Considerations for Cannabis Use to Treat Pain in Sickle Cell Disease
Abstract
1. Introduction
2. Cannabinoids and Their Receptors
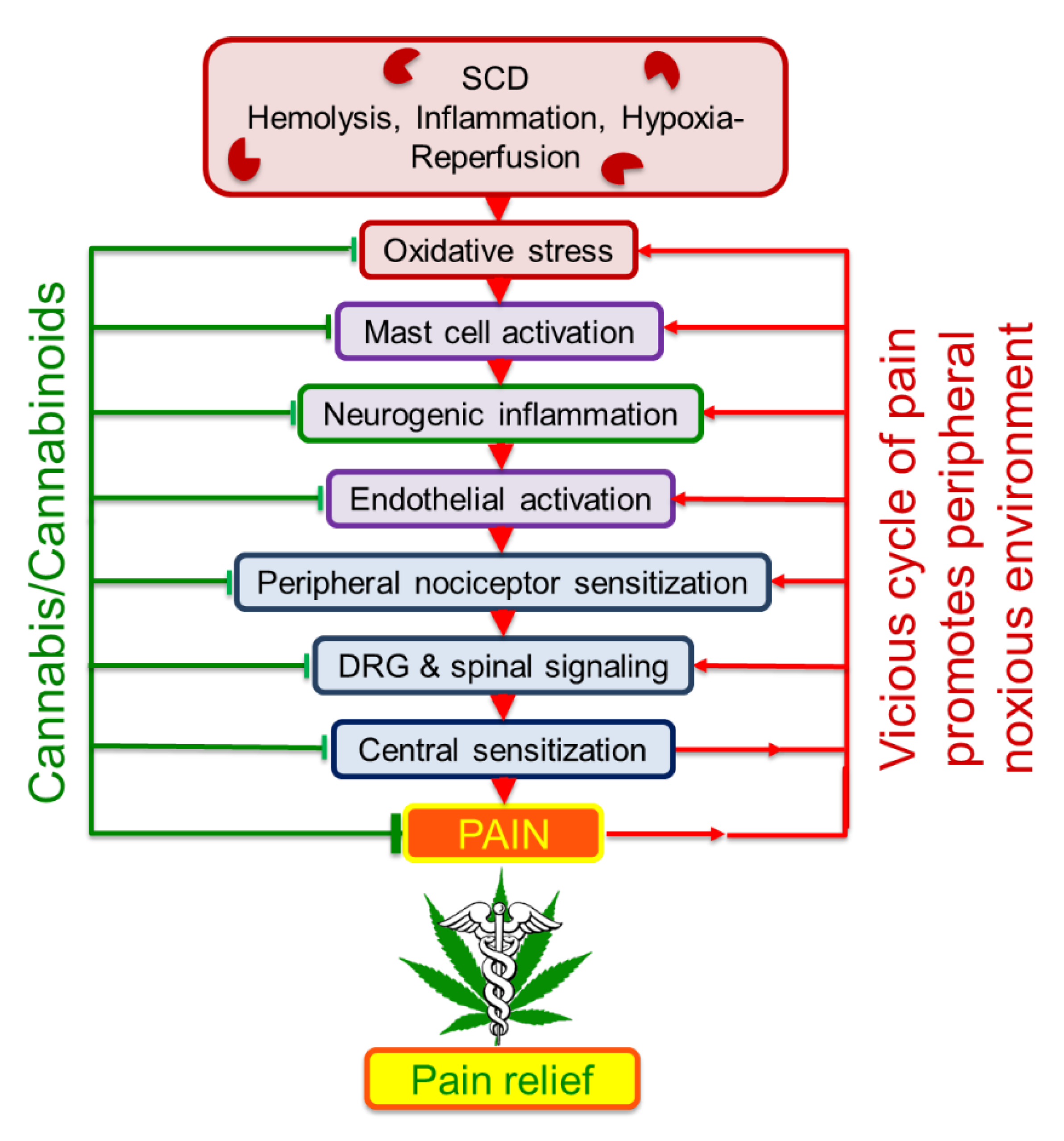
3. Mechanisms of Pain in Sickle Cell Disease
3.1. Mechanisms Involving the Nervous System
3.2. Neuroimmune Mechanisms
4. Effect of Cannabinoids on Sickle Pain and Sickle Pathobiology
5. Clinical Studies on the Effect of Cannabinoids on Pain
6. Clinical Use of Cannabis in Sickle Cell Disease
6.1. Fewer Admissions in Cannabis Users with Pain in SCD
6.2. Prospective Trial Shows Vaporized Cannabis Is Well Tolerated in Adults with SCD
6.3. Self-Management of Pain with Cannabis
6.4. Juvenile Use of Cannabis in SCD
6.5. Urine Screening Revealed Use of Cannabis and Other Drugs
6.6. Cannabis Use and Other Comorbidities of SCD
6.7. Cannabis Use in Sickle Cell Trait (SCT)
7. Risks vs. Benefits?
7.1. Tsunami of Cannabinoids
7.2. Major Challenges in the Study and Use of Cannabis in SCD
8. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ballas, S.K.; Gupta, K.; Adams-Graves, P. Sickle cell pain: A critical reappraisal. Blood 2012, 120, 3647–3656. [Google Scholar] [CrossRef] [PubMed]
- Smith, W.R.; Penberthy, L.T.; Bovbjerg, V.E.; McClish, D.K.; Roberts, J.D.; Dahman, B.; Aisiku, I.P.; Levenson, J.L.; Roseff, S.D. Daily Assessment of Pain in Adults with Sickle Cell Disease. Ann. Intern. Med. 2008, 148, 94–101. [Google Scholar] [CrossRef] [PubMed]
- Darbari, D.S.; Wang, Z.; Kwak, M.; Hildesheim, M.; Nichols, J.; Allen, D.; Seamon, C.; Peters-Lawrence, M.; Conrey, A.; Hall, M.K.; et al. Severe Painful Vaso-Occlusive Crises and Mortality in a Contemporary Adult Sickle Cell Anemia Cohort Study. PLoS ONE 2013, 8, e79923. [Google Scholar] [CrossRef] [PubMed]
- Gupta, M.; Msambichaka, L.; Ballas, S.K.; Gupta, K. Morphine for the Treatment of Pain in Sickle Cell Disease. Sci. World J. 2015, 2015, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Tran, H.; Sagi, V.; Song-Naba, W.L.; Wang, Y.; Mittal, A.; Lamarre, Y.; Zhang, L.; Gupta, K. Effect of chronic opioid therapy on pain and survival in a humanized mouse model of sickle cell disease. Blood Adv. 2019, 3, 869–873. [Google Scholar] [CrossRef]
- Ballas, S.K.; Kanter, J.; Agodoa, I.; Howard, R.; Wade, S.; Noxon, V.; Dampier, C. Opioid utilization patterns in United States individuals with sickle cell disease. Am. J. Hematol. 2018, 93, E345–E347. [Google Scholar] [CrossRef]
- Ruta, N.S.; Ballas, S.K. The Opioid Drug Epidemic and Sickle Cell Disease: Guilt by Association. Pain Med. 2016, 17, 1793–1798. [Google Scholar] [CrossRef]
- Russo, E.B. Cannabinoids in the management of difficult to treat pain. Ther. Clin. Risk Manag. 2008, 4, 245–259. [Google Scholar] [CrossRef]
- Ren, M.; Tang, Z.; Wu, X.; Spengler, R.; Jiang, H.; Yang, Y.; Boivin, N. The origins of cannabis smoking: Chemical residue evidence from the first millennium BCE in the Pamirs. Sci. Adv. 2019, 5, eaaw1391. [Google Scholar] [CrossRef]
- Gabay, M. The Federal Controlled Substances Act: Schedules and Pharmacy Registration. Hosp. Pharm. 2013, 48, 473–474. [Google Scholar] [CrossRef]
- Whiting, P.F.; Wolff, R.F.; Deshpande, S.; Di Nisio, M.; Duffy, S.; Hernandez, A.V.; Keurentjes, J.C.; Lang, S.; Misso, K.; Ryder, S.; et al. Cannabinoids for Medical Use: A Systematic Review and Meta-analysis. JAMA 2015, 313, 2456–2473. [Google Scholar] [CrossRef] [PubMed]
- Laux, L.C.; Bebin, E.M.; Checketts, D.; Chez, M.; Flamini, R.; Marsh, E.D.; Miller, I.; Nichol, K.; Park, Y.; Segal, E.; et al. Long-term safety and efficacy of cannabidiol in children and adults with treatment resistant Lennox-Gastaut syndrome or Dravet syndrome: Expanded access program results. Epilepsy Res. 2019, 154, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Maccallum, C.A.; Russo, E.B. Practical considerations in medical cannabis administration and dosing. Eur. J. Intern. Med. 2018, 49, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Ishida, J.H.; Wong, P.O.; Cohen, B.E.; Vali, M.; Steigerwald, S.; Keyhani, S. Substitution of marijuana for opioids in a national survey of US adults. PLoS ONE 2019, 14, e0222577. [Google Scholar] [CrossRef]
- Bachhuber, M.; Saloner, B.; Cunningham, C.O.; Barry, C.L. Medical Cannabis Laws and Opioid Analgesic Overdose Mortality in the United States, 1999–2010. JAMA Intern. Med. 2014, 174, 1668–1673. [Google Scholar] [CrossRef]
- Lopez, A.K.K.; Nichols, S.D.; Chung, D.Y.; Kaufman, D.E.; McCall, K.L.; Piper, B.J. Prescription Opioid Distribution after the Legalization of Recreational Marijuana in Colorado. Int. J. Environ. Res. Public Health 2020, 17, 3251. [Google Scholar] [CrossRef]
- Lopez, C.D.; Boddapati, V.; Jobin, C.M.; Hickernell, T.R. State Medical Cannabis Laws Associated with Reduction in Opioid Prescriptions by Orthopaedic Surgeons in Medicare Part D Cohort. J. Am. Acad. Orthop. Surg. 2020. [Google Scholar] [CrossRef]
- Howard, J.; Anie, K.A.; Holdcroft, A.; Israeli-Korn, S.; Davies, S.C. Cannabis use in sickle cell disease: A questionnaire study. Br. J. Haematol. 2005, 131, 123–128. [Google Scholar] [CrossRef]
- Frisch, S. Medical cannabis: US researchers battle for access to the plant. BMJ 2014, 349, g6997. [Google Scholar] [CrossRef]
- Roberts, J.D.; Spodick, J.; Cole, J.; Bozzo, J.; Curtis, S.A.; Forray, A. Marijuana Use in Adults Living with Sickle Cell Disease. Cannabis Cannabinoid Res. 2018, 3, 162–165. [Google Scholar] [CrossRef]
- Sinha, C.B.; Bakshi, N.; Ross, D.; Krishnamurti, L. Management of Chronic Pain in Adults Living with Sickle Cell Disease in the Era of the Opioid Epidemic: A Qualitative Study. JAMA Netw. Open 2019, 2, e194410. [Google Scholar] [CrossRef] [PubMed]
- Fearon, A.; Marsh, A.; Kim, J.; Treadwell, M. Pediatric residents’ perceived barriers to opioid use in sickle cell disease pain management. Pediatr. Blood Cancer 2019, 66, e27535. [Google Scholar] [CrossRef] [PubMed]
- Al Achkar, M.; Revere, D.; Dennis, B.; Mackie, P.; Gupta, S.; Grannis, S. Exploring perceptions and experiences of patients who have chronic pain as state prescription opioid policies change: A qualitative study in Indiana. BMJ Open 2017, 7, e015083. [Google Scholar] [CrossRef] [PubMed]
- Nichols, V.P.; Toye, F.; Eldabe, S.; Sandhu, H.K.; Underwood, M.; Seers, K. Experiences of people taking opioid medication for chronic non-malignant pain: A qualitative evidence synthesis using meta-ethnography. BMJ Open 2020, 10, e032988. [Google Scholar] [CrossRef] [PubMed]
- Toye, F.; Seers, K.; Tierney, S.; Barker, K.L. A qualitative evidence synthesis to explore healthcare professionals’ experience of prescribing opioids to adults with chronic non-malignant pain. BMC Fam. Pract. 2017, 18, 94. [Google Scholar] [CrossRef] [PubMed]
- Kohli, D.R.; Li, Y.; Khasabov, S.G.; Gupta, P.; Kehl, L.J.; Ericson, M.E.; Nguyen, J.; Gupta, V.; Hebbel, R.P.; Simone, D.A.; et al. Pain-related behaviors and neurochemical alterations in mice expressing sickle hemoglobin: Modulation by cannabinoids. Blood 2010, 116, 456–465. [Google Scholar] [CrossRef] [PubMed]
- Cain, D.M.; Vang, D.; Simone, D.A.; Hebbel, R.P.; Gupta, K. Mouse models for studying pain in sickle disease: Effects of strain, age, and acuteness. Br. J. Haematol. 2011, 156, 535–544. [Google Scholar] [CrossRef]
- Vincent, L.; Vang, D.; Nguyen, J.; Benson, B.; Lei, J.; Gupta, K. Cannabinoid receptor-specific mechanisms to alleviate pain in sickle cell anemia via inhibition of mast cell activation and neurogenic inflammation. Haematology 2015, 101, 566–577. [Google Scholar] [CrossRef]
- Argueta, D.A.; Ventura, C.M.; Kiven, S.; Sagi, V.; Gupta, K. A Balanced Approach for Cannabidiol Use in Chronic Pain. Front. Pharmacol. 2020, 11, 561. [Google Scholar] [CrossRef]
- Abrams, D.I.; Couey, P.; Dixit, N.; Sagi, V.; Hagar, W.; Vichinsky, E.; Kelly, M.E.; Connett, J.E.; Gupta, K. Effect of Inhaled Cannabis for Pain in Adults With Sickle Cell Disease: A Randomized Clinical Trial. JAMA Netw. Open 2020, 3, e2010874. [Google Scholar] [CrossRef]
- Mechoulam, R.; Ben-Shabat, S.; Hanus, L.; Ligumsky, M.; Kaminski, N.E.; Schatz, A.R.; Gopher, A.; Almog, S.; Martin, B.R.; Compton, D.R.; et al. Identification of an endogenous 2-monoglyceride, present in canine gut, that binds to cannabinoid receptors. Biochem. Pharmacol. 1995, 50, 83–90. [Google Scholar] [CrossRef]
- Munro, S.; Thomas, K.L.; Abu-Shaar, M. Molecular characterization of a peripheral receptor for cannabinoids. Nat. Cell Biol. 1993, 365, 61–65. [Google Scholar] [CrossRef] [PubMed]
- Tai, S.; Fantegrossi, W.E. Synthetic Cannabinoids: Pharmacology, Behavioral Effects, and Abuse Potential. Curr. Addict. Rep. 2014, 1, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Atakan, Z. Cannabis, a complex plant: Different compounds and different effects on individuals. Ther. Adv. Psychopharmacol. 2012, 2, 241–254. [Google Scholar] [CrossRef]
- Lauckner, J.E.; Jensen, J.B.; Chen, H.-Y.; Lu, H.-C.; Hille, B.; Mackie, K. GPR55 is a cannabinoid receptor that increases intracellular calcium and inhibits M current. Proc. Natl. Acad. Sci. USA 2008, 105, 2699–2704. [Google Scholar] [CrossRef]
- Morales, P.; Reggio, P.H. An Update on Non-CB1, Non-CB2 Cannabinoid Related G-Protein-Coupled Receptors. Cannabis Cannabinoid Res. 2017, 2, 265–273. [Google Scholar] [CrossRef]
- Mechoulam, R.; Shvo, Y.; Hashish, I. The structure of cannabidiol. Tetrahedron 1963, 19, 2073–2078. [Google Scholar] [CrossRef]
- Mechoulam, R.; Gaoni, Y. A total synthesis of dl-delta-1-tetrahydrocannabinol, the active constituent of hashish. J. Am. Chem. Soc. 1965, 87, 3273–3275. [Google Scholar] [CrossRef]
- DiPatrizio, N.V.; Piomelli, D. The thrifty lipids: Endocannabinoids and the neural control of energy conservation. Trends Neurosci. 2012, 35, 403–411. [Google Scholar] [CrossRef]
- Donvito, G.; Nass, S.R.; Wilkerson, J.L.; Curry, Z.A.; Schurman, L.D.; Kinsey, S.G.; Lichtman, A.H. The Endogenous Cannabinoid System: A Budding Source of Targets for Treating Inflammatory and Neuropathic Pain. Neuropsychopharmacology 2018, 43, 52–79. [Google Scholar] [CrossRef]
- Fowler, C. Monoacylglycerol lipase—A target for drug development? Br. J. Pharmacol. 2012, 166, 1568–1585. [Google Scholar] [CrossRef] [PubMed]
- Pertwee, R.G. Handbook of Cannabis, 1st ed.; Oxford University Press: Oxford, UK, 2014. [Google Scholar]
- Piomelli, D. The molecular logic of endocannabinoid signalling. Nat. Rev. Neurosci. 2003, 4, 873–884. [Google Scholar] [CrossRef] [PubMed]
- Argueta, D.A.; Perez, P.A.; Makriyannis, A.; DiPatrizio, N.V. Cannabinoid CB1 Receptors Inhibit Gut-Brain Satiation Signaling in Diet-Induced Obesity. Front. Physiol. 2019, 10, 704. [Google Scholar] [CrossRef] [PubMed]
- Turcotte, C.; Blanchet, M.-R.; LaViolette, M.; Flamand, N. The CB2 receptor and its role as a regulator of inflammation. Cell. Mol. Life Sci. 2016, 73, 4449–4470. [Google Scholar] [CrossRef]
- Dotsey, E.; Ushach, I.; Pone, E.; Nakajima, R.; Jasinskas, A.; Argueta, D.A.; Dillon, A.; DiPatrizio, N.; Davies, H.; Zlotnik, A.; et al. Transient Cannabinoid Receptor 2 Blockade during Immunization Heightens Intensity and Breadth of Antigen-specific Antibody Responses in Young and Aged mice. Sci. Rep. 2017, 7, 42584. [Google Scholar] [CrossRef]
- Kato, G.J.; Piel, F.B.; Reid, C.D.; Gaston, M.H.; Ohene-Frempong, K.; Krishnamurti, L.; Smith, W.R.; Panepinto, J.A.; Weatherall, D.J.; Costa, F.F.; et al. Sickle cell disease. Nat. Rev. Dis. Primers 2018, 4, 18010. [Google Scholar] [CrossRef]
- Martí-Carvajal, A.J.; Solà, I.; Agreda-Pérez, L.H. Treatment for avascular necrosis of bone in people with sickle cell disease. Cochrane Database Syst. Rev. 2019, 12, CD004344. [Google Scholar] [CrossRef]
- Kato, G.J.; Steinberg, M.H.; Gladwin, M.T. Intravascular hemolysis and the pathophysiology of sickle cell disease. J. Clin. Investig. 2017, 127, 750–760. [Google Scholar] [CrossRef]
- Dampier, C. New and emerging treatments for vaso-occlusive pain in sickle cell disease. Expert Rev. Hematol. 2019, 12, 857–872. [Google Scholar] [CrossRef]
- Manwani, D.; Frenette, P.S. Vaso-occlusion in sickle cell disease: Pathophysiology and novel targeted therapies. Blood 2013, 122, 3892–3898. [Google Scholar] [CrossRef]
- Takaoka, K.; Cyril, A.C.; Jinesh, S.; Radhakrishnan, R. Mechanisms of pain in sickle cell disease. Br. J. Pain 2020. [Google Scholar] [CrossRef]
- Sadler, K.E.; Stucky, C.L. Neuronal transient receptor potential (TRP) channels and noxious sensory detection in sickle cell disease. Neurosci. Lett. 2019, 694, 184–191. [Google Scholar] [CrossRef] [PubMed]
- Sagi, V.; Mittal, A.; Gupta, M.; Gupta, K. Immune cell neural interactions and their contributions to sickle cell disease. Neurosci. Lett. 2019, 699, 167–171. [Google Scholar] [CrossRef] [PubMed]
- Tran, H.; Gupta, M.; Gupta, K. Targeting novel mechanisms of pain in sickle cell disease. Blood 2017, 130, 2377–2385. [Google Scholar] [CrossRef] [PubMed]
- Vincent, L.; Vang, D.; Nguyen, J.; Gupta, M.; Luk, K.; Ericson, M.E.; Simone, D.A.; Gupta, K. Mast cell activation contributes to sickle cell pathobiology and pain in mice. Blood 2013, 122, 1853–1862. [Google Scholar] [CrossRef] [PubMed]
- Hillery, C.A.; Kerstein, P.C.; Vilceanu, D.; Barabas, M.E.; Retherford, D.; Brandow, A.M.; Wandersee, N.J.; Stucky, C.L. Transient receptor potential vanilloid 1 mediates pain in mice with severe sickle cell disease. Blood 2011, 118, 3376–3383. [Google Scholar] [CrossRef] [PubMed]
- Cataldo, G.; Rajput, S.; Gupta, K.; Simone, D.A. Sensitization of nociceptive spinal neurons contributes to pain in a transgenic model of sickle cell disease. Pain 2015, 156, 722–730. [Google Scholar] [CrossRef]
- Uhelski, M.L.; Gupta, K.; Simone, D.A. Sensitization of C-fiber nociceptors in mice with sickle cell disease is decreased by local inhibition of anandamide hydrolysis. Pain 2017, 158, 1711–1722. [Google Scholar] [CrossRef]
- Valverde, Y.; Benson, B.; Gupta, M.; Gupta, K. Spinal glial activation and oxidative stress are alleviated by treatment with curcumin or coenzyme Q in sickle mice. Haematology 2015, 101, e44–e47. [Google Scholar] [CrossRef]
- Lei, J.; Paul, J.A.; Wang, Y.; Gupta, M.; Vang, D.; Thompson, S.; Jha, R.; Nguyen, J.; Valverde, Y.; Lamarre, Y.; et al. Heme causes pain in sickle mice via toll-like receptor 4-mediated ROS- and endoplasmic reticulum stress-induced glial activation. Antioxid. Redox Signal. 2020. [Google Scholar] [CrossRef]
- Tran, H.; Mittal, A.; Sagi, V.; Luk, K.; Nguyen, A.; Gupta, M.; Nguyen, J.; Lamarre, Y.; Lei, J.; Guedes, A.; et al. Mast Cells Induce Blood Brain Barrier Damage in SCD by Causing Endoplasmic Reticulum Stress in the Endothelium. Front. Cell. Neurosci. 2019, 13, 56. [Google Scholar] [CrossRef] [PubMed]
- Manci, E.A. Pathology of Berkeley sickle cell mice: Similarities and differences with human sickle cell disease. Blood 2006, 107, 1651–1658. [Google Scholar] [CrossRef]
- Kiven, S.; Wang, Y.; Aich, A.; Argueta, D.A.; Lei, J.; Sagi, V.; Tennakoon, M.; Bedros, S.J.; Lambrecht, N.; Gupta, K. Spatiotemporal Alterations in Gait in Humanized Transgenic Sickle Mice. Front. Immunol. 2020, 11, 561947. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Almeida, L.E.; Batista, C.M.D.S.; Khaibullina, A.; Xu, N.; Albani, S.; Guth, K.A.; Seo, J.S.; Quezado, M.; Quezado, Z.M. Cognitive and behavior deficits in sickle cell mice are associated with profound neuropathologic changes in hippocampus and cerebellum. Neurobiol. Dis. 2016, 85, 60–72. [Google Scholar] [CrossRef] [PubMed]
- Brandow, A.M.; Wandersee, N.J.; Dasgupta, M.; Hoffmann, R.G.; Hillery, C.A.; Stucky, C.L.; Panepinto, J.A. Substance P is increased in patients with sickle cell disease and associated with haemolysis and hydroxycarbamide use. Br. J. Haematol. 2016, 175, 237–245. [Google Scholar] [CrossRef] [PubMed]
- Michaels, L.A.; Ohene-Frempong, K.; Zhao, H.; Douglas, S.D. Serum levels of substance P are elevated in patients with sickle cell disease and increase further during vaso-occlusive crisis. Blood 1998, 92, 3148–3151. [Google Scholar] [CrossRef] [PubMed]
- Albo, C.; Kumar, S.; Pope, M.; Kidwell, K.M.; Xu, H.; Bowman, L.; Wells, L.; Barrett, N.; Fields, S.; Bora, P.; et al. Characteristics and potential biomarkers of adult sickle cell patients with chronic pain. Eur. J. Haematol. 2020. [Google Scholar] [CrossRef]
- Savage, W.J.; Barron-Casella, E.; Fu, Z.; Dulloor, P.; Williams, L.; Crain, B.J.; White, D.A.; Jennings, J.M.; Van Eyk, J.E.; DeBaun, M.R.; et al. Plasma glial fibrillary acidic protein levels in children with sickle cell disease. Am. J. Hematol. 2011, 86, 427–429. [Google Scholar] [CrossRef]
- Romero-Sandoval, A.; Horvath, R.J.; DeLeo, J.A. Neuroimmune interactions and pain: Focus on glial-modulating targets. Curr. Opin. Investig. Drugs 2008, 9, 726–734. [Google Scholar]
- Gupta, K.; Jahagirdar, O.; Gupta, K. Targeting pain at its source in sickle cell disease. Am. J. Physiol. Integr. Comp. Physiol. 2018, 315, R104–R112. [Google Scholar] [CrossRef]
- Ezenwa, M.O.; Molokie, R.E.; Wang, Z.J.; Yao, Y.; Suarez, M.L.; Pullum, C.; Schlaeger, J.M.; Fillingim, R.B.; Wilkie, D.J. Safety and Utility of Quantitative Sensory Testing among Adults with Sickle Cell Disease: Indicators of Neuropathic Pain? Pain Pract. 2016, 16, 282–293. [Google Scholar] [CrossRef] [PubMed]
- Sharma, D.; Brandow, A.M. Neuropathic pain in individuals with sickle cell disease. Neurosci. Lett. 2020, 714, 134445. [Google Scholar] [CrossRef] [PubMed]
- Orhurhu, M.S.; Chu, R.; Claus, L.; Roberts, J.; Salisu, B.; Urits, I.; Orhurhu, E.; Viswanath, O.; Kaye, A.D.; Kaye, A.J.; et al. Neuropathic Pain and Sickle Cell Disease: A Review of Pharmacologic Management. Curr. Pain Headache Rep. 2020, 24, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Ballas, S.K.; Darbari, D.S. Review/overview of pain in sickle cell disease. Complement. Ther. Med. 2020, 49, 102327. [Google Scholar] [CrossRef] [PubMed]
- Brandow, A.M.; Stucky, C.L.; Hillery, C.A.; Hoffmann, R.G.; Panepinto, J.A. Patients with sickle cell disease have increased sensitivity to cold and heat. Am. J. Hematol. 2013, 88, 37–43. [Google Scholar] [CrossRef]
- Campbell, C.M.; Carroll, C.P.; Kiley, K.; Han, D.; Haywood, C., Jr.; Lanzkron, S.; Swedberg, L.; Edwards, R.R.; Page, G.G.; Haythornthwaite, J.A. Quantitative sensory testing and pain-evoked cytokine reactivity: Comparison of patients with sickle cell disease to healthy matched controls. Pain 2016, 157, 949–956. [Google Scholar] [CrossRef]
- Campbell, C.M.; Moscou-Jackson, G.; Carroll, C.P.; Kiley, K.; Haywood, C.; Lanzkron, S.; Hand, M.; Edwards, R.R.; Haythornthwaite, J.A. An Evaluation of Central Sensitization in Patients with Sickle Cell Disease. J. Pain 2016, 17, 617–627. [Google Scholar] [CrossRef]
- Kuei, B.N.; Patel, M.N.; Xu, H.; Wells, M.L.; Bowman, B.L.; Bora, P.; Natrajan, M.K.; Kutlar, A. Characteristics and Potential Biomarkers for Chronic Pain in Patients with Sickle Cell Disease. Blood 2015, 126, 986. [Google Scholar] [CrossRef]
- Stojanovic, K.S.; Thioliere, B.; Garandeau, E.; LeComte, I.; Bachmeyer, C.; Lionnet, F. Chronic myeloid leukaemia and sickle cell disease: Could imatinib prevent vaso-occlusive crisis? Br. J. Haematol. 2011, 155, 271–272. [Google Scholar] [CrossRef]
- Close, J.L.; Lottenberg, R. Effectiveness of Imatinib Therapy for a Patient with Sickle Cell Anemia and Chronic Myelocytic Leukemia. Blood 2009, 114, 2559. [Google Scholar] [CrossRef]
- Murphy, M.; Close, J.; Lottenberg, R.; Rajasekhar, A. Effectiveness of Imatinib Therapy for Sickle Cell Anemia and Chronic Myeloid Leukemia. Am. J. Med. Sci. 2014, 347, 254–255. [Google Scholar] [CrossRef] [PubMed]
- Calignano, A.; La Rana, G.; Giuffrida, A.; Piomelli, D. Control of pain initiation by endogenous cannabinoids. Nat. Cell Biol. 1998, 394, 277–281. [Google Scholar] [CrossRef] [PubMed]
- Costa, B.; Trovato, A.E.; Comelli, F.; Giagnoni, G.; Colleoni, M. The non-psychoactive cannabis constituent cannabidiol is an orally effective therapeutic agent in rat chronic inflammatory and neuropathic pain. Eur. J. Pharmacol. 2007, 556, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Zoppi, S.; Madrigal, J.L.M.; Caso, J.R.; García-Gutiérrez, M.S.; Manzanares, J.; Leza, J.C.; García-Bueno, B. Regulatory role of the cannabinoid CB2receptor in stress-induced neuroinflammation in mice. Br. J. Pharmacol. 2014, 171, 2814–2826. [Google Scholar] [CrossRef] [PubMed]
- Hebbel, R.P. Ischemia-reperfusion injury in sickle cell anemia: Relationship to acute chest syndrome, endothelial dysfunction, arterial vasculopathy, and inflammatory pain. Hematol. Oncol. Clin. 2014, 28, 181–198. [Google Scholar] [CrossRef]
- Frenette, P.S. Sickle cell vasoocclusion: Heterotypic, multicellular aggregations driven by leukocyte adhesion. Microcirculation 2004, 11, 167–177. [Google Scholar] [CrossRef] [PubMed]
- Hebbel, R.P.; Osarogiagbon, R.; Kaul, D. The endothelial biology of sickle cell disease: Inflammation and a chronic vasculopathy. Microcirculation 2004, 11, 129–151. [Google Scholar] [CrossRef]
- Giacoppo, S.; Mandolino, G.; Galuppo, M.; Bramanti, P.; Mazzon, E. Cannabinoids: New Promising Agents in the Treatment of Neurological Diseases. Molecules 2014, 19, 18781–18816. [Google Scholar] [CrossRef]
- Nyakundi, B.B.; Erdei, J.; Tóth, A.; Balogh, E.; Nagy, A.; Nagy, B.; Novák, L.; Bognár, L.; Paragh, G.; Kappelmayer, J.; et al. Formation and Detection of Highly Oxidized Hemoglobin Forms in Biological Fluids during Hemolytic Conditions. Oxidative Med. Cell. Longev. 2020, 2020, 1–13. [Google Scholar] [CrossRef]
- Rangel-López, E.; Colín-González, A.; Paz-Loyola, A.; Pinzón, E.; Torres, I.; Serratos, I.; Castellanos, P.; Wajner, M.; Souza, D.; Santamaría, A. Cannabinoid receptor agonists reduce the short-term mitochondrial dysfunction and oxidative stress linked to excitotoxicity in the rat brain. Neuroscience 2015, 285, 97–106. [Google Scholar] [CrossRef]
- Casarejos, M.J.; Perucho, J.; Gomez, A.; Muñoz, M.P.; Fernandez-Estevez, M.; Sagredo, O.; Ruiz, J.F.; Guzmán, M.; De Yebenes, J.G.; Mena, M. Natural Cannabinoids Improve Dopamine Neurotransmission and Tau and Amyloid Pathology in a Mouse Model of Tauopathy. J. Alzheimer Dis. 2013, 35, 525–539. [Google Scholar] [CrossRef] [PubMed]
- Kassim, A.A.; DeBaun, M.R. Sickle Cell Disease, Vasculopathy, and Therapeutics. Annu. Rev. Med. 2013, 64, 451–466. [Google Scholar] [CrossRef] [PubMed]
- Rees, D.C.; Williams, T.N.; Gladwin, M.T. Sickle-cell disease. Lancet 2010, 376, 2018–2031. [Google Scholar] [CrossRef]
- Stanley, C.; O’Sullivan, S.E. Vascular targets for cannabinoids: Animal and human studies. Br. J. Pharmacol. 2014, 171, 1361–1378. [Google Scholar] [CrossRef]
- Stockings, E.; Campbell, G.; Hall, W.D.; Nielsen, S.; Zagic, D.; Rahman, R.; Murnion, B.; Farrell, M.; Weier, M.; Degenhardt, L. Cannabis and cannabinoids for the treatment of people with chronic noncancer pain conditions: A systematic review and meta-analysis of controlled and observational studies. Pain 2018, 159, 1932–1954. [Google Scholar] [CrossRef]
- Abrams, D.I.; Jay, C.A.; Shade, S.B.; Vizoso, H.; Reda, H.; Press, S.; Kelly, M.E.; Rowbotham, M.C.; Petersen, K.L. Cannabis in painful HIV-associated sensory neuropathy: A randomized placebo-controlled trial. Neurology 2007, 68, 515–521. [Google Scholar] [CrossRef]
- Cooper, Z.D.; Comer, S.D.; Haney, M. Comparison of the Analgesic Effects of Dronabinol and Smoked Marijuana in Daily Marijuana Smokers. Neuropsychopharmacology 2013, 38, 1984–1992. [Google Scholar] [CrossRef]
- Wilsey, B.; Marcotte, T.; Tsodikov, A.; Millman, J.; Bentley, H.; Gouaux, B.; Fishman, S. A Randomized, Placebo-Controlled, Crossover Trial of Cannabis Cigarettes in Neuropathic Pain. J. Pain 2008, 9, 506–521. [Google Scholar] [CrossRef]
- Johnson, J.R.; Lossignol, D.; Burnell-Nugent, M.; Fallon, M.T. An Open-Label Extension Study to Investigate the Long-Term Safety and Tolerability of THC/CBD Oromucosal Spray and Oromucosal THC Spray in Patients With Terminal Cancer-Related Pain Refractory to Strong Opioid Analgesics. J. Pain Symptom Manag. 2013, 46, 207–218. [Google Scholar] [CrossRef]
- De Vries, M.; Van Rijckevorsel, D.C.; Vissers, K.C.; Wilder-Smith, O.H.; Van Goor, H. Tetrahydrocannabinol Does Not Reduce Pain in Patients With Chronic Abdominal Pain in a Phase 2 Placebo-controlled Study. Clin. Gastroenterol. Hepatol. 2017, 15, 1079–1086.e4. [Google Scholar] [CrossRef]
- Langford, R.M.; Mares, J.; Novotna, A.; Vachova, M.; Novakova, I.; Notcutt, W.; Ratcliffe, S. A double-blind, randomized, placebo-controlled, parallel-group study of THC/CBD oromucosal spray in combination with the existing treatment regimen, in the relief of central neuropathic pain in patients with multiple sclerosis. J. Neurol. 2013, 260, 984–997. [Google Scholar] [CrossRef] [PubMed]
- Rog, D.J.; Nurmikko, T.J.; Friede, T.; Young, C.A. Randomized, controlled trial of cannabis-based medicine in central pain in multiple sclerosis. Neurology 2005, 65, 812–819. [Google Scholar] [CrossRef] [PubMed]
- Serpell, M.; Ratcliffe, S.; Hovorka, J.; Schofield, M.; Taylor, L.; Lauder, H.; Ehler, E. A double-blind, randomized, placebo-controlled, parallel group study of THC/CBD spray in peripheral neuropathic pain treatment. Eur. J. Pain 2014, 18, 999–1012. [Google Scholar] [CrossRef] [PubMed]
- Berman, J.S.; Symonds, C.; Birch, R. Efficacy of two cannabis based medicinal extracts for relief of central neuropathic pain from brachial plexus avulsion: Results of a randomised controlled trial. Pain 2004, 112, 299–306. [Google Scholar] [CrossRef]
- Nurmikko, T.; Serpell, M.G.; Hoggart, B.; Toomey, P.J.; Morlion, B.J.; Haines, D. Sativex successfully treats neuropathic pain characterised by allodynia: A randomised, double-blind, placebo-controlled clinical trial. Pain 2007, 133, 210–220. [Google Scholar] [CrossRef]
- Fisher, E.; Eccleston, C.; Degenhardt, L.; Finn, D.P.; Finnerup, N.B.; Gilron, I.; Haroutounian, S.; Krane, E.; Rice, A.S.C.; Rowbotham, M.; et al. Cannabinoids, cannabis, and cannabis-based medicine for pain management: A systematic review of randomised controlled trials. Pain Rep. 2019. [Google Scholar] [CrossRef]
- Bhuniya, D.; Kharul, R.K.; Hajare, A.; Shaikh, N.; Bhosale, S.; Balwe, S.; Begum, F.; De, S.; Athavankar, S.; Joshi, D.; et al. Discovery and evaluation of novel FAAH inhibitors in neuropathic pain model. Bioorgan. Med. Chem. Lett. 2019, 29, 238–243. [Google Scholar] [CrossRef]
- Singh, S.; Kaur, R.; Sidhu, P. What failed BIA 10-2474 Phase I clinical trial? Global speculations and recommendations for future Phase I trials. J. Pharmacol. Pharmacother. 2016, 7, 120–126. [Google Scholar] [CrossRef]
- Curtis, S.A.; Lew, D.; Spodick, J.; Roberts, J.D. Medical Marijuana for Sickle Cell Disease: Results of Two Years of Certification in an Adult Sickle Cell Center. Blood 2018, 132, 858. [Google Scholar] [CrossRef]
- Curtis, S.A.; Brandow, A.M.; Deveaux, M.; Zeltermam, D.; Devine, L.; Roberts, J.D. Daily Cannabis Users with Sickle Cell Disease Show Fewer Admissions than Others with Similar Pain Complaints. Cannabis Cannabinoid Res. 2020, 5, 255–262. [Google Scholar] [CrossRef]
- Curtis, S.A.; Lew, D.; Spodick, J.; Hendrickson, J.E.; Minniti, C.P.; Roberts, J.D. Medical marijuana certification for patients with sickle cell disease: A report of a single center experience. Blood Adv. 2020, 4, 3814–3821. [Google Scholar] [CrossRef] [PubMed]
- Wilson, J.D.; Pecker, L.H.; Lanzkron, S.; Bediako, S.M.; Han, D.; Beach, M.C. Marijuana use and health behaviors in a US clinic sample of patients with sickle cell disease. PLoS ONE 2020, 15, e0235192. [Google Scholar] [CrossRef] [PubMed]
- Wilson, J.D.; Lanzkron, S.; Pecker, L.H.; Bediako, S.M.; Han, D.; Beach, M.C. Psychosocial and Clinical Risk Factors Associated with Substance Use in Observational Cohort of Patients with Sickle Cell Disease. Subst. Use Misuse 2020, 55, 2205–2212. [Google Scholar] [CrossRef] [PubMed]
- Ballas, S.K. The Use of Cannabis by Patients with Sickle Cell Disease Increased the Frequency of Hospitalization due to Vaso-Occlusive Crises. Cannabis Cannabinoid Res. 2017, 2, 197–201. [Google Scholar] [CrossRef] [PubMed]
- Zheng, C.Y.; Minniti, C.P.; Chaitowitz, M.H. Sickle Cell Crisis Complicated by Synthetic Cannabinoid Abuse: A Case Report. Hemoglobin 2016, 40, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Matta, A.; Tandra, P.K.; Berim, L. Priapism in a patient with sickle cell trait using marijuana. BMJ Case Rep. 2014, 2014. [Google Scholar] [CrossRef] [PubMed]
- Birnbaum, B.F.; Pinzone, J.J. Sickle cell trait and priapism: A case report and review of the literature. Cases J. 2008, 1, 429. [Google Scholar] [CrossRef]
- Knight-Madden, J.; Lewis, N.; Hambleton, I. The prevalence of marijuana smoking in young adults with sickle cell disease: A longitudinal study. West Indian Med. J. 2006, 55, 224–227. [Google Scholar] [CrossRef]
- Britto, M.T.; Garrett, J.M.; Dugliss, M.A.J.; Daeschner, C.W., Jr.; Johnson, C.A.; Leigh, M.W.; Majure, J.M.; Schultz, W.H.; Konrad, T.R. Risky Behavior in Teens With Cystic Fibrosis or Sickle Cell Disease: A Multicenter Study. Pediatrics 1998, 101, 250–256. [Google Scholar] [CrossRef]
- Román, M.E.; Highland, J.; Retherford, D.; Pan, A.Y.; Panepinto, J.A.; Brandow, A.M. Neuropathic pain is associated with poor health-related quality of life in adolescents with sickle cell disease: A preliminary report. Pediatr. Blood Cancer 2020, 67, e28698. [Google Scholar] [CrossRef]
- Ballas, S.K. How I treat acute and persistent sickle cell pain. Mediterr. J. Hematol. Infect. Dis. 2020, 12, e2020064. [Google Scholar] [CrossRef] [PubMed]
- Luis, C.V.S.; Nobleza, C.O.S.; Shekhar, S.; Sugg, R.; Villareal, D.J.; Mehta, T.; Gangadhara, S. Association between recent cannabinoid use and acute ischemic stroke. Neurol. Clin. Pract. 2020, 10, 333–339. [Google Scholar] [CrossRef] [PubMed]
- Wood, K.E. Exposure to Bath Salts and Synthetic Tetrahydrocannabinol from 2009 to 2012 in the United States. J. Pediatr. 2013, 163, 213–216. [Google Scholar] [CrossRef] [PubMed]
- Lichtman, A.H.; Lux, E.A.; McQuade, R.; Rossetti, S.; Sanchez, R.; Sun, W.; Wright, S.; Kornyeyeva, E.; Fallon, M.T. Results of a Double-Blind, Randomized, Placebo-Controlled Study of Nabiximols Oromucosal Spray as an Adjunctive Therapy in Advanced Cancer Patients with Chronic Uncontrolled Pain. J. Pain Symptom Manag. 2018, 55, 179–188.e1. [Google Scholar] [CrossRef]
- Fallon, M.; Lux, E.A.; McQuade, R.; Rossetti, S.; Sanchez, R.; Sun, W.; Wright, S.; Lichtman, A.H.; Kornyeyeva, E. Sativex oromucosal spray as adjunctive therapy in advanced cancer patients with chronic pain unalleviated by optimized opioid therapy: Two double-blind, randomized, placebo-controlled phase 3 studies. Br. J. Pain 2017, 11, 119–133. [Google Scholar] [CrossRef] [PubMed]
- Lynch, M.E.; Cesar-Rittenberg, P.; Hohmann, A.G. A Double-Blind, Placebo-Controlled, Crossover Pilot Trial With Extension Using an Oral Mucosal Cannabinoid Extract for Treatment of Chemotherapy-Induced Neuropathic Pain. J. Pain Symptom Manag. 2014, 47, 166–173. [Google Scholar] [CrossRef] [PubMed]
- Young-Wolff, K.C.; Tucker, L.-Y.; Alexeeff, S.; Armstrong, M.A.; Conway, A.; Weisner, C.; Goler, N. Trends in Self-reported and Biochemically Tested Marijuana Use among Pregnant Females in California from 2009–2016. JAMA 2017, 318, 2490–2491. [Google Scholar] [CrossRef]
- Young-Wolff, K.C.; Sarovar, V.; Tucker, L.-Y.; Conway, A.; Alexeeff, S.; Weisner, C.; Armstrong, M.A.; Goler, N. Self-reported Daily, Weekly, and Monthly Cannabis Use Among Women Before and During Pregnancy. JAMA Netw. Open 2019, 2, e196471. [Google Scholar] [CrossRef]
- Young-Wolff, K.C.; Adams, S.R.; Wi, S.; Weisner, C.; Conway, A. Routes of cannabis administration among females in the year before and during pregnancy: Results from a pilot project. Addict. Behav. 2020, 100, 106125. [Google Scholar] [CrossRef]
- Young-Wolff, K.C.; Sarovar, V.; Tucker, L.-Y.; Goler, N.; Conway, A.; Weisner, C.; Armstrong, M.A.; Alexeeff, S. Validity of Self-reported Cannabis Use Among Pregnant Females in Northern California. J. Addict. Med. 2019, 14, 287–292. [Google Scholar] [CrossRef]
- Sampson, M.; Archibong, A.E.; Powell, A.; Strange, B.; Roberson, S.I.; Hills, E.R.; Bourne, P. Perturbation of the Developmental Potential of Preimplantation Mouse Embryos by Hydroxyurea. Int. J. Environ. Res. Public Health 2010, 7, 2033–2044. [Google Scholar] [CrossRef] [PubMed]
- Fish, E.W.; Murdaugh, L.B.; Zhang, C.; Boschen, K.E.; Boa-Amponsem, O.; Mendoza-Romero, H.N.; Tarpley, M.; Chdid, L.; Mukhopadhyay, S.; Cole, G.J.; et al. Cannabinoids Exacerbate Alcohol Teratogenesis by a CB1-Hedgehog Interaction. Sci. Rep. 2019, 9, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Brigante, T.A.V.; Abe, F.R.; Zuardi, A.W.; Hallak, J.E.C.; Crippa, J.A.S.; De Oliveira, D.P. Cannabidiol did not induce teratogenicity or neurotoxicity in exposed zebrafish embryos. Chem. Interact. 2018, 291, 81–86. [Google Scholar] [CrossRef]
- Volkow, N.D.; Baler, R.D.; Compton, W.M.; Weiss, S.R. Adverse Health Effects of Marijuana Use. N. Engl. J. Med. 2014, 370, 2219–2227. [Google Scholar] [CrossRef] [PubMed]
- Gorfinkel, L.R.; Stohl, M.; Hasin, D. Association of Depression With Past-Month Cannabis Use among US Adults Aged 20 to 59 Years, 2005 to 2016. JAMA Netw. Open 2020, 3, e2013802. [Google Scholar] [CrossRef]
- Bonn-Miller, M.O.; Loflin, M.J.E.; Thomas, B.F.; Marcu, J.P.; Hyke, T.; Vandrey, R. Labeling Accuracy of Cannabidiol Extracts Sold Online. JAMA 2017, 318, 1708–1709. [Google Scholar] [CrossRef]
- Rubin, R. Cannabidiol Products Are Everywhere, but Should People Be Using Them? JAMA 2019, 322, 2156. [Google Scholar] [CrossRef]
- Arepally, G.M.; Ortel, T.L. Bad weed: Synthetic cannabinoid-associated coagulopathy. Blood 2019, 133, 902–905. [Google Scholar] [CrossRef]
- Bahouth, M.N.; Kraus, P.; Dane, K.; Montana, M.P.; Tsao, W.; Tabaac, B.; Jasem, J.; Schmidlin, H.; Einstein, E.; Streiff, M.B.; et al. Synthetic cannabinoid-associated coagulopathy secondary to long-acting anticoagulant rodenticides: Observational case series and management recommendations. Medicine 2019, 98, e17015. [Google Scholar] [CrossRef]
- Okie, S. Medical Marijuana and the Supreme Court. N. Engl. J. Med. 2005, 353, 648–651. [Google Scholar] [CrossRef]
- Hoffmann, D.E.; Weber, E. Medical Marijuana and the Law. N. Engl. J. Med. 2010, 362, 1453–1457. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, O.; Caudy, M.S. Examining Racial Disparities in Drug Arrests. Justice Q. 2015, 32, 288–313. [Google Scholar] [CrossRef]
- NCSL. State Medical Marijuana Laws. 2020. Available online: https://www.ncsl.org/research/health/state-medical-marijuana-laws.aspx (accessed on 19 November 2020).
- Abrams, D.I. The therapeutic effects of Cannabis and cannabinoids: An update from the National Academies of Sciences, Engineering and Medicine report. Eur. J. Intern. Med. 2018, 49, 7–11. [Google Scholar] [CrossRef] [PubMed]
- Groce, E. The Health Effects of Cannabis and Cannabinoids: The Current State of Evidence and Recommendations for Research. J. Med. Regul. 2018, 104, 32. [Google Scholar] [CrossRef]
| Study | Year | Sample Size | Key Finding/Summary | Strengths | Limitations |
|---|---|---|---|---|---|
| Abrams et al. [30] | 2020 | 23 | Cannabis was safe and significantly improved mood compared to placebo. There was no statistically significant difference, but there was a trend towards reduction in pain interference ratings in cannabis compared to placebo. | Randomized, double-blind, placebo-controlled clinical trial specifically assessing cannabis use for pain in SCD patients. | Small sample size and short duration of treatment. |
| Curtis et al. [111] | 2020 | 49 | SCD patients who use cannabis daily reported more severe pain but had fewer ER visits/hospital admissions compared to non-daily cannabis users with similar pain and disease severity. Daily and non-daily cannabis users reported pain relief as the most common reason for using cannabis. | Cross-sectional survey that examines the link between cannabis use and SCD pain severity and uses propensity score matching to adjust for confounders. | Route and amount of cannabis used was not controlled and presents a confounding factor in this study. Authors report possible selection bias because patients were recruited in clinic visits, and patients with greater disease severity likely present to clinic more often, so they would be more likely to be recruited. |
| Curtis et al. [112] | 2020 | 75 | SCD patients who obtained medical marijuana showed a decrease in admission rates in the 6 months after obtaining medical marijuana, compared to patients who did not obtain it. Patients who obtained medical marijuana had higher baseline use of opioids and illicit cannabis, but neither group demonstrated changes in opioid use. | Retrospective study that evaluates if obtaining medical marijuana is associated with changes in opioids dispensed or in health care utilization. | The method and amount of cannabis used was not controlled. It is unknown if patients who obtained medical marijuana also used illicit marijuana, or used other illicit drugs. |
| Wilson et al. [113] | 2020 | 291 | 16.9% of young SCD patients (<25 years) and 21.8% of older SCD patients (≥25 years) used marijuana. Younger patients had lower SCD-related self-care scores and were more likely to have hospital admissions for pain compared to non-users. Older patients using marijuana had more days treating pain at home. | Retrospective study that establishes a link between marijuana use and SCD self-care behaviors and pain management. | Patients may have under-reported illicit marijuana use, as legal medical marijuana was not available during the study period. Information on baseline levels of disease severity was not reported, and presents a confounding factor in the two groups. The data is observational, so a causative relationship between age of marijuana users and self-care/pain management cannot be established. |
| Wilson et al. [114] | 2020 | 258 | 24.9% of SCD patients reported substance use. The most commonly used substance was marijuana, with 22.5% of the sample reporting marijuana use, and 15% reporting use in the past 30 days. SCD patients reporting substance use had more stress/distress, higher rates of depression, and poorer quality of life. | Observational study that investigates the relationship between psychosocial/clinical risk factors and substance use in SCD patients. | Patient’s were self-reporting illicit drug use, so may have under-reported. Motivation for substance use and the perceived health benefits of substance use were not investigated. As this is an observational study, it is not possible to establish a causative relationship between substance use and psychosocial/clinical risk factors. |
| Sinha et al. [21] | 2019 | 15 | SCD patients reported increased barriers to opioids for pain management, decreased opioid dosing, physician preoccupation with opiod dosage, and a lack of access to non-opioid therapies, such as marijuana, for pain. | The small sample size allowed for a detailed account of each participant’s point of view in this qualitative study. | This study’s format as a qualitative study with a small, mostly, female sample size makes it difficult to draw conclusions about the greater population of SCD patients. The researchers did not have access to participants’ medical records, so there was no verification of participants’ accounts of opioid experiences. |
| Curtis et al. [110] | 2018 | 50 | Medical marijuana users were more likely to have genotypes associated with more clinically severe SCD, showed a decrease in hospital admissions in the 6 months after obtaining medical marijuana compared to the 6 months prior. | Prospective study that establishes a correlation between medical marijuana use and decreased hospital admissions in SCD patients. | The increased likelihood of patients with more severe SCD to obtain medical marijuana is a confounding variable in this study. The frequency and method of marijuana ingestion were not controlled, making it difficult to establish a causative link between medical marijuana and decreased hospital admissions. Illicit use of other drugs was not evaluated. |
| Roberts et al. [20] | 2018 | 58 (survey)/57 (urine drug testing) | 42% of surveyed SCD patients reported marijuana use, with the majority of users citing medical indications for use. A majority of marijuana users also indicated reduced use of pain medications. On urine drug tests, 18% of patients tested positive for cannabinoids only, 5% tested positive for cannabinoids and cocaine/phencyclidine, and 12% tested positive for cocaine/phencyclidine only. | A survey study that examined the frequency of and reasons for marijuana use in SCD patients. | Patients were self-reporting on illicit marijuana use, so some patients may not have reported their use at all or may have overstated the medicinal benefits of marijuana use. Urine testing was used to estimate marijuana use, so infrequent testing would not account for occasional users. Also, testing was often based on clinician concerns during routine care, so there may have been a sampling bias as patients more likely to show signs of drug abuse may have been more likely to have urine drug testing performed. |
| Ballas [115] | 2017 | 72 (270 drug screen tests from 72 patients) | SCD patients using cannabis had a higher frequency of VOCs than non-users. | A retrospective study that examines the association between cannabis use and frequency of acute vaso-occlusive crises resulting in hospitalization. | There were statistically significant differences in mixed drug use, frequency of clinic visits, and age of males when comparing the patients who tested positive and negative for cannabinoids. Baseline severity of SCD was not evaluated in the groups of cannabinoid users vs. non-users. These confounders preclude the establishment of a causative link between cannabis use and frequency of VOCs. |
| Zheng et al. [116] | 2016 | 1 | Failure in relieving pain with opioids in a patient with a history of synthetic cannabinoid (K2) use. | Case study that examines the interactions between synthetic cannabinoid use, altered mental status and pain. | Single patient with a history of prior frontal stroke makes it difficult to attribute the patient’s altered mental status solely to the K2 synthetic cannabinoid. However, the toxicity of synthetic drugs raises a concern for safety. |
| Matta et al. [117] | 2014 | 1 | Male Sickle Cell Trait patient with a history of alcohol, tobacco, and cannabis use presented to the emergency department with priapism. | Another case study that examines drug interactions and potential adverse effects associated with cannabis use. | Mixed drug use makes it difficult to extrapolate causation between cannabis and priapism. |
| Birnbaum, Pinzone [118] | 2008 | 1 | Male SCD patient with a history of substance abuse with alcohol, cocaine, and cannabis attempted suicide by quetiapine ingestion, and subsequently developed priapism. | Case study that examines drug interactions and potential adverse effects associated with cannabis use. | Single patient with mixed drug use makes it difficult to extrapolate causation between cannabis and priapism. |
| Knight-Madden et al. [119] | 2006 | n = 288, n = 234 (in 2000 and 2004 respectively) | Among homozygous sickle cell (SS) patients and sickle cell hemoglobin-C disease (SC) patients, the prevalence of smoking marijuana was higher in males and in SC patients. The prevalence of marijuana smoking increased from 2000 to 2004 in both sexes, and marijuana use was not related to SCD complications. | Longitudinal questionnaire-based study that demonstrated high prevalence of marijuana use in SCD patients. | Patients were specifically asked about smoking marijuana, so other methods of use were not represented, and patients were self-reporting on illicit marijuana use, so use may be underreported. The frequency of marijuana use and the type/frequency of SCD complications were not investigated, so motivation for marijuana use remains unclear. Some participants were lost to followup by 2004, while some (n = 8) responded only in 2004; it is unclear if these patients were included in the reported results. |
| Howard et al. [18] | 2005 | 86 | 36% (n = 31) of SCD patients reported using cannabis to relieve pain, anxiety, or depression. | Questionnaire-based survey. | Only 34% of eligible patients completed the questionnaire. Respondents had variable frequency of cannabis use, and there was no quantitative testing to establish baseline pain levels or opioid use prior to cannabis use. Cannabis use may be underreported due to its status as an illicit drug. |
| Britto et al. [120] | 1998 | 321 | Teens with SCD and cystic fibrosis reported significantly less lifetime and current use of marijuana, tobacco, alcohol and fewer other risky behaviors than their healthy peers. | This crossurvey study demonstrates that cannabis use is apparent in SCD teens. | Teens were self-reporting on illicit behaviors, so their responses may not be reliable. Motivation for marijuana use and other risky behaviors were not assessed. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Argueta, D.A.; Aich, A.; Muqolli, F.; Cherukury, H.; Sagi, V.; DiPatrizio, N.V.; Gupta, K. Considerations for Cannabis Use to Treat Pain in Sickle Cell Disease. J. Clin. Med. 2020, 9, 3902. https://doi.org/10.3390/jcm9123902
Argueta DA, Aich A, Muqolli F, Cherukury H, Sagi V, DiPatrizio NV, Gupta K. Considerations for Cannabis Use to Treat Pain in Sickle Cell Disease. Journal of Clinical Medicine. 2020; 9(12):3902. https://doi.org/10.3390/jcm9123902
Chicago/Turabian StyleArgueta, Donovan A., Anupam Aich, Fjolla Muqolli, Hemanth Cherukury, Varun Sagi, Nicholas V. DiPatrizio, and Kalpna Gupta. 2020. "Considerations for Cannabis Use to Treat Pain in Sickle Cell Disease" Journal of Clinical Medicine 9, no. 12: 3902. https://doi.org/10.3390/jcm9123902
APA StyleArgueta, D. A., Aich, A., Muqolli, F., Cherukury, H., Sagi, V., DiPatrizio, N. V., & Gupta, K. (2020). Considerations for Cannabis Use to Treat Pain in Sickle Cell Disease. Journal of Clinical Medicine, 9(12), 3902. https://doi.org/10.3390/jcm9123902




