Impact of Tranexamic Acid on Chondrocytes and Osteogenically Differentiated Human Mesenchymal Stromal Cells (hMSCs) In Vitro
Abstract
1. Introduction
2. Materials and Methods
2.1. Isolation and Culture of Chondrocytes
2.2. Isolation and Culture of Bone Marrow-Derived MSCs
2.3. Osteogenic Differentiation of MSCs
2.4. Tranexamic Acid Treatment of Chondrocytes and MSCs
2.5. Biochemical Assays
2.6. Alizarin Red S Staining
2.7. RNA Isolation and Semiquantitative RT-PCR
2.8. Statistical Analysis
3. Results
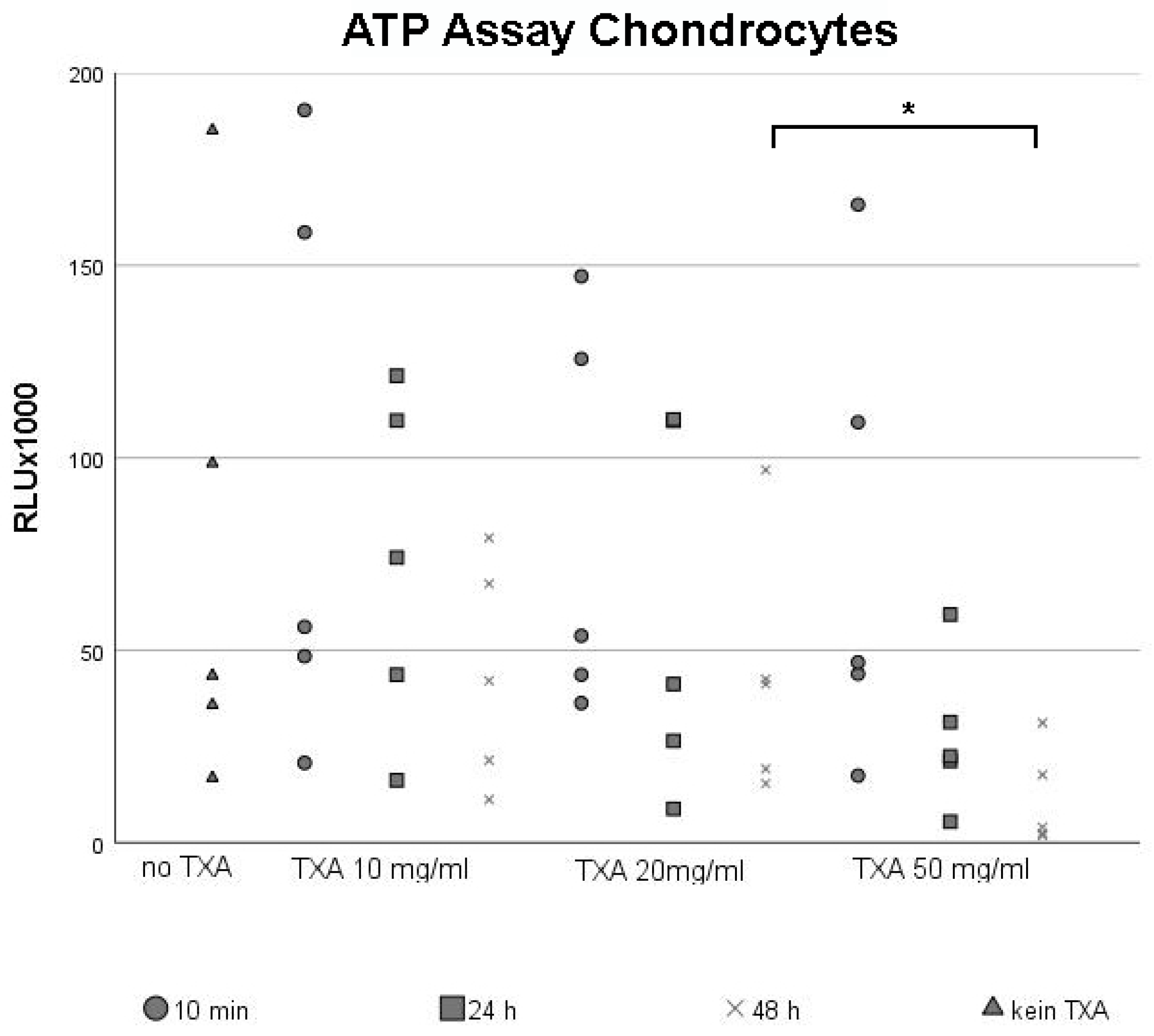
3.1. ATP Assay of Chondrocytes
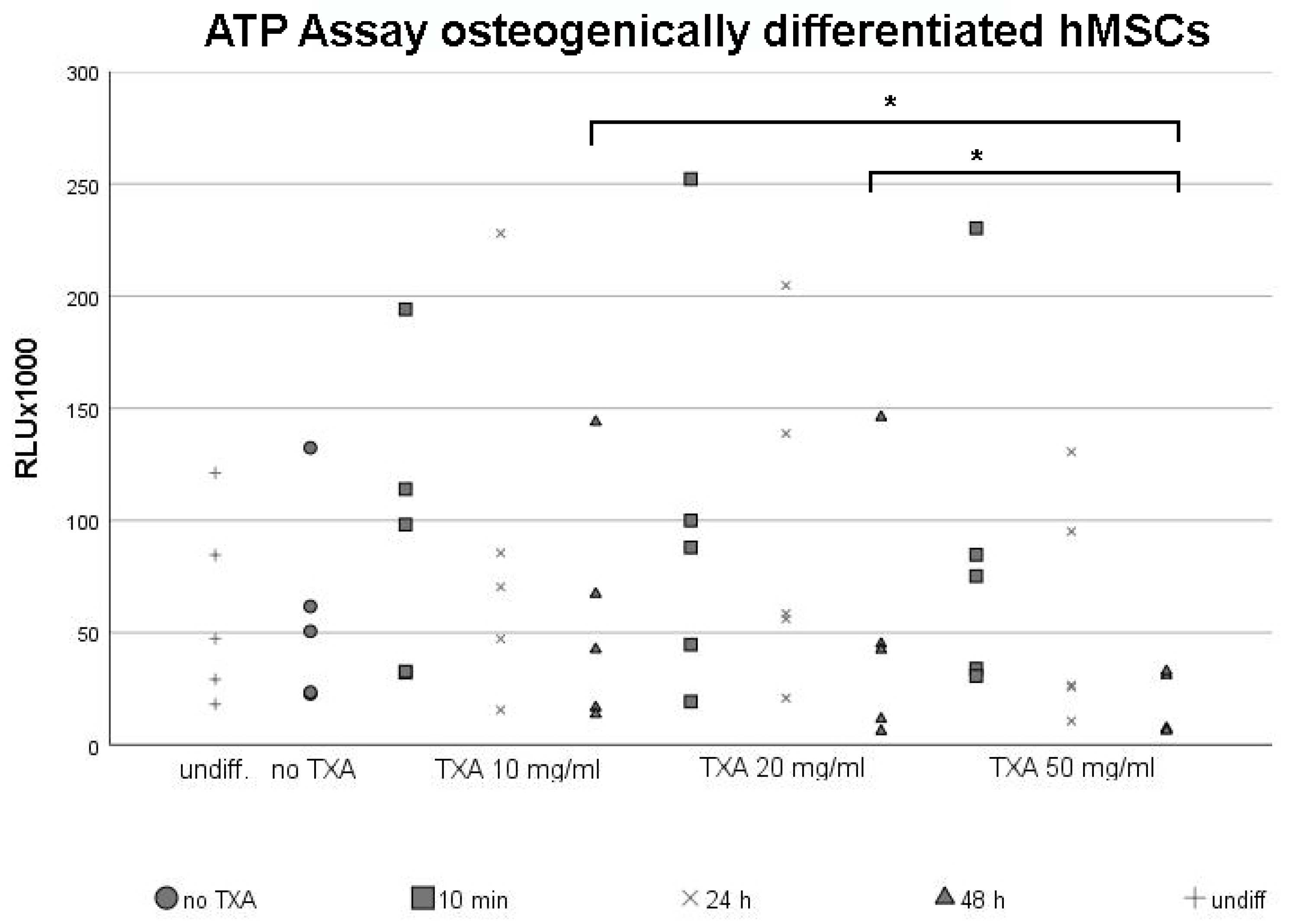
3.2. ATP Assay of Osteogenically Differentiated hMSCs
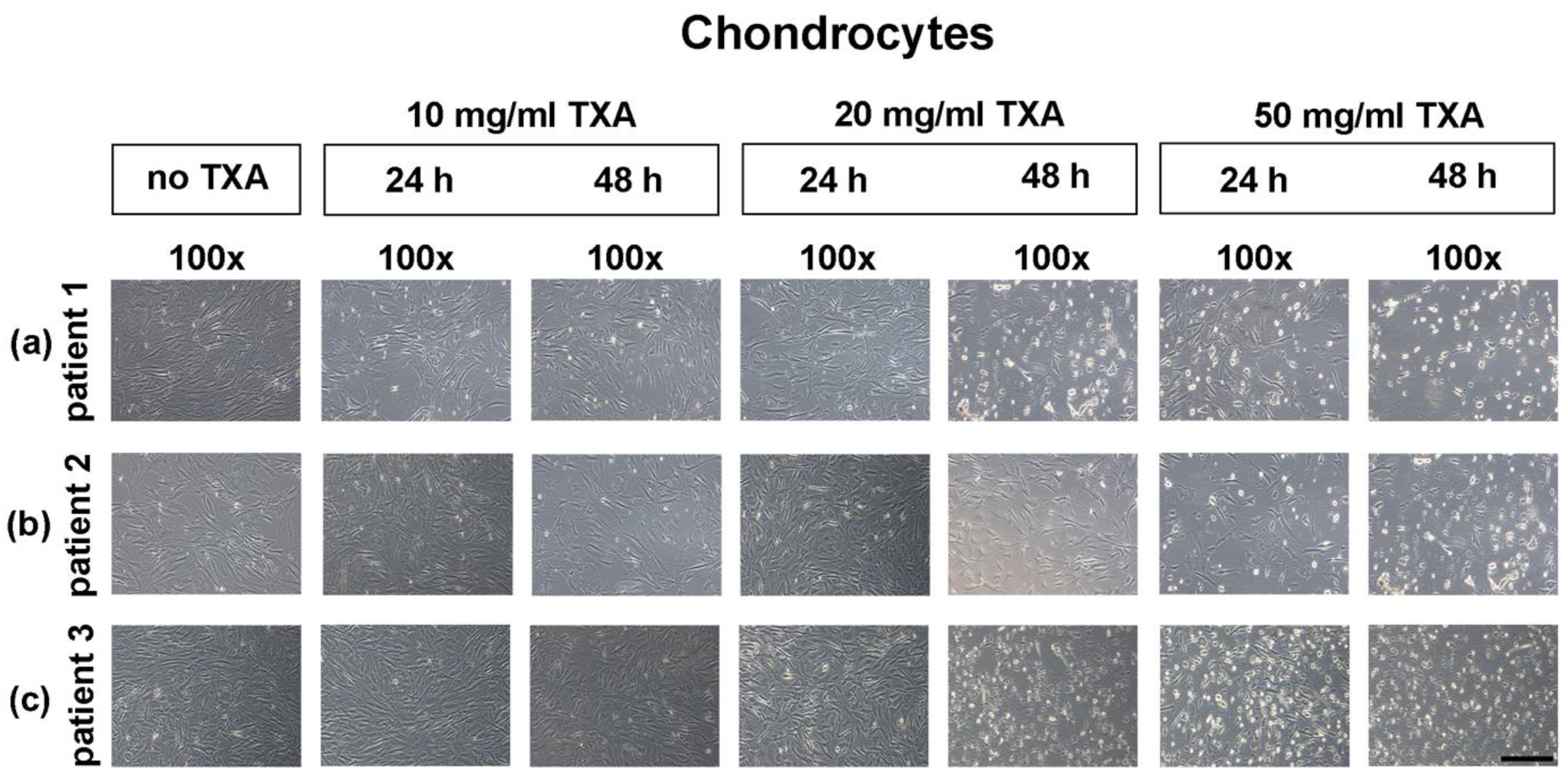
3.3. Microscopical Examination of Chondrocyte Morphology
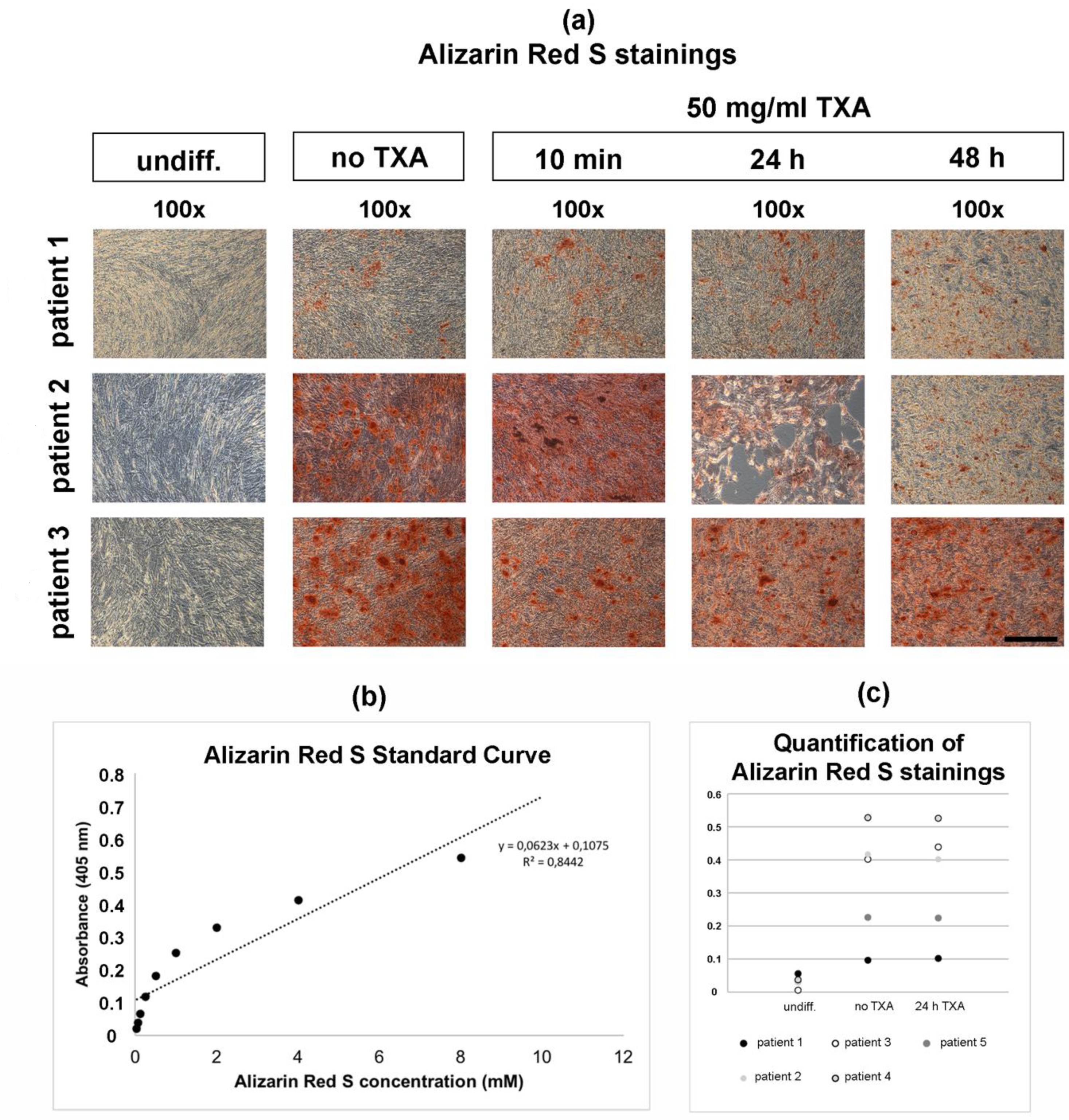
3.4. Alizarin Red S Staining of Osteogenically Differentiated hMSCs
3.5. Expression of Chondrogenic Marker Genes in Chondrocytes
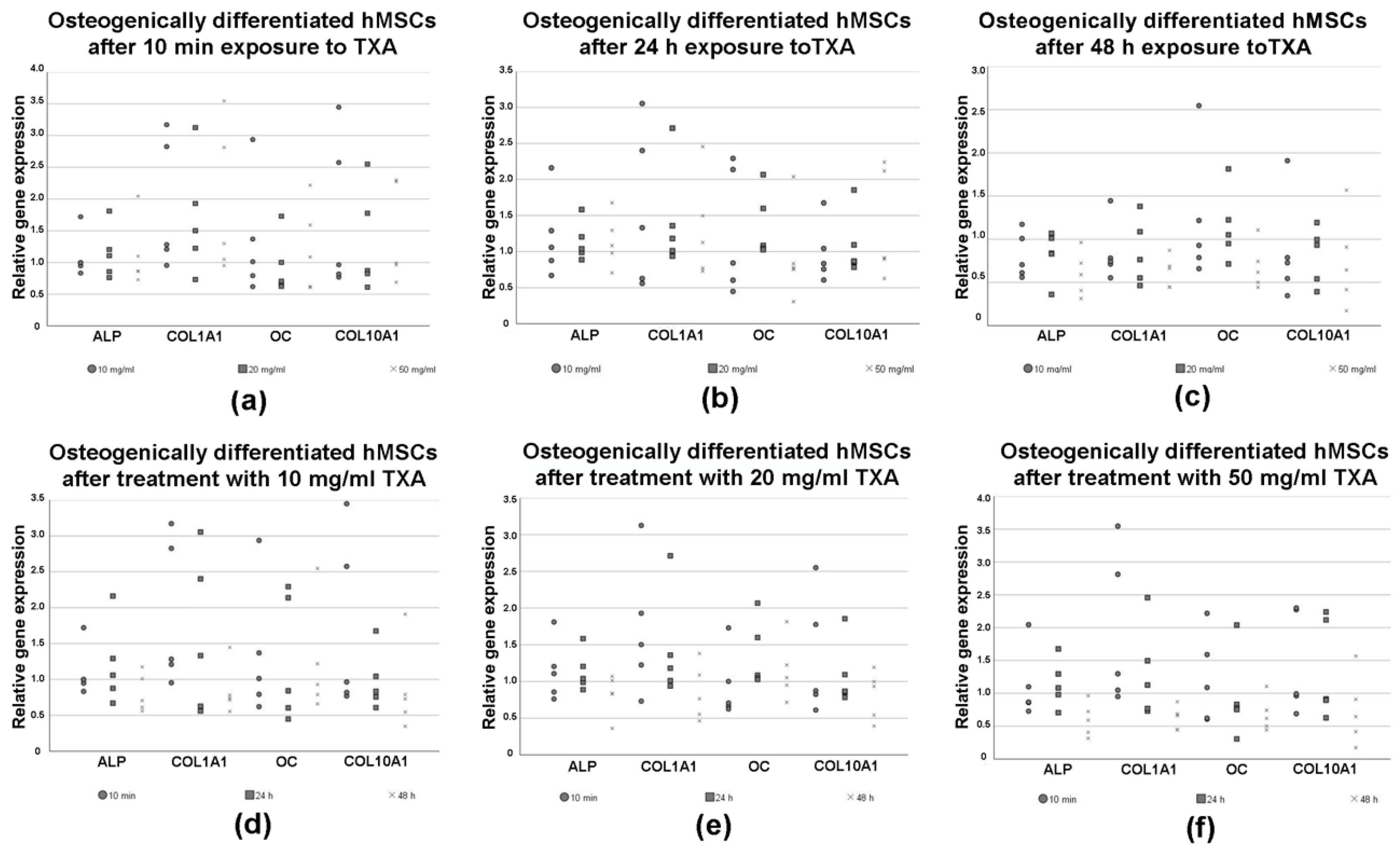
3.6. Expression of Osteogenic Marker Genes in Differentiated hMSCs
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ATP | adenosine 5’-triphosphate |
| AGG | aggrecan |
| ALP | alkaline phosphatase |
| COMP | cartilage oligomeric matrix protein |
| COL1A1 | collagen type I alpha 1 chain |
| COL2A1 | collagen type II alpha 1 |
| COL10A1 | collagen type X alpha 1 |
| d | days |
| DMEM | Dulbecco’s modified Eagle medium |
| EEF1A1 | eukaryotic elongation factor 1α |
| FBS | fetal bovine serum |
| h | hours |
| min | minutes |
| hMSCs | human mesenchymal stromal cells |
| OECD | Organization for Economic Co-operation and Development |
| OC | osteocalcin |
| PS | penicillin/streptomycin |
| PBS | phosphate-buffered saline |
| SOX9 | sex-determining region Y-box 9 |
| TXA | tranexamic acid |
References
- Fernandes, A.C. OECD Indicators. In Health at a Glance 2019; OECD Publishing: Paris, France, 2019. [Google Scholar]
- Carling, M.S.; Jeppsson, A.; Eriksson, B.I.; Brisby, H. Transfusions and blood loss in total hip and knee arthroplasty: A prospective observational study. J. Orthop Surg Res. 2015, 10, 48. [Google Scholar] [CrossRef] [PubMed]
- McLean, M.; McCall, K.; Smith, I.D.M.; Blyth, M.; Kitson, S.M.; Crowe, L.A.N.; Leach, W.J.; Rooney, B.P.; Spencer, S.J.; Mullen, M.; et al. Tranexamic acid toxicity in human periarticular tissues. Bone Joint Res. 2019, 8, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Raut, S.; Mertes, S.C.; Muniz-Terrera, G.; Khanduja, V. Factors associated with prolonged length of stay following a total knee replacement in patients aged over 75. Int. Orthop. 2012, 36, 1601–1608. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zhang, H.; Chen, J.; Chen, F.; Que, W. The effect of tranexamic acid on blood loss and use of blood products in total knee arthroplasty: A meta-analysis. Knee Surg. Sports Traumatol. Arthrosc. Off. J. ESSKA 2012, 20, 1742–1752. [Google Scholar] [CrossRef]
- Oremus, K. Tranexamic acid for the reduction of blood loss in total knee arthroplasty. Ann. Transl Med. 2015, 3, S40. [Google Scholar]
- Sassoon, A.; Nam, D.; Jackups, R.; Johnson, S.R.; Nunley, R.M.; Barrack, R.L. Tranexamic acid: Optimal blood loss management in surface replacement arthroplasty. Bone Joint J. 2016, 98, 173–178. [Google Scholar] [CrossRef]
- Jacob, B.; Kloss, N.; Böhle, S.; Kirschberg, J.; Zippelius, T.; Heinecke, M.; Matziolis, G.; Röhner, E. Tranexamic acid is toxic on human chondrocytes, In Vitro. J. Orthop. 2020, 20, 1–5. [Google Scholar] [CrossRef]
- Yuan, Z.F.; Yin, H.; Ma, W.P.; Xing, D.L. The combined effect of administration of intravenous and topical tranexamic acid on blood loss and transfusion rate in total knee arthroplasty: Combined tranexamic acid for TKA. Bone Joint Res. 2016, 5, 353–361. [Google Scholar] [CrossRef]
- Marmotti, A.; Mattia, S.; Mangiavini, L.; Bonasia, D.E.; Bruzzone, M.; Dettoni, F.; Rosso, F.; Blonna, D.; Rossi, R.; Castoldi, F.; et al. Tranexamic acid effects on cartilage and synovial tissue: An In Vitro study for a possible safe intra-articular use. J. Biol. Regul. Homeost. Agents 2016, 30, 33–40. [Google Scholar]
- Goderecci, R.; Giusti, I.; Necozione, S.; Cinque, B.; D’Ascenzo, S.; Dolo, V.; Calvisi, V. Short exposure to tranexamic acid does not affect, In Vitro, the viability of human chondrocytes. Eur. J. Med. Res. 2019, 24, 15. [Google Scholar] [CrossRef]
- Ambra, L.F.; de Girolamo, L.; Niu, W.; Phan, A.; Spector, M.; Gomoll, A.H. No effect of topical application of tranexamic acid on articular cartilage. Knee Surg. Sports Traumatol. Arthrosc. Off. J. ESSKA 2019, 27, 931–935. [Google Scholar] [CrossRef] [PubMed]
- Parker, J.D.; Lim, K.S.; Kieser, D.C.; Woodfield, T.B.F.; Hooper, G.J. Is tranexamic acid toxic to articular cartilage when administered topically? What is the safe dose? Bone Joint J. 2018, 100, 404–412. [Google Scholar] [CrossRef] [PubMed]
- Tuttle, J.R.; Feltman, P.R.; Ritterman, S.A.; Ehrlich, M.G. Effects of tranexamic acid cytotoxicity on In Vitro chondrocytes. Am. J. Orthop. 2015, 44, 497–502. [Google Scholar]
- Wagenbrenner, M.; Heinz, T.; Horas, K.; Jakuscheit, A.; Arnholdt, J.; Herrmann, M.; Rudert, M.; Holzapfel, B.M.; Steinert, A.F.; Weißenberger, M. The human arthritic hip joint is a source of mesenchymal stromal cells (MSCs) with extensive multipotent differentiation potential. BMC Musculoskelet. Disord. 2020, 21, 297. [Google Scholar] [CrossRef]
- Sakaguchi, Y.; Sekiya, I.; Yagishita, K.; Muneta, T. Comparison of human stem cells derived from various mesenchymal tissues: Superiority of synovium as a cell source. Arthritis Rheum. 2005, 52, 2521–2529. [Google Scholar] [CrossRef]
- McCutchan, A.; Dobson, G.P.; Stewart, N.; Letson, H.L.; Grant, A.L.; Jovanovic, I.A.; Hazratwala, K.; Wilkinson, M.; McEwen, P.; Morris, J. Absence of cytotoxic and inflammatory effects following In Vitro exposure of chondrogenically-differentiated human mesenchymal stem cells to adenosine, lidocaine and Mg(2+) solution. J. Exp. Orthop. 2019, 6, 16. [Google Scholar] [CrossRef]
- Horwitz, E.M.; Le Blanc, K.; Dominici, M.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.C.; Deans, R.J.; Krause, D.S.; Keating, A. Clarification of the nomenclature for MSC: The international society for cellular therapy position statement. Cytotherapy 2005, 7, 393–395. [Google Scholar] [CrossRef]
- Han, Y.; Li, X.; Zhang, Y.; Han, Y.; Chang, F.; Ding, J. Mesenchymal stem cells for regenerative medicine. Cells 2019, 8, 886. [Google Scholar] [CrossRef]
- Pajarinen, J.; Lin, T.H.; Nabeshima, A.; Jämsen, E.; Lu, L.; Nathan, K.; Yao, Z.; Goodman, S.B. Mesenchymal stem cells in the aseptic loosening of total joint replacements. J. Biomed. Mat. Res. Part. A 2017, 105, 1195–1207. [Google Scholar] [CrossRef]
- Noth, U.; Tuli, R.; Osyczka, A.M.; Danielson, K.G.; Tuan, R.S. In Vitro engineered cartilage constructs produced by press-coating biodegradable polymer with human mesenchymal stem cells. Tissue Eng. 2002, 8, 131–144. [Google Scholar] [CrossRef]
- Steinert, A.F.; Proffen, B.; Kunz, M.; Hendrich, C.; Ghivizzani, S.C.; Nöth, U.; Rethwilm, A.; Eulert, J.; Evans, C.H. Hypertrophy is induced during the In Vitro chondrogenic differentiation of human mesenchymal stem cells by bone morphogenetic protein-2 and bone morphogenetic protein-4 gene transfer. Arthr. Res. Ther. 2009, 11, 148. [Google Scholar] [CrossRef] [PubMed]
- Reichert, J.C.; Schmalzl, J.; Prager, P.; Gilbert, F.; Quent, V.M.; Steinert, A.F.; Rudert, M.; Nöth, U. Synergistic effect of Indian hedgehog and bone morphogenetic protein-2 gene transfer to increase the osteogenic potential of human mesenchymal stem cells. Stem Cell Res. Ther. 2013, 4, 105. [Google Scholar] [CrossRef] [PubMed]
- Steinert, A.F.; Kunz, M.; Prager, P.; Barthel, T.; Jakob, F.; Nöth, U.; Murray, M.M.; Evans, C.H.; Porter, R.M. Mesenchymal stem cell characteristics of human anterior cruciate ligament outgrowth cells. Tissue Eng. Part. A 2011, 17, 1375–1388. [Google Scholar] [CrossRef] [PubMed]
- Steinert, A.F.; Weissenberger, M.; Kunz, M.; Gilbert, F.; Ghivizzani, S.C.; Göbel, S.; Jakob, F.; Nöth, U.; Rudert, M. Indian hedgehog gene transfer is a chondrogenic inducer of human mesenchymal stem cells. Arthr. Res. Ther. 2012, 14, 168. [Google Scholar] [CrossRef]
- Drosos, G.I.; Ververidis, A.; Valkanis, C.; Tripsianis, G.; Stavroulakis, E.; Vogiatzaki, T.; Kazakos, K. A randomized comparative study of topical versus intravenous tranexamic acid administration in enhanced recovery after surgery (ERAS) total knee replacement. J. Orthop. 2016, 13, 127–131. [Google Scholar] [CrossRef][Green Version]
- Aggarwal, A.K.; Singh, N.; Sudesh, P. Topical vs intravenous tranexamic acid in reducing blood loss after bilateral total knee arthroplasty: A prospective study. J. Arthroplast. 2016, 31, 1442–1448. [Google Scholar] [CrossRef]
- Barrett, C.D.; Moore, H.B.; Kong, Y.W.; Chapman, M.P.; Sriram, G.; Lim, D.; Moore, E.E.; Yaffe, M.B. Tranexamic acid mediates proinflammatory and anti-inflammatory signaling via complement C5a regulation in a plasminogen activator-dependent manner. J. Trauma Acute Care Surg. 2019, 86, 101–107. [Google Scholar] [CrossRef]
- Wu, K.T.; Siu, K.K.; Ko, J.Y.; Chou, W.Y.; Kuo, S.J.; Hsu, Y.H. Tranexamic acid reduces total blood loss and inflammatory response in computer-assisted navigation total knee arthroplasty. Biomed. Res. Int. 2019, 2019, 5207517. [Google Scholar] [CrossRef]
- Tzatzairis, T.K.; Drosos, G.I.; Kotsios, S.E.; Ververidis, A.N.; Vogiatzaki, T.D.; Kazakos, K.I. Intravenous vs topical tranexamic acid in total knee arthroplasty without tourniquet application: A randomized controlled study. J. Arthroplast. 2016, 31, 2465–2470. [Google Scholar] [CrossRef]
- Alshryda, S.; Sukeik, M.; Sarda, P.; Blenkinsopp, J.; Haddad, F.S.; Mason, J.M. A systematic review and meta-analysis of the topical administration of tranexamic acid in total hip and knee replacement. Bone Joint J. 2014, 96, 1005–1015. [Google Scholar] [CrossRef]
- Kim, T.K.; Chang, C.B.; Koh, I.J. Practical issues for the use of tranexamic acid in total knee arthroplasty: A systematic review. Knee Surg. Sports Traumatol. Arthrosc. Off. J. ESSKA 2014, 22, 1849–1858. [Google Scholar] [CrossRef] [PubMed]
- Astedt, B. Clinical pharmacology of tranexamic acid. Scand. J. Gastroenterol. Suppl. 1987, 137, 22–25. [Google Scholar] [CrossRef] [PubMed]
- Chan, G.K.; Kleinheinz, T.L.; Peterson, D.; Moffat, J.G. A simple high-content cell cycle assay reveals frequent discrepancies between cell number and ATP and MTS proliferation assays. PLoS ONE 2013, 8, 63583. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.G.; Saris, D.B.; Geuze, R.E.; van Rijen, M.H.P.; van der Helm, Y.J.M.; Verbout, A.J.; Creemers, L.B.; Dhert, W.J.A. Altered In Vitro chondrogenic properties of chondrocytes harvested from unaffected cartilage in osteoarthritic joints. Osteoarthr. Cartil. 2006, 14, 561–570. [Google Scholar] [CrossRef] [PubMed]

| Gene | Primer Sequences (5’–3’) | Annealing Temperature (°C) | Product Size (Base Pairs) | Cycles | MgCl2 |
|---|---|---|---|---|---|
| housekeeping gene for internal control | |||||
| EEF1A1 | Sense: AGGTGATTATCCTGAACCATCC Antisense: AAAGGTGGATAGTCTGAGAAGC | 54.0 | 234 | 21 | 1 x |
| osteogenic marker genes | |||||
| COL1A1 | Sense: GGACACAATGGATTGCAAGG Antisense: TAACCACTGCTCCACTCTGG | 55.0 | 461 | 22 | 2x |
| COL10A1 | Sense: CCCTTTTTGCTGCTAGTATCC Antisense: CTGTTGTCCAGGTTTTCCTGGCAC | 54.0 | 468 | 40 | 1x |
| ALP | Sense: TGGAGCTTCAGAAGCTCAACACCA Antisense: ATCTCGTTGTCTGAGTACCAGTCC | 51.0 | 454 | 33 | 1x |
| OC | Sense: ATGAGAGCCCTCACACTCCTC Antisense: GCCGTAGAAGCGCCGATAGGC | 62.0 | 293 | 35 | 2x |
| chondrogenic marker genes | |||||
| ACAN | Sense: GCCTTGAGCAGTTCACCTTC Antisense: CTCTTCTACGGGGACAGCAG | 54.0 | 400 | 35 | 1x |
| COL2A1 | Sense: TTTCCCAGGTCAAGATGGTC Antisense: CTTCAGCACCTGTCCACCA | 51.0 | 155 | 31 | 1x |
| SOX9 | Sense: ATCTGAAGAAGGAGAGCGAG Antisense: TCAGAAGTCTCCAGAGCTTG | 60.0 | 263 | 31 | 1x |
| COMP | Sense: CAGGACGACTTTGATGCAGA Antisense: AAGCTGGAGCTGTCTGGTA | 54.0 | 312 | 32 | 1x |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wagenbrenner, M.; Heinz, T.; Horas, K.; Jakuscheit, A.; Arnholdt, J.; Mayer-Wagner, S.; Rudert, M.; Holzapfel, B.M.; Weißenberger, M. Impact of Tranexamic Acid on Chondrocytes and Osteogenically Differentiated Human Mesenchymal Stromal Cells (hMSCs) In Vitro. J. Clin. Med. 2020, 9, 3880. https://doi.org/10.3390/jcm9123880
Wagenbrenner M, Heinz T, Horas K, Jakuscheit A, Arnholdt J, Mayer-Wagner S, Rudert M, Holzapfel BM, Weißenberger M. Impact of Tranexamic Acid on Chondrocytes and Osteogenically Differentiated Human Mesenchymal Stromal Cells (hMSCs) In Vitro. Journal of Clinical Medicine. 2020; 9(12):3880. https://doi.org/10.3390/jcm9123880
Chicago/Turabian StyleWagenbrenner, Mike, Tizian Heinz, Konstantin Horas, Axel Jakuscheit, Joerg Arnholdt, Susanne Mayer-Wagner, Maximilian Rudert, Boris M. Holzapfel, and Manuel Weißenberger. 2020. "Impact of Tranexamic Acid on Chondrocytes and Osteogenically Differentiated Human Mesenchymal Stromal Cells (hMSCs) In Vitro" Journal of Clinical Medicine 9, no. 12: 3880. https://doi.org/10.3390/jcm9123880
APA StyleWagenbrenner, M., Heinz, T., Horas, K., Jakuscheit, A., Arnholdt, J., Mayer-Wagner, S., Rudert, M., Holzapfel, B. M., & Weißenberger, M. (2020). Impact of Tranexamic Acid on Chondrocytes and Osteogenically Differentiated Human Mesenchymal Stromal Cells (hMSCs) In Vitro. Journal of Clinical Medicine, 9(12), 3880. https://doi.org/10.3390/jcm9123880








