Autism Spectrum Disorder and Disruptive Behavior Disorders Comorbidities Delineate Clinical Phenotypes in Attention-Deficit Hyperactivity Disorder: Novel Insights from the Assessment of Psychopathological and Neuropsychological Profiles
Abstract
1. Introduction
2. Experimental Section
2.1. Participants and Diagnostic Procedures
2.2. Inclusion and Exclusion Criteria
2.3. Clinical Groups
2.4. Measures
2.5. Statistical Analysis
3. Results
3.1. Clinical and Demographic Characteristics
3.2. Child Behavior Checklist
3.3. Behavior Rating Inventory of Executive Function
3.4. Principal Component Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Biederman, J.; Faraone, S.V.; Spencer, T.; Wilens, T.; Norman, D.; Lapey, K.A.; Mick, E.; Lehman, B.K.; Doyle, A. Patterns of psychiatric comorbidity, cognition, and psychosocial functioning in adults with attention deficit hyperactivity disorder. Am. J. Psychiatry 1993, 150, 1792–1798. [Google Scholar] [CrossRef] [PubMed]
- Klein, R.G.; Mannuzza, S.; Ramos Olazagasti, M.A.; Roizen, E.; Hutchison, J.A.; Lashua, E.C.; Castellanos, F.X. Clinical and functional outcome of childhood attention-deficit/ hyperactivity disorder 33 years later. Arch. Gen. Psychiatry 2012, 69, 1295–1303. [Google Scholar] [CrossRef] [PubMed]
- Mrug, S.; Brooke, B.S.; Hoza, B.; Gerdes, A.C.; Hinshaw, S.P.; Hechtman, L.; Arnold, L.E. Peer rejection and friendships in children with attention-deficit/ hyperactivity disorder: Contributions to long-term outcomes. J. Abnorm. Child Psychol. 2012, 40, 1013–1026. [Google Scholar] [CrossRef] [PubMed]
- Barkley, R.; Fischer, M.; Edelbrock, C.S.; Smallish, L. The adolescent outcome of hyperactive children diagnosed by research criteria: I. An 8-year prospective follow-up study. J. Am. Acad. Child Adolesc. Psychiatry 1990, 29, 546–557. [Google Scholar] [CrossRef] [PubMed]
- Masi, G.; Mucci, M.; Pfanner, C.; Berloffa, S.; Magazù, A.; Perugi, G. Developmental pathways for different subtypes of early-onset bipolarity in youths. J. Clin. Psychiatry 2012, 73, 1335–1341. [Google Scholar] [CrossRef] [PubMed]
- Green, J.L.; Sciberras, E.; Anderson, V.; Efron, D.; Rinehart, N. Association between autism symptoms and functioning in children with ADHD. Arch. Dis. Child. 2016, 101, 922–928. [Google Scholar] [CrossRef] [PubMed]
- Jensen, C.M.; Steinhausen, H.C. Comorbid mental disorders in children and adolescents with attention-deficit/hyperactivity disorder in a large nationwide study. ADHD Atten. Deficit Hyperact. Disord. 2015, 7, 27–38. [Google Scholar] [CrossRef]
- Gnanavel, S.; Sharma, P.; Kaushal, P.; Hussain, S. Attention deficit hyperactivity disorder and comorbidity: A review of literature. World J. Clin. Cases 2019, 7, 2420–2426. [Google Scholar] [CrossRef]
- Scandurra, V.; Emberti Gialloreti, L.; Barbanera, F.; Scordo, M.R.; Pierini, A.; Canitano, R. Neurodevelopmental Disorders and Adaptive Functions: A Study of Children with Autism Spectrum Disorders (ASD) and/or Attention Deficit and Hyperactivity Disorder (ADHD). Front. Psychiatry 2019, 10. [Google Scholar] [CrossRef]
- Cooper, M.; Martin, J.; Langley, K.; Hamshere, M.; Thapar, A. Autistic traits in children with ADHD index clinical and cognitive problems. Eur. Child Adolesc. Psychiatry 2014, 23, 23–34. [Google Scholar] [CrossRef]
- Maskey, M.; Warnell, F.; Parr, J.R.; Le Couteur, A.; McConachie, H. Emotional and behavioural problems in children with autism spectrum disorder. J. Autism Dev. Disord. 2013, 43, 851–859. [Google Scholar] [CrossRef]
- Yamawaki, K.; Ishitsuka, K.; Suyama, S.; Suzumura, S.; Yamashita, H.; Kanba, S. Clinical characteristics of boys with comorbid autism spectrum disorder and attention deficit/hyperactivity disorder. Pediatr. Int. 2020, 62, 151–157. [Google Scholar] [CrossRef]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th ed.; CBS Publishers: New Delhi, India, 2013. [Google Scholar]
- Stevens, M.C.; Gaynor, A.; Bessette, K.L.; Pearlson, G.D. A preliminary study of the effects of working memory training on brain function. Brain Imaging Behav. 2016, 10, 387–407. [Google Scholar] [CrossRef] [PubMed]
- Sprenger, L.; Bühler, E.; Poustka, L.; Bach, C.; Heinzel-Gutenbrunner, M.; Kamp-Becker, I.; Bachmann, C. Impact of ADHD symptoms on autism spectrum disorder symptom severity. Res. Dev. Disabil. 2013, 34, 3545–3552. [Google Scholar] [CrossRef] [PubMed]
- Atherton, O.E.; Lawson, K.M.; Ferrer, E.; Robins, R.W. The role of effortful control in the development of ADHD, ODD, and CD symptoms. J. Pers. Soc. Psychol. 2019. [Google Scholar] [CrossRef] [PubMed]
- Erskine, H.E.; Norman, R.E.; Ferrari, A.J.; Chan, G.C.K.; Copeland, W.E.; Whiteford, H.A.; Scott, J.G. Long-Term Outcomes of Attention-Deficit/Hyperactivity Disorder and Conduct Disorder: A Systematic Review and Meta-Analysis. J. Am. Acad. Child Adolesc. Psychiatry 2016, 55, 841–850. [Google Scholar] [CrossRef] [PubMed]
- Moffitt, T.E.; Silva, P.A. Self-Reported Delinquency, neuropsychological deficit, and history of attention deficit disorder. J. Abnorm. Child Psychol. 1988, 16, 553–569. [Google Scholar] [CrossRef]
- Barkley, R.; Fischer, M.; Smallish, L.; Fletcher, K. Young adult follow-up of hyperactive children: Antisocial activities and drug use. J. Child Psychol. Psychiatry Allied Discip. 2004, 45, 195–211. [Google Scholar] [CrossRef]
- Sibley, M.H.; Waxmonsky, J.G.; Robb, J.A.; Pelham, W.E. Implications of Changes for the Field: ADHD. J. Learn. Disabil. 2013, 46, 34–42. [Google Scholar] [CrossRef]
- Ter-Stepanian, M.; Grizenko, N.; Cornish, K.; Talwar, V.; Mbekou, V.; Schmitz, N.; Joober, R. Attention and Executive Function in Children Diagnosed with Attention Deficit Hyperactivity Disorder and Comorbid Disorders. J. Can. Acad. Child Adolesc. Psychiatry 2017, 26, 21–30. [Google Scholar]
- Craig, F.; Margari, F.; Legrottaglie, A.R.; Palumbi, R.; de Giambattista, C.; Margari, L. A review of executive function deficits in autism spectrum disorder and attention-deficit/hyperactivity disorder. Neuropsychiatr. Dis. Treat. 2016, 12, 1191–1202. [Google Scholar] [PubMed]
- Berenguer, C.; Roselló, B.; Colomer, C.; Baixauli, I.; Miranda, A. Children with autism and attention deficit hyperactivity disorder. Relationships between symptoms and executive function, theory of mind, and behavioral problems. Res. Dev. Disabil. 2018, 83, 260–269. [Google Scholar] [CrossRef] [PubMed]
- Antonini, T.N.; Becker, S.P.; Tamm, L.; Epstein, J.N. Hot and Cool Executive Functions in Children with Attention-Deficit/Hyperactivity Disorder and Comorbid Oppositional Defiant Disorder. J. Int. Neuropsychol. Soc. 2015, 21, 584–595. [Google Scholar] [CrossRef]
- Glenn, A.L.; Remmel, R.J.; Ong, M.Y.; Lim, N.S.J.; Ang, R.P.; Threadgill, A.H.; Ryerson, N.; Raine, A.; Fung, D.; Ooi, Y.P. Neurocognitive characteristics of youth with noncomorbid and comorbid forms of conduct disorder and attention deficit hyperactivity disorder. Compr. Psychiatry 2017, 77, 60–70. [Google Scholar] [CrossRef] [PubMed]
- Schachar, R.; Mota, V.L.; Logan, G.D.; Tannock, R.; Klim, P. Confirmation of an inhibitory control deficit in attention- deficit/hyperactivity disorder. J. Abnorm. Child Psychol. 2000, 28, 227–235. [Google Scholar] [CrossRef]
- Shuai, L.; Wang, Y. Executive function characteristic in boys with attention deficit hyperactivity disorder comorbid learning disabilities. Beijing Da Xue Xue Bao 2007, 39, 526–530. [Google Scholar] [PubMed]
- Van der Meere, J.; Marzocchi, G.M.; De Meo, T. Response inhibition and attention deficit hyperactivity disorder with and without oppositional defiant disorder screened from a community sample. Dev. Neuropsychol. 2005, 28, 459–472. [Google Scholar] [CrossRef]
- Clark, C.; Prior, M.; Kinsella, G.J. Do executive function deficits differentiate between adolescents with ADHD and Oppositional Defiant/Conduct Disorder? A neuropsychological study using the Six Elements Test and Hayling Sentence Completion Test. J. Abnorm. Child Psychol. 2000, 28, 403–414. [Google Scholar] [CrossRef]
- Qian, Y.; Shuai, L.; Cao, Q.; Chan, R.C.K.; Wang, Y. Do executive function deficits differentiate between children with Attention Deficit Hyperactivity Disorder (ADHD) and ADHD comorbid with oppositional defiant disorder? A cross-cultural study using performance-based tests and the behavior rating inventory. Clin. Neuropsychol. 2010, 24, 793–810. [Google Scholar] [CrossRef]
- Gioia, G.A.; Isquith, P.K.; Guy, S.C.; Kenworthy, L.; Baron, I.S. Behavior rating inventory of executive function. Child Neuropsychol. 2000, 6, 235–238. [Google Scholar] [CrossRef]
- Hobson, C.W.; Scott, S.; Rubia, K. Investigation of cool and hot executive function in ODD/CD independently of ADHD. J. Child Psychol. Psychiatry Allied Discip. 2011, 52, 1035–1043. [Google Scholar] [CrossRef] [PubMed]
- Zelazo, P.D.; Carlson, S.M. Hot and Cool Executive Function in Childhood and Adolescence: Development and Plasticity. Child Dev. Perspect. 2012. [Google Scholar] [CrossRef]
- Carter Leno, V.; Chandler, S.; White, P.; Pickles, A.; Baird, G.; Hobson, C.; Smith, A.B.; Charman, T.; Rubia, K.; Simonoff, E. Testing the specificity of executive functioning impairments in adolescents with ADHD, ODD/CD and ASD. Eur. Child Adolesc. Psychiatry 2018, 27, 899–908. [Google Scholar] [CrossRef] [PubMed]
- Tye, C.; Bedford, R.; Asherson, P.; Ashwood, K.L.; Azadi, B.; Bolton, P.; McLoughlin, G. Callous-unemotional traits moderate executive function in children with ASD and ADHD: A pilot event-related potential study. Dev. Cogn. Neurosci. 2017, 26, 84–90. [Google Scholar] [CrossRef] [PubMed]
- Hudziak, J.J.; Achenbach, T.M.; Althoff, R.R.; Pine, D.S. A dimensional approach to developmental psychopathology. Int. J. Methods Psychiatr. Res. Int. J. Methods Psychiatr. Res 2007, 16, 16–23. [Google Scholar] [CrossRef] [PubMed]
- Achenbach, T.M.; Ivanova, M.Y.; Rescorla, L.A.; Turner, L.V.; Althoff, R.R. Internalizing/Externalizing Problems: Review and Recommendations for Clinical and Research Applications. J. Am. Acad. Child Adolesc. Psychiatry 2016, 55, 647–656. [Google Scholar] [CrossRef]
- Van Dam, N.T.; O’Connor, D.; Marcelle, E.T.; Ho, E.J.; Cameron Craddock, R.; Tobe, R.H.; Gabbay, V.; Hudziak, J.J.; Xavier Castellanos, F.; Leventhal, B.L.; et al. Data-Driven Phenotypic Categorization for Neurobiological Analyses: Beyond DSM-5 Labels. Biol. Psychiatry 2017, 81, 484–494. [Google Scholar] [CrossRef]
- Kaufman, J.; Birmaher, B.; Brent, D.; Rao, U.; Flynn, C.; Moreci, P.; Williamson, D.; Ryan, N. Schedule for affective disorders and schizophrenia for school-age children-present and lifetime version (K-SADS-PL): Initial reliability and validity data. J. Am. Acad. Child Adolesc. Psychiatry 1997, 36, 980–988. [Google Scholar] [CrossRef]
- Wechsler, D. The Wechsler Intelligence Scale for Children—Fourth Edition; Springer: Berlin, Germany, 2004; ISBN 9780470135389. [Google Scholar]
- Lord, C.; Rutter, M.; Di Lavore, P.; Risi, S.; Gotham, K.; Bishop, S. Autism Diagnostic Observation Schedule—Second Edition (ADOS-2). Available online: https://www.wpspublish.com/ados-2-autism-diagnostic-observation-schedule-second-edition (accessed on 22 February 2020).
- Rutter, M.; LeCouteur, A.; Lord, C. Autism Diagnostic Interview-Revised (ADI-R). Available online: https://www.wpspublish.com/adi-r-autism-diagnostic-interview-revised (accessed on 22 February 2020).
- Achenbach, T.; Rescorla, L. Manual for the ASEBA School-Age Forms and Profiles: An Integrated System of Multi-Informant Assessment; University of Vermont, Research Center for Children, Youth, & Families: Burlington, VT, USA, 2001. [Google Scholar]
- Gioia, G.A.; Isquith, P.K.; Retzlaff, P.D.; Espy, K.A. Confirmatory factor analysis of the Behavior Rating Inventory of Executive Function (BRIEF) in a clinical sample. Child Neuropsychol. 2002, 8, 249–257. [Google Scholar] [CrossRef]
- Willcutt, E.G.; Doyle, A.E.; Nigg, J.T.; Faraone, S.V.; Pennington, B.F. Validity of the Executive Function Theory of Attention-Deficit/Hyperactivity Disorder: A Meta-Analytic Review. Biol. Psychiatry 2005, 57, 1336–1346. [Google Scholar] [CrossRef]
- Stringaris, A.; Goodman, R. Longitudinal outcome of youth oppositionality: Irritable, headstrong, and hurtful behaviors have distinctive predictions. J. Am. Acad. Child Adolesc. Psychiatry 2009, 48, 404–412. [Google Scholar] [CrossRef] [PubMed]
- Thapar, A.; Harrington, R.; McGuffin, P. Examining the comorbidity of ADHD-related behaviours and conduct problems using a twin study design. Br. J. Psychiatry 2001, 179, 224–229. [Google Scholar] [CrossRef] [PubMed]
- Drechsler, R.; Zulauf Logoz, M.; Walitza, S.; Steinhausen, H.C. The Relations Between Temperament, Character, and Executive Functions in Children With ADHD and Clinical Controls. J. Atten. Disord. 2018, 22, 764–775. [Google Scholar] [CrossRef] [PubMed]
- Craig, F.; Lamanna, A.L.; Margari, F.; Matera, E.; Simone, M.; Margari, L. Overlap Between Autism Spectrum Disorders and Attention Deficit Hyperactivity Disorder: Searching for Distinctive/Common Clinical Features. Autism Res. 2015, 8, 328–337. [Google Scholar] [CrossRef] [PubMed]
- Mansour, R.; Dovi, A.T.; Lane, D.M.; Loveland, K.A.; Pearson, D.A. ADHD severity as it relates to comorbid psychiatric symptomatology in children with Autism Spectrum Disorders (ASD). Res. Dev. Disabil. 2017, 60, 52–64. [Google Scholar] [CrossRef]
- Insel, T.R. The nimh research domain criteria (RDOC) project: Precision medicine for psychiatry. Am. J. Psychiatry 2014, 171, 395–397. [Google Scholar] [CrossRef]
- Cuthbert, B.N. The RDoC framework: Facilitating transition from ICD/DSM to dimensional approaches that integrate neuroscience and psychopathology. World Psychiatry 2014, 13, 28–35. [Google Scholar] [CrossRef]
- Di Martino, A.; Zuo, X.N.; Kelly, C.; Grzadzinski, R.; Mennes, M.; Schvarcz, A.; Rodman, J.; Lord, C.; Castellanos, F.X.; Milham, M.P. Shared and distinct intrinsic functional network centrality in autism and attention-deficit/hyperactivity disorder. Biol. Psychiatry 2013, 74, 623–632. [Google Scholar] [CrossRef]
- Kernbach, J.M.; Satterthwaite, T.D.; Bassett, D.S.; Smallwood, J.; Margulies, D.; Krall, S.; Shaw, P.; Varoquaux, G.; Thirion, B.; Konrad, K.; et al. Shared endo-phenotypes of default mode dsfunction in attention deficit/hyperactivity disorder and autism spectrum disorder. Transl. Psychiatry 2018, 8. [Google Scholar] [CrossRef]
- Beauchaine, T.P.; Hinshaw, S.P. RDoC and Psychopathology among Youth: Misplaced Assumptions and an Agenda for Future Research. J. Clin. Child Adolesc. Psychol. 2020, 49, 322–340. [Google Scholar] [CrossRef]
- Garvey, M.; Avenevoli, S.; Anderson, K. The National Institute of Mental Health Research Domain Criteria and Clinical Research in Child and Adolescent Psychiatry. J. Am. Acad. Child Adolesc. Psychiatry 2016, 55, 93–98. [Google Scholar] [CrossRef] [PubMed]
| ADHD | ADHD+ASD | ADHD+ODD/CD | ADHD+ASD+ODD/CD | p-Values | |
|---|---|---|---|---|---|
| Subjects a | 64 (42.38) | 19 (12.58) | 43 (28.48) | 25 (16.56) | |
| Age b | 10.02 ± 2.49 | 9.58 ± 2.69 | 9.37 ± 2.95 | 8.40 ± 2.24 | 0.0760 |
| Adolescentsa | 19 (29.69) | 6 (31.58) | 10 (23.26) | 4 (16) | 0.5238 |
| Gender a | 8 (12.5) | 1 (5.26) | 4 (9.3) | 1 (4) | 0.6998 |
| WISC-IV | |||||
| VCIb | 104.89 ± 17.41 | 104.76 ± 17 | 104.5 ± 14.61 | 107.32 ± 19.24 | 0.9440 |
| PRIb | 99.11 ± 12.76 | 104.76 ± 17.07 | 103.82 ± 18.17 | 107.77 ± 16.19 | 0.2860 |
| WMIb | 87.07 ± 13.38 | 88.07 ± 7.32 | 88.95 ± 11.53 | 90.33 ± 17.45 | 0.8710 |
| PSIb | 83.56 ± 15.28 | 83.73 ± 13.97 | 89.47 ± 18.46 | 83.1 ± 14.89 | 0.5470 |
| FSIQ or GAI b | 93 ± 14.98 | 92.69 ± 17 | 96.86 ± 16.05 | 98.94 ± 18.06 | 0.5440 |
| Comorbidities | |||||
| Mood Disa | 8 (12.50) * | 7 (36.84) | 14 (32.56) | 13 (52.00) * | 0.0011 * |
| Anxiety Disa | 7 (10.94) * | 8 (42.11) * | 7 (16.28) | 8 (32.00) | 0.0087 * |
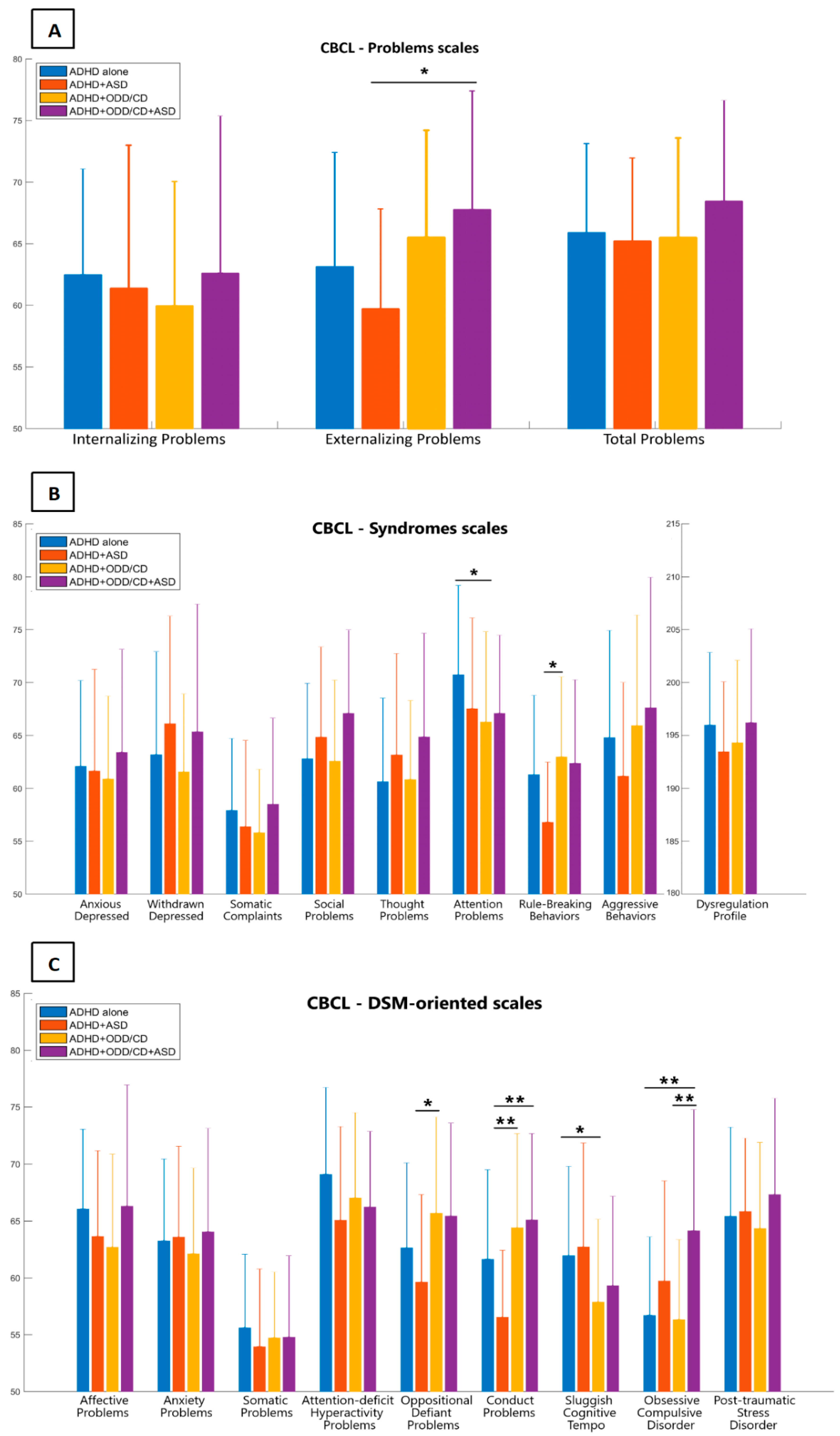
| CBCL – 6/18 | ADHD | ADHD+ASD | ADHD+ODD/CD | ADHD+ASD+ODD/CD | F Value | p-Values |
|---|---|---|---|---|---|---|
| Internalizing P | 62.47 ± 8.59 | 61.37 ± 11.62 | 59.95 ± 10.09 | 62.61 ± 12.75 | 0.612 | 0.608 |
| Externalizing P | 63.13 ± 9.28 | 59.72 ± 8.10 | 65.51 ± 8.69 | 67.77 ± 9.63 | 3.222 | 0.025 * |
| Total Problems | 65.89 ± 7.24 | 65.22 ± 6.73 | 65.49 ± 8.09 | 68.45 ± 8.18 | 0.896 | 0.445 |
| Anxious/Dep | 62.09 ± 8.09 | 61.63 ± 9.62 | 60.88 ± 7.84 | 63.41 ± 9.72 | 0.455 | 0.714 |
| Withdrawn/Dep | 63.19 ± 9.73 | 66.11 ± 10.19 | 61.56 ± 7.37 | 65.35 ± 12.07 | 1.361 | 0.257 |
| Somatic C | 57.90 ± 6.81 | 56.37 ± 8.17 | 55.81 ± 5.99 | 58.48 ± 8.16 | 1.121 | 0.343 |
| Social Problems | 62.78 ± 7.14 | 64.84 ± 8.53 | 62.59 ± 7.66 | 67.09 ± 7.88 | 2.239 | 0.086 |
| Thought P | 60.63 ± 7.91 | 63.16 ± 9.59 | 60.84 ± 7.47 | 64.87 ± 9.79 | 1.814 | 0.147 |
| Attention P | 70.73 ± 8.45 | 67.53 ± 8.59 | 66.26 ± 8.55 | 67.10 ± 7.39 | 2.786 | 0.043 * |
| Rule-Breaking B | 61.28 ± 7.51 | 56.79 ± 5.68 | 62.98 ± 7.57 | 62.36 ± 7.91 | 3.252 | 0.024 * |
| Aggressive B | 64.78 ± 10.16 | 61.16 ± 8.85 | 65.93 ± 10.46 | 67.61 ± 12.35 | 1.448 | 0.231 |
| DP Index | 197.94 ± 20.62 | 190.32 ± 19.90 | 192.93 ± 23.40 | 198.64 ± 26.56 | 0.930 | 0.428 |
| Affective P | 66.05 ± 7.02 | 63.63 ± 7.54 | 62.70 ± 8.18 | 66.30 ± 10.62 | 1.885 | 0.135 |
| Anxious P | 63.25 ± 7.20 | 63.58 ± 7.99 | 62.12 ± 7.54 | 64.04 ± 9.09 | 0.380 | 0.767 |
| Somatic P | 55.60 ± 6.47 | 53.95 ± 6.83 | 54.72 ± 5.79 | 54.78 ± 7.15 | 0.389 | 0.761 |
| ADHD P | 69.09 ± 7.62 | 65.06 ± 8.24 | 67.02 ± 7.48 | 66.22 ± 6.65 | 1.870 | 0.137 |
| ODD P | 62.63 ± 7.47 | 59.63 ± 7.68 | 65.67 ± 8.48 | 65.43 ± 8.17 | 3.316 | 0.022 * |
| Conduct P | 61.63 ± 7.86 | 56.53 ± 5.89 | 64.40 ± 8.29 | 65.09 ± 7.58 | 5.693 | 0.001 ** |
| SCT | 61.94 ± 7.86 | 62.72 ± 9.13 | 57.88 ± 7.27 | 59.30 ± 7.88 | 2.922 | 0.036 * |
| OCD P | 56.69 ± 6.91 | 59.71 ± 8.82 | 56.33 ± 7.02 | 64.14 ± 10.63 | 5.952 | 0.001 ** |
| PTSD P | 65.40 ± 7.85 | 65.83 ± 6.44 | 64.33 ± 7.56 | 67.32 ± 8.44 | 0.741 | 0.529 |
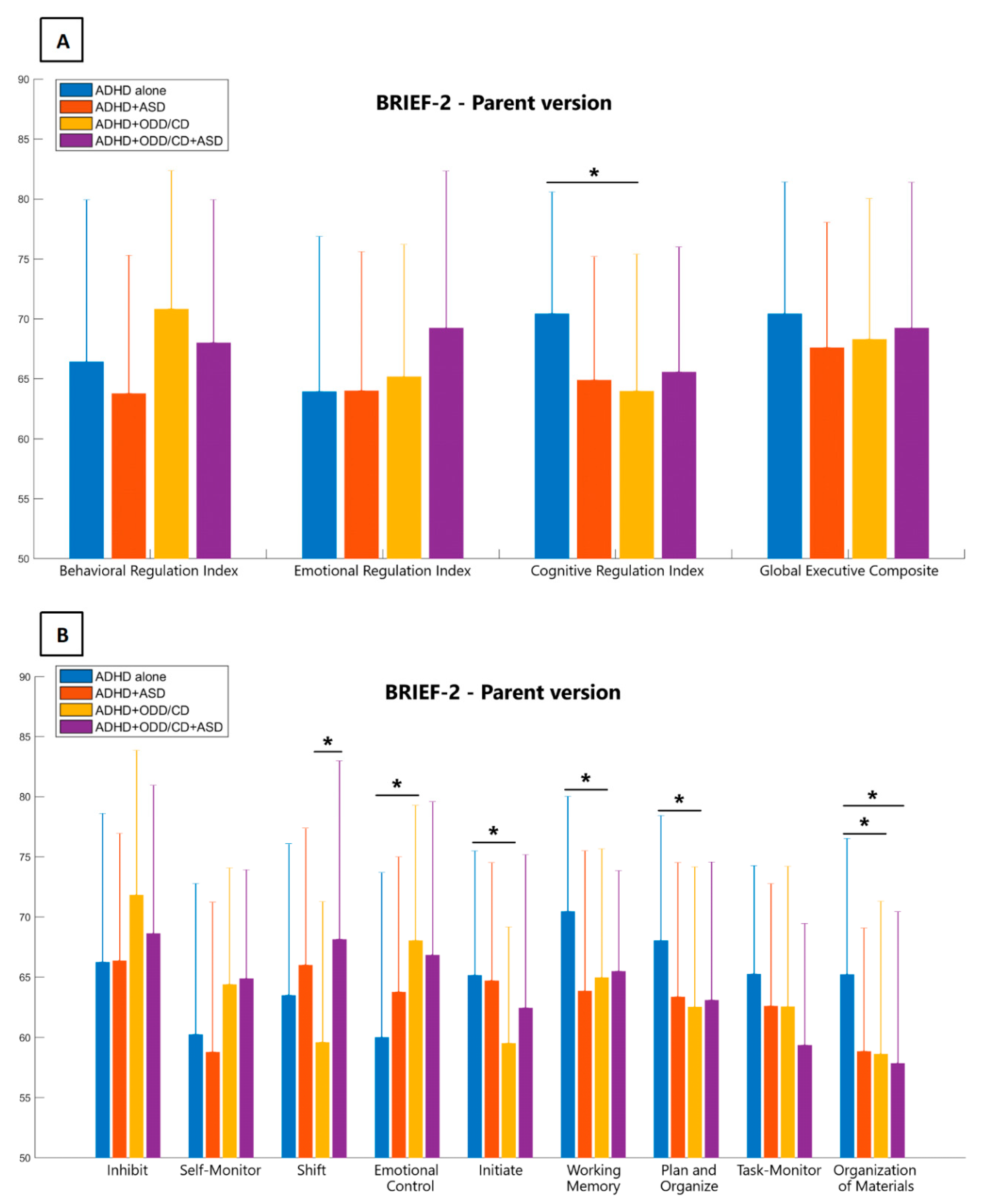
| BRIEF-2 | ADHD | ADHD+ASD | ADHD+ODD/CD | ADHD+ASD+ODD/CD | F Value | p-Values |
|---|---|---|---|---|---|---|
| ERI | 63.92 ± 12.95 | 64.00 ± 11.60 | 65.16 ± 11.04 | 69.22 ± 13.10 | 1.086 | 0.358 |
| BRI | 66.40 ± 13.51 | 63.76 ± 11.54 | 70.82 ± 11.53 | 68.00 ± 11.93 | 1.562 | 0.201 |
| CRI | 70.42 ± 10.16 | 64.88 ± 10.30 | 63.97 ± 11.41 | 65.57 ± 10.42 | 3.551 | 0.016 * |
| GEC | 70.40 ± 11.00 | 67.59 ± 10.47 | 68.29 ± 11.73 | 69.22 ± 12.17 | 0.426 | 0.735 |
| Inhibition | 66.23 ± 12.37 | 66.35 ± 10.58 | 71.82 ± 12.03 | 68.61 ± 12.34 | 1.809 | 0.149 |
| Self-Monitor | 60.22 ± 12.55 | 58.76 ± 12.47 | 64.39 ± 9.68 | 64.87 ± 9.06 | 2.036 | 0.112 |
| Shift | 63.48 ± 12.58 | 66.00 ± 11.37 | 59.58 ± 11.69 | 68.13 ± 14.85 | 2.408 | 0.059 § |
| Emotional C | 59.98 ± 13.69 | 63.76 ± 11.22 | 68.03 ± 11.25 | 66.83 ± 12.75 | 3.698 | 0.014 * |
| Initiate | 65.15 ± 10.34 | 64.71 ± 9.80 | 59.50 ± 9.66 | 62.43 ± 12.73 | 2.423 | 0.059 § |
| Working M | 70.45 ± 9.57 | 63.82 ± 11.67 | 64.95 ± 10.70 | 65.48 ± 8.37 | 3.705 | 0.013 * |
| Plan/Organize | 68.02 ± 10.40 | 63.35 ± 11.17 | 62.5 ± 11.65 | 63.09 ± 11.48 | 2.549 | 0.050 * |
| Org of Materials | 65.21 ± 11.31 | 58.82 ± 10.25 | 58.61 ± 12.70 | 57.83 ± 12.61 | 3.802 | 0.012 * |
| Task Monitor | 65.24 ± 9.01 | 62.59 ± 10.18 | 62.52 ± 11.68 | 59.35 ± 10.10 | 2.032 | 0.112 |
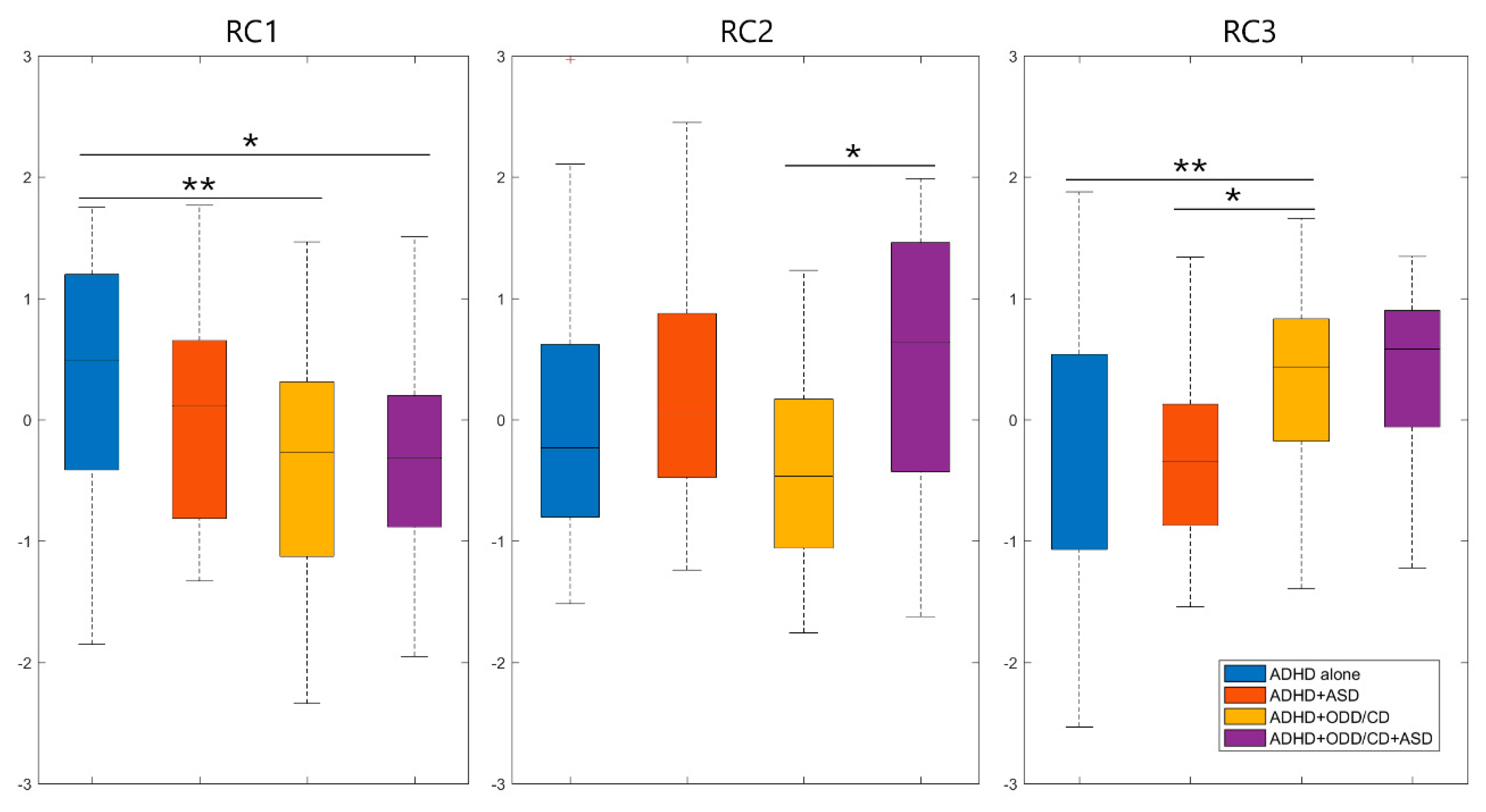
| RC1 | RC2 | RC3 | |
|---|---|---|---|
| BRIEF-2—Plan/Organize | 0.8420 | 0.2389 | 0.1921 |
| BRIEF-2—Task Monitor | 0.8019 | 0.0576 | 0.2487 |
| BRIEF-2—Working Memory | 0.7848 | 0.0050 | 0.1382 |
| BRIEF-2—Organization of Materials | 0.6903 | −0.0165 | 0.1760 |
| BRIEF-2—Initiate | 0.6900 | 0.3306 | 0.0691 |
| CBCL—Attention Problems | 0.5421 | 0.3778 | 0.2531 |
| CBCL—Anxious/Depressed | 0.0162 | 0.8254 | 0.1641 |
| CBCL—Somatic Complaints | 0.2113 | 0.7475 | −0.0119 |
| CBCL—Social Problems | 0.0169 | 0.7259 | 0.3128 |
| CBCL—Withdrawn/Depressed | 0.1771 | 0.7107 | 0.1029 |
| CBCL—Thought Problems | 0.0625 | 0.6922 | 0.2486 |
| BRIEF-2—Shift | 0.4150 | 0.5162 | 0.1894 |
| BRIEF-2—Inhibit | 0.2657 | 0.0520 | 0.8497 |
| CBCL—Rule-Breaking Behaviors | 0.2006 | 0.1169 | 0.7782 |
| CBCL—Aggressive Behaviors | 0.1491 | 0.3767 | 0.7615 |
| BRIEF-2—Emotional Control | 0.1613 | 0.3199 | 0.6968 |
| BRIEF-2—Self-Monitor | 0.1731 | 0.1165 | 0.6913 |
| Unadjusted Eigenvalue | 6.6114 | 2.2253 | 1.7460 |
| Adjusted Eigenvalue | 5.9195 | 1.6879 | 1.3212 |
| Proportion Variance | 0.2151 | 0.2121 | 0.1954 |
| Cumulative Variance | 0.2151 | 0.4271 | 0.6225 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sesso, G.; Cristofani, C.; Berloffa, S.; Cristofani, P.; Fantozzi, P.; Inguaggiato, E.; Narzisi, A.; Pfanner, C.; Ricci, F.; Tacchi, A.; et al. Autism Spectrum Disorder and Disruptive Behavior Disorders Comorbidities Delineate Clinical Phenotypes in Attention-Deficit Hyperactivity Disorder: Novel Insights from the Assessment of Psychopathological and Neuropsychological Profiles. J. Clin. Med. 2020, 9, 3839. https://doi.org/10.3390/jcm9123839
Sesso G, Cristofani C, Berloffa S, Cristofani P, Fantozzi P, Inguaggiato E, Narzisi A, Pfanner C, Ricci F, Tacchi A, et al. Autism Spectrum Disorder and Disruptive Behavior Disorders Comorbidities Delineate Clinical Phenotypes in Attention-Deficit Hyperactivity Disorder: Novel Insights from the Assessment of Psychopathological and Neuropsychological Profiles. Journal of Clinical Medicine. 2020; 9(12):3839. https://doi.org/10.3390/jcm9123839
Chicago/Turabian StyleSesso, Gianluca, Chiara Cristofani, Stefano Berloffa, Paola Cristofani, Pamela Fantozzi, Emanuela Inguaggiato, Antonio Narzisi, Chiara Pfanner, Federica Ricci, Annalisa Tacchi, and et al. 2020. "Autism Spectrum Disorder and Disruptive Behavior Disorders Comorbidities Delineate Clinical Phenotypes in Attention-Deficit Hyperactivity Disorder: Novel Insights from the Assessment of Psychopathological and Neuropsychological Profiles" Journal of Clinical Medicine 9, no. 12: 3839. https://doi.org/10.3390/jcm9123839
APA StyleSesso, G., Cristofani, C., Berloffa, S., Cristofani, P., Fantozzi, P., Inguaggiato, E., Narzisi, A., Pfanner, C., Ricci, F., Tacchi, A., Valente, E., Viglione, V., Milone, A., & Masi, G. (2020). Autism Spectrum Disorder and Disruptive Behavior Disorders Comorbidities Delineate Clinical Phenotypes in Attention-Deficit Hyperactivity Disorder: Novel Insights from the Assessment of Psychopathological and Neuropsychological Profiles. Journal of Clinical Medicine, 9(12), 3839. https://doi.org/10.3390/jcm9123839






