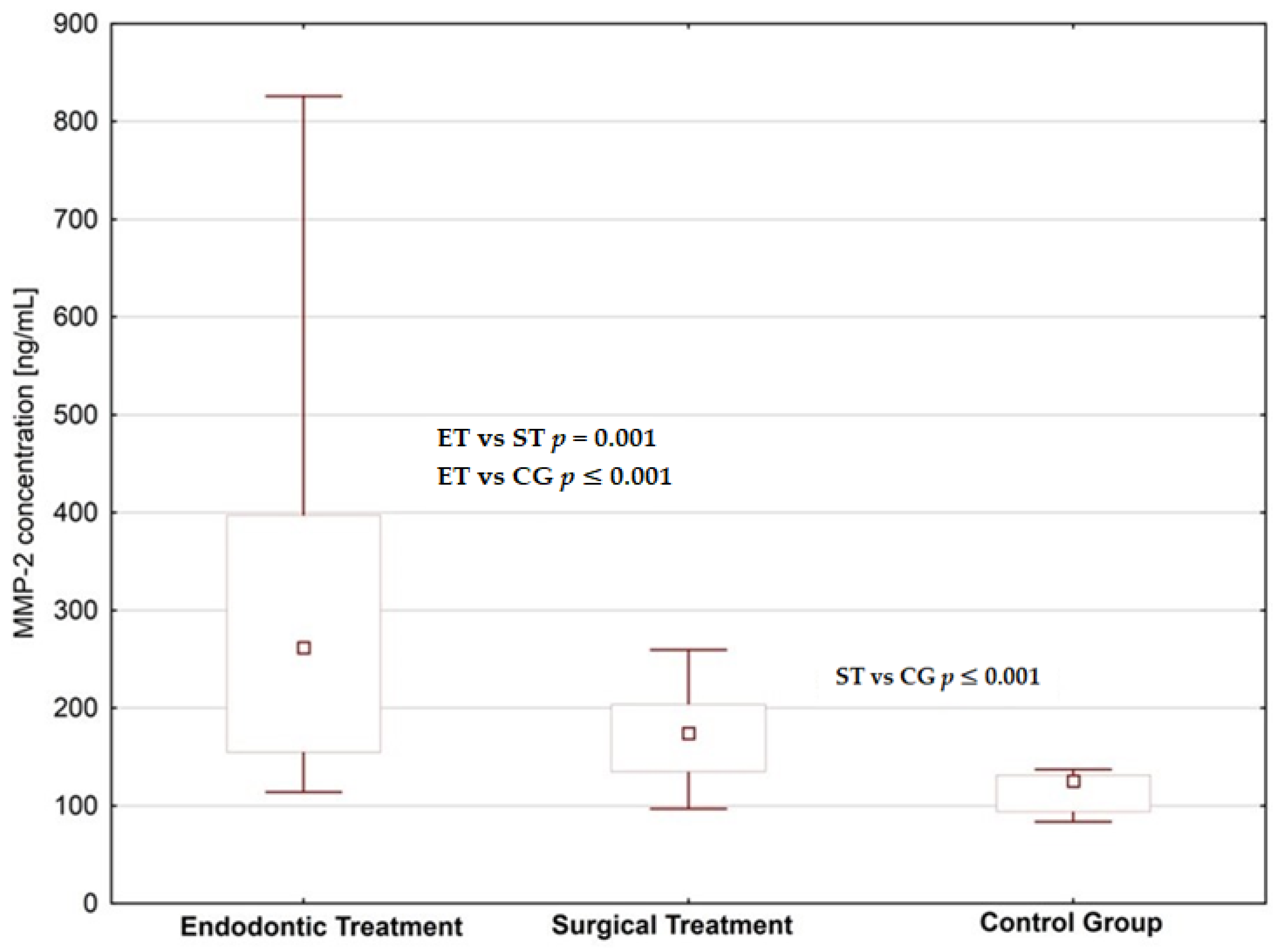
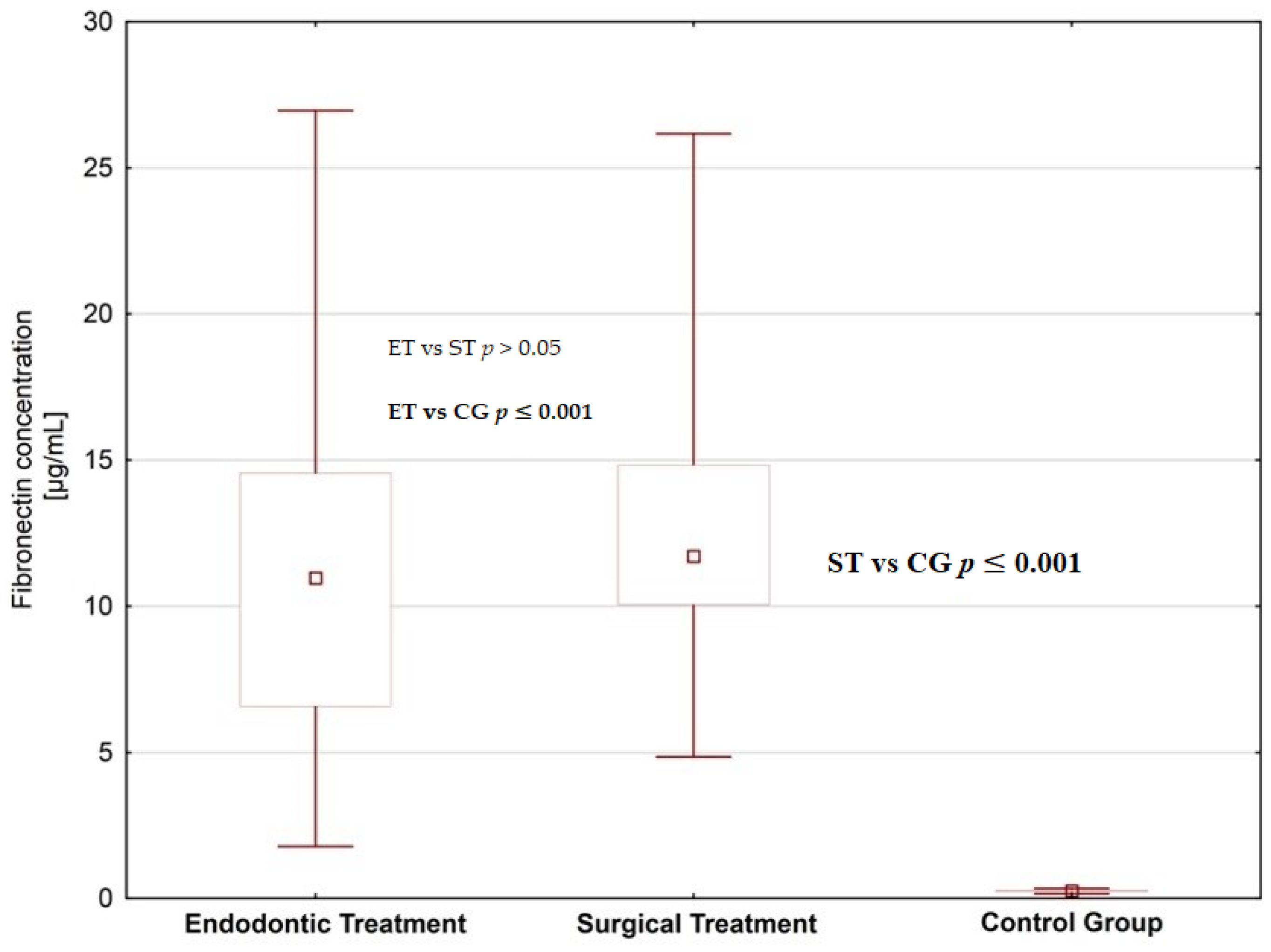
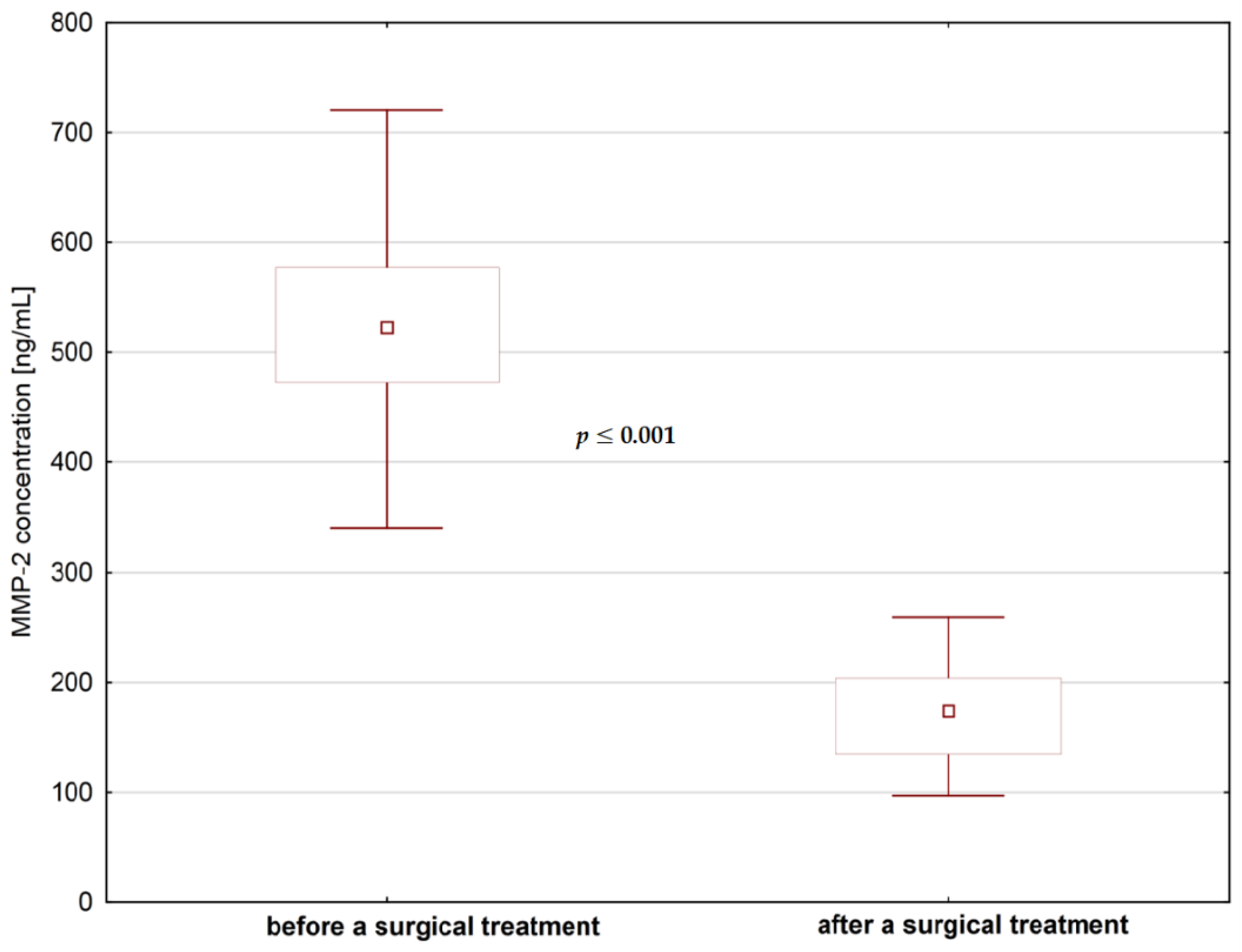
4. Results
5. Discussion
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Loo, J.; Yan, W.; Ramachan, P.; Wong, D. Comparative human salivary and plasma proteomes. J. Dent. Res. 2010, 89, 1016–1023. [Google Scholar] [CrossRef] [PubMed]
- Buczko, P.; Knaś, M.; Grycz, M.; Szarmach, I.; Zalewska, A. Orthodontic treatment modifies the oxidant-antioxidant balance in saliva of clinically healthy subjects. Adv. Med. Sci. 2017, 62, 129–135. [Google Scholar] [CrossRef] [PubMed]
- Weremijewicz, A.; Matuszczak, E.; Sankiewicz, A.; Tylicka, M.; Komarowska, M.; Tokarzewicz, A.; Debek, W.; Gorodkiewicz, E.; Hermanowicz, A. Matrix metalloproteinase-2 and its correlation with basal membrane components laminin-5 and collagen type IV in paediatric burn patients measured with Surface Plasmon Resonance Imaging (SPRI) biosensors. Burns 2018, 4, 931–940. [Google Scholar] [CrossRef] [PubMed]
- Chaussain-Miller, C.; Fioretti, F.; Goldberg, M.; Menashi, S. The role of matrix metalloproteinases (MMPs) in human caries. J. Dent. Res. 2006, 8, 22–32. [Google Scholar] [CrossRef]
- Jäsberg, H.; Tervahartiala, T.; Sorsa, T.; Söderling, E.; Haukioja, A. Probiotic intervention influences the salivary levels of Matrix Metalloproteinase (MMP)-9 and Tissue Inhibitor of metalloproteinases (TIMP)-1 in healthy adults. Arch. Oral Biol. 2018, 85, 58–63. [Google Scholar] [CrossRef]
- Visse, R.; Nagase, H. Matrix metalloproteinases and tissue inhibitors of metalloproteinases: Structure, function, and biochemistry. Circ. Res. 2003, 92, 827–839. [Google Scholar] [CrossRef]
- van Strijp, A.J.P.; Jansen, D.C.; DeGroot, J.; ten Cate, J.M.; Everts, V. Host-derived proteinases and degradation of dentine collagen in situ. Caries Res. 2003, 37, 58–65. [Google Scholar] [CrossRef]
- Tjäderhane, L.; Larjava, H.; Sorsa, T.; Uitto, V.J.; Larmas, M.; Salo, T. The activation and function of host matrix metalloproteinases in dentin matrix breakdown in caries lesions. J. Dent. Res. 1998, 77, 1622–1629. [Google Scholar] [CrossRef]
- Mazzoni, A.; Tjäderhane, L.; Checchi, V.; Di Lenarda, R.; Salo, T.; Tay, F.R.; Pashley, D.H.; Breschi, L. Role of dentin MMPs in caries progression and bond stability. J. Dent. Res. 2015, 94, 241–251. [Google Scholar] [CrossRef]
- Chaussain, C.; Boukpessi, T.; Khaddam, M.; Tjaderhane, L.; George, A.; Menashi, S. Dentin matrix degradation by host matrix metalloproteinases: Inhibition and clinical perspectives toward regeneration. Front. Physiol. 2013, 4, 308. [Google Scholar] [CrossRef]
- Sorsa, T.; Tjaderhane, I.; Salo, T. Matrix metalloproteinases (MMPs) in oral diseases. Oral Dis. 2004, 10, 311–318. [Google Scholar] [CrossRef]
- Verstappen, J.; Von den Hoff, J.W. Tissue inhibitors of metalloproteinases (TIMPs): Their biological functions and involvement in oral disease. J. Dent. Res. 2006, 85, 1074–1084. [Google Scholar] [CrossRef]
- Birkedal-Hansen, H. Role of matrix metalloproteinases in human periodontal diseases. J. Periodontol. 1993, 64, 474–484. [Google Scholar] [PubMed]
- Gursoy, U.; EKönönen, E.; Pradhan Palikhe, P.; Tervahartiala, T.; Pussinen, P.; Suominen Taipale, L.; Sorsa, T. Salivary MMP-8, TIMP-1, and ICTP as markers of advanced periodontitis. J. Clin. Periodontol. 2010, 37, 487–493. [Google Scholar] [CrossRef] [PubMed]
- Murakami, Y.; Machino, M.; Fujisawa, S. Porphyromonas gingivalis Fimbria-Induced Expression of Inflammatory Cytokines and Cyclooxygenase-2 in Mouse Macrophages and Its Inhibition by the Bioactive Compounds Fibronectin and Melatonin. ISRN Dent. 2012, 2012, 350859. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Murakami, Y.; Yuhara, K.; Takada, N.; Arai, T.; Tsuda, S.; Takamatsu, S.; Machino, M.; Fujisawa, S. Effect of melatonin on cyclooxygenase-2 expression and nuclear factor-kappa B activation in RAW264.7 macrophage-like cells stimulated with fimbriae of Porphyromonas gingivalis. In Vivo 2011, 25, 641–647. [Google Scholar]
- Huynh, Q.N.; Wang, S.; Tafolla, E.; Gansky, S.A.; Kapila, S.; Armitage, G.C.; Kapila, Y.L. Specific fibronectin fragments as markers of periodontal disease status. J. Periodontol. 2002, 73, 1101–1110. [Google Scholar] [CrossRef]
- Kapila, Y.L.; Wang, S.; Dazin, P.; Tafolla, E.; Mass, M.J. The heparin-binding domain and V region of fibronectin regulate apoptosis by suppression of p53 and c-myc in human primary cells. J. Biol. Chem. 2002, 277, 8482–8491. [Google Scholar] [CrossRef]
- Ghosh, A.; Joo, N.E.; Chen, T.C.; Kapila, Y.L. Proapoptotic fibronectin fragment induces the degradation of ubiquitinated p53 via proteasomes in periodontal ligament cells. J. Periodontal Res. 2010, 45, 481–487. [Google Scholar] [CrossRef]
- Llena-Puy, M.C.; Montañana-Llorens, C.; Forner-Navarro, L. Optimal assay conditions for quantifying fibronectin in saliva. Med. Oral. 2004, 9, 191–196. [Google Scholar]
- Vanden-Abbeele, A.; Courtois, P.; Pourtois, M. The antiseptic role of saliva. Rev. Belg. Med. Dent. 1992, 47, 52–58. [Google Scholar]
- Sojar, H.T.; Lee, J.Y.; Genco, R.J. Fibronectin binding domain of P. Gingivalis fimbriae. Biochem. Biophyis. Res. Commun. 1995, 216, 785–792. [Google Scholar] [CrossRef] [PubMed]
- Su, H.; Boackle, R.J. Interaction of the envelope glycoprotein of human immunodeficiency virus with C1q and fibronectin under conditions present in human saliva. Mol. Immunol. 1991, 28, 811–817. [Google Scholar] [CrossRef]
- Lyons, A.J.; Cui, N. Salivary oncofetal fibronectin and oral squamous cell carcinoma. J. Oral Pathol. Med. 2000, 29, 267–270. [Google Scholar] [CrossRef] [PubMed]
- Matuszczak, E.; Tylicka, M.; Hermanowicz, A.; Debek, W.; Sankiewicz, A.; Gorodkiewicz, E. Application of SPR Imaging Biosensor for the Measurement of 20S Proteasomes in Blood Plasma of Children with Thermal Injury. Ann. Clin. Lab. Sci. 2016, 4, 407–411. [Google Scholar]
- Tokarzewicz, A.; Romanowicz, L.; Sveklo, I.; Matuszczak, E.; Hermanowicz, A.; Gorodkiewicz, E. SPRI biosensors for quantitative determination of matrix metalloproteinase-2. Anal. Mathods 2017, 9, 2407–2414. [Google Scholar] [CrossRef]
- Tokarzewicz, A.; Romanowicz, L.; Sveklo, I.; Gorodkiewicz, E. The development of a matrix metalloproteinase-1 biosensor based on the surface plasmon resonance imaging technique. Anal. Met. 2016, 8, 6428–6435. [Google Scholar] [CrossRef]
- Sankiewicz, A.; Romanowicz, L.; Pyca, M.; Hermanowicz, A.; Gorodkiewicz, E. SPR imaging biosensor for the quantitation of fibronectin concentration in blood samples. J. Pharm. Biomed. Anal. 2018, 15, 1–8. [Google Scholar] [CrossRef]
- Matuszczak, E.; Tylicka, M.; Debek, W.; Sankiewicz, A.; Gorodkiewicz, E.; Hermanowicz, A. Overexpression of ubiquitin carboxyl-terminal hydrolase L1 (UCHL1) in serum of children after thermal injury. Adv. Med. Sci. 2017, 62, 83–86. [Google Scholar] [CrossRef]
- Saarinen, R.; Pitkäranta, A.; Kolho, K.; Tervahartiala, T.; Sorsa, T.; Lauhio, A. Decreased salivary matrix metalloproteinase-8 reflecting a defensive potential in juvenile parotitis. J. Ped. Otorhin. 2016, 80, 74–77. [Google Scholar] [CrossRef]
- Overall, C.M.; Lopez-Otín, C. Strategies for MMP inhibition in cancer: Innovations for the post-trial era. Nat. Rev. Cancer 2002, 2, 657–672. [Google Scholar] [CrossRef] [PubMed]
- Nagase, H.; Woessner, J.F. Matrix metalloproteinases. J. Biol. Chem. 1999, 274, 21491–21494. [Google Scholar] [CrossRef] [PubMed]
- Karsdal, M.A.; Larsen, L.; Engsig, M.T.; Lou, H.; Ferreras, M.; Lochter, A.; Delaissé, J.M.; Foged, N.T. Matrix metalloproteinase-dependent activation of latent transforming growth factor-beta controls the conversion of osteoblasts into osteocytes by blocking osteoblast apoptosis. J. Biol. Chem. 2002, 277, 44061–44067. [Google Scholar] [CrossRef] [PubMed]
- Overall, C.M.; McQuibban, G.A.; Clark-Lewis, I. Discovery of chemokine substrates for matrix metalloproteinases by exosite scanning: A new tool for degradomics. Biol. Chem. 2002, 383, 1059–1066. [Google Scholar] [CrossRef] [PubMed]
- Strand, S.; Vollmer, P.; van den Abeelen, L.; Gottfried, D.; Alla, V.; Heid, H.; Kuball, J.; Theobald, M.; Galle, P.R.; Strand, D. Cleavage of CD95 by matrix metalloproteinase-7 induces apoptosis resistance in tumour cells. Oncogene 2004, 23, 3732–3736. [Google Scholar] [CrossRef]
- Mu, D.; Cambier, S.; Fjellbirkeland, L.; Baron, J.L.; Munger, J.S.; Kawakatsu, H.; Sheppard, D.; Broaddus, V.C.; Nishimura, S.L. The integrin alpha(v)beta8 mediates epithelial homeostasis through MT1-MMP-dependent activation of TGF-beta1. J. Cell Biol. 2002, 157, 493–507. [Google Scholar] [CrossRef]
- Sadowski, T.; Dietrich, S.; Koschinsky, F.; Sedlacek, R. Matrix metalloproteinase 19 regulates insulin-like growth factor-mediated proliferation, migration, and adhesion in human keratinocytes through proteolysis of insulin-like growth factor binding protein-3. Mol. Biol. Cell 2003, 14, 4569–4580. [Google Scholar] [CrossRef]
- Caron, C.; Xue, J.; Sun, X.; Simmer, J.P.; Bartlett, J.D. Gelatinase A (MMP-2) in developing tooth tissues and amelogenin hydrolysis. J. Dent. Res. 2001, 80, 1660–1664. [Google Scholar] [CrossRef]
- Goldberg, M.; Rapoport, O.; Septier, D.; Palmier, K.; Hall, R.; Embery, G.; Young, M.; Ameye, L. Proteoglycans in predentin: The last 15 micrometers before mineralization. Connect. Tissue Res. 2003, 44 (Suppl. 1), 184–188. [Google Scholar] [CrossRef]
- Bourd-Boittin, K.; Septier, D.; Hall, R.; Goldberg, M.; Menashi, S. Immunolocalization of enamelysin (matrix metalloproteinase-20) in the forming rat incisor. J. Histochem. Cytochem. 2004, 52, 437–445. [Google Scholar] [CrossRef]
- Randall, L.E.; Hall, R.C. Temperospatial expression of matrix metalloproteinases 1, 2, 3, and 9 during early tooth development. Connect. Tissue Res. 2002, 43, 205–211. [Google Scholar] [CrossRef] [PubMed]
- Ingman, T.; Tervahartiala, T.; Ding, Y.; Tschesche, H.; Haerian, A.; Kinane, D.F.; Konttinen, Y.T.; Sorsa, T. Matrix metalloproteinases and their inhibitors in gingival crevicular fluid and saliva of periodontitis patients. J. Clin. Periodontol. 1996, 23, 1127–1132. [Google Scholar] [CrossRef] [PubMed]
- Nascimento, F.D.; Minciotti, C.L.; Geraldeli, S.; Carrilho, M.R.; Pashley, D.H.; Tay, F.R.; Nader, H.B.; Salo, T.; Tjäderhane, L.; Tersariol, I.L.S. Cysteine Cathepsins in Human Carious Dentin. J. Dent. Res. 2011, 90, 506–511. [Google Scholar] [CrossRef] [PubMed]
- Tersariol, I.L.; Geraldeli, S.; Minciotti, C.L.; Nascimento, F.D.; Paakkonen, V.; Martins, M.T.; Carrilho, M.R.; Pashley, D.H.; Tay, F.R.; Salo, T.; et al. Cysteine cathepsins in human dentin-pulp complex. J. Endod. 2010, 36, 475–481. [Google Scholar] [CrossRef] [PubMed]
- Steinman, R.R. Physiologic activity of the pulp-dentin complex. Quintessence Int. 1985, 16, 723–726. [Google Scholar]
- Steinman, R.R.; Leonora, J.; Singh, R.J. The effect of desalivation upon pulpal function and dental caries in rats. J. Dent. Res. 1980, 59, 176–185. [Google Scholar] [CrossRef]


| Value | MMP-1 Concentration (ng/mL) | MMP-2 Concentration (ng/mL) | ||
|---|---|---|---|---|
| Before Dental Treatment | After Dental Treatment | Before Dental Treatment | After Dental Treatment | |
| Median | 12.27 | 13.48 | 438.62 | 204.70 |
| Minimum | 2.45 | 0.90 | 96.72 | 97.04 |
| Maximum | 21.78 | 28.91 | 720.43 | 825.95 |
| Percentiles (25–75%) | 8.72–16.94 | 7.37–18.23 | 338.68–532.02 | 152.78–318.12 |
| p-value * | p > 0.05 | p ≤ 0.001 | ||
| Difference ** | 1.1 | 2.14 | ||
| Value | Fibronectin Concentration [µg/mL] | |
|---|---|---|
| Before Dental Treatment | After Dental Treatment | |
| Median | 9.97 | 11.43 |
| Minimum | 1.27 | 1.77 |
| Maximum | 21.48 | 26.96 |
| Percentiles (25–75%) | 6.91–13.33 | 8.51–14.56 |
| p value * | p = 0.057 | |
| Difference ** | 1.15 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matuszczak, E.; Cwalina, I.; Tylicka, M.; Wawrzyn, K.; Nowosielska, M.; Sankiewicz, A.; Ołdak, Ł.; Gorodkiewicz, E.; Hermanowicz, A. Levels of Selected Matrix Metalloproteinases—MMP-1, MMP-2 and Fibronectin in the Saliva of Patients Planned for Endodontic Treatment or Surgical Extraction. J. Clin. Med. 2020, 9, 3971. https://doi.org/10.3390/jcm9123971
Matuszczak E, Cwalina I, Tylicka M, Wawrzyn K, Nowosielska M, Sankiewicz A, Ołdak Ł, Gorodkiewicz E, Hermanowicz A. Levels of Selected Matrix Metalloproteinases—MMP-1, MMP-2 and Fibronectin in the Saliva of Patients Planned for Endodontic Treatment or Surgical Extraction. Journal of Clinical Medicine. 2020; 9(12):3971. https://doi.org/10.3390/jcm9123971
Chicago/Turabian StyleMatuszczak, Ewa, Izabela Cwalina, Marzena Tylicka, Katarzyna Wawrzyn, Magdalena Nowosielska, Anna Sankiewicz, Łukasz Ołdak, Ewa Gorodkiewicz, and Adam Hermanowicz. 2020. "Levels of Selected Matrix Metalloproteinases—MMP-1, MMP-2 and Fibronectin in the Saliva of Patients Planned for Endodontic Treatment or Surgical Extraction" Journal of Clinical Medicine 9, no. 12: 3971. https://doi.org/10.3390/jcm9123971
APA StyleMatuszczak, E., Cwalina, I., Tylicka, M., Wawrzyn, K., Nowosielska, M., Sankiewicz, A., Ołdak, Ł., Gorodkiewicz, E., & Hermanowicz, A. (2020). Levels of Selected Matrix Metalloproteinases—MMP-1, MMP-2 and Fibronectin in the Saliva of Patients Planned for Endodontic Treatment or Surgical Extraction. Journal of Clinical Medicine, 9(12), 3971. https://doi.org/10.3390/jcm9123971







