Vitamin D Receptor Polymorphisms and Non-Melanoma Skin Cancer Risk: A Case-Control Study
Abstract
1. Introduction
2. Experimental Section
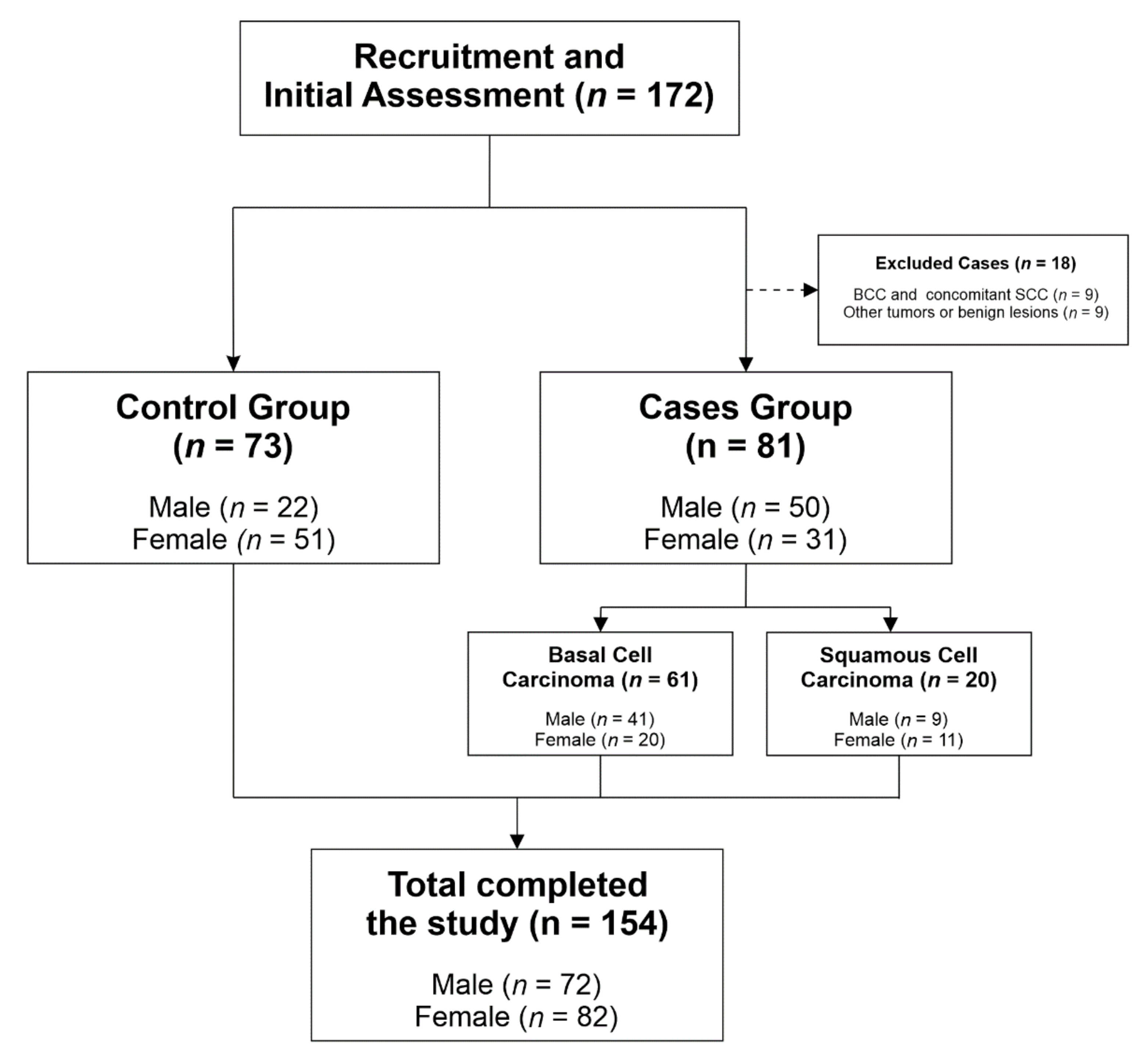
2.1. Study Design, Setting and Participants
2.2. Selection of Polymorphisms and Genotyping
2.3. Skin Samples
2.4. Statistical Methods
3. Results
3.1. Descriptive Characteristics of Cases and Controls
3.2. VDR SNPs, Age and Gender and Non-Melanoma Skin Cancer Risk
3.3. The Combined BsmI/ApaI Genotypes in the Patients with BCC or SCC and the Controls
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Giovannucci, E. The epidemiology of vitamin D and cancer incidence and mortality: A review (United States). Cancer Causes Control 2005, 16, 83–95. [Google Scholar] [CrossRef]
- Holick, M.F. Vitamin D: Importance in the prevention of cancers, type 1 diabetes, heart disease, and osteoporosis. Am. J. Clin. Nutr. 2004, 79, 362–371. [Google Scholar] [CrossRef]
- Holick, M.F. Vitamin D and sunlight: Strategies for cancer prevention and other health benefits. Clin. J. Am. Soc. Nephrol. 2008, 3, 1548–1554. [Google Scholar] [CrossRef] [PubMed]
- Lomas, A.; Leonardi-Bee, J.; Bath-Hextall, F. A systematic review of worldwide incidence of nonmelanoma skin cancer. Br. J. Dermatol. 2012, 166, 1069–1080. [Google Scholar] [CrossRef] [PubMed]
- International Agency for Research on Cancer Working Group. Vitamin D and Cancer; International Agency for Research on Cancer: Lyon, France, 2008. [Google Scholar]
- Reichrath, J. The challenge resulting from positive and negative effects of sunlight: How much solar UV exposure is appropriate to balance between risks of vitamin D deficiency and skin cancer? Prog. Biophys. Mol. Biol. 2006, 92, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Van der Pols, J.C.; Russell, A.; Bauer, U.; Neale, R.E.; Kimlin, M.G.; Green, A.C. Vitamin D status and skin cancer risk independent of time outdoors: 11-year prospective study in an Australian community. J. Investig. Dermatol. 2013, 133, 637–641. [Google Scholar] [CrossRef]
- Dehghan, M.; Pourahmad-Jaktaji, R. The Effect of Some Polymorphisms in Vitamin D Receptor Gene in Menopausal Women with Osteoporosis. J. Clin. Diagn. Res. 2016, 10, Rc06–Rc10. [Google Scholar] [CrossRef]
- Hustmyer, F.G.; DeLuca, H.F.; Peacock, M. ApaI, BsmI, EcoRV and TaqI polymorphisms at the human vitamin D receptor gene locus in Caucasians, blacks and Asians. Hum. Mol. Genet. 1993, 2, 487. [Google Scholar] [CrossRef]
- Kostner, K.; Denzer, N.; Muller, C.S.; Klein, R.; Tilgen, W.; Reichrath, J. The relevance of vitamin D receptor (VDR) gene polymorphisms for cancer: A review of the literature. Anticancer Res. 2009, 29, 3511–3536. [Google Scholar]
- Denzer, N.; Vogt, T.; Reichrath, J. Vitamin D receptor (VDR) polymorphisms and skin cancer: A systematic review. Derm. -Endocrinol. 2011, 3, 205–210. [Google Scholar] [CrossRef]
- Cohen, J. Statistical Power Analysis for the Behavioural Science. In Statistical Power Analysis for the Behavioural Science, 2nd ed.; Lawrence Erlbaum Associates: New York, NY, USA, 1988; ISBN 080580283. [Google Scholar]
- von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gotzsche, P.C.; Vandenbroucke, J.P.; Initiative, S. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies. Epidemiology 2007, 18, 800–804. [Google Scholar] [CrossRef] [PubMed]
- Brodland, D.G.; Zitelli, J.A. Surgical margins for excision of primary cutaneous squamous cell carcinoma. J. Am. Acad. Dermatol. 1992, 27, 241–248. [Google Scholar] [CrossRef]
- Gulleth, Y.; Goldberg, N.; Silverman, R.P.; Gastman, B.R. What is the best surgical margin for a Basal cell carcinoma: A meta-analysis of the literature. Plast. Reconstr. Surg. 2010, 126, 1222–1231. [Google Scholar] [CrossRef] [PubMed]
- Garcovich, S.; Colloca, G.; Sollena, P.; Andrea, B.; Balducci, L.; Cho, W.C.; Bernabei, R.; Peris, K. Skin Cancer Epidemics in the Elderly as an Emerging Issue in Geriatric Oncology. Aging Dis. 2017, 8, 643–661. [Google Scholar] [CrossRef] [PubMed]
- Joseph, A.K.; Mark, T.L.; Mueller, C. The period prevalence and costs of treating nonmelanoma skin cancers in patients over 65 years of age covered by medicare. Dermatol. Surg. 2001, 27, 955–959. [Google Scholar] [PubMed]
- Pascual, J.C.; Belinchon, I.; Ramos, J.M.; Blanes, M.; Betlloch, I. Skin tumors in patients aged 90 years and older. Dermatol. Surg. 2004, 30, 1017–1019, discussion 1019-1020. [Google Scholar] [CrossRef] [PubMed]
- Oberyszyn, T.M. Non-melanoma skin cancer: Importance of gender, immunosuppressive status and vitamin D. Cancer Lett. 2008, 261, 127–136. [Google Scholar] [CrossRef]
- Scrivener, Y.; Grosshans, E.; Cribier, B. Variations of basal cell carcinomas according to gender, age, location and histopathological subtype. Br. J. Dermatol. 2002, 147, 41–47. [Google Scholar] [CrossRef]
- Cakir, B.O.; Adamson, P.; Cingi, C. Epidemiology and economic burden of nonmelanoma skin cancer. Facial Plast. Surg. Clin. N. Am. 2012, 20, 419–422. [Google Scholar] [CrossRef]
- Duarte, A.F.; Sousa-Pinto, B.; Freitas, A.; Delgado, L.; Costa-Pereira, A.; Correia, O. Skin cancer healthcare impact: A nation-wide assessment of an administrative database. Cancer Epidemiol. 2018, 56, 154–160. [Google Scholar] [CrossRef]
- English, D.R.; Armstrong, B.K.; Kricker, A.; Fleming, C. Sunlight and cancer. Cancer Causes Control 1997, 8, 271–283. [Google Scholar] [CrossRef] [PubMed]
- Ravanat, J.L.; Douki, T.; Cadet, J. Direct and indirect effects of UV radiation on DNA and its components. J. Photochem. Photobiol. B 2001, 63, 88–102. [Google Scholar] [CrossRef]
- Hutchinson, P.E.; Osborne, J.E.; Lear, J.T.; Smith, A.G.; Bowers, P.W.; Morris, P.N.; Jones, P.W.; York, C.; Strange, R.C.; Fryer, A.A. Vitamin D receptor polymorphisms are associated with altered prognosis in patients with malignant melanoma. Clin. Cancer Res. 2000, 6, 498–504. [Google Scholar] [PubMed]
- Colston, K.; Colston, M.J.; Feldman, D. 1,25-dihydroxyvitamin D3 and malignant melanoma: The presence of receptors and inhibition of cell growth in culture. Endocrinology 1981, 108, 1083–1086. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.Y.; Xiao, T.Z.; Oda, Y.; Chang, K.S.; Shpall, E.; Wu, A.; So, P.L.; Hebert, J.; Bikle, D.; Epstein, E.H., Jr. Vitamin D3 inhibits hedgehog signaling and proliferation in murine Basal cell carcinomas. Cancer Prev. Res. (Phila. PA) 2011, 4, 744–751. [Google Scholar] [CrossRef]
- Raimondi, S.; Johansson, H.; Maisonneuve, P.; Gandini, S. Review and meta-analysis on vitamin D receptor polymorphisms and cancer risk. Carcinogenesis 2009, 30, 1170–1180. [Google Scholar] [CrossRef]
- Gnagnarella, P.; Pasquali, E.; Serrano, D.; Raimondi, S.; Disalvatore, D.; Gandini, S. Vitamin D receptor polymorphism FokI and cancer risk: A comprehensive meta-analysis. Carcinogenesis 2014, 35, 1913–1919. [Google Scholar] [CrossRef]
- Li, C.; Liu, Z.; Wang, L.E.; Gershenwald, J.E.; Lee, J.E.; Prieto, V.G.; Duvic, M.; Grimm, E.A.; Wei, Q. Haplotype and genotypes of the VDR gene and cutaneous melanoma risk in non-Hispanic whites in Texas: A case-control study. Int. J. Cancer 2008, 122, 2077–2084. [Google Scholar] [CrossRef]
- Orlow, I.; Reiner, A.S.; Thomas, N.E.; Roy, P.; Kanetsky, P.A.; Luo, L.; Paine, S.; Armstrong, B.K.; Kricker, A.; Marrett, L.D.; et al. Vitamin D receptor polymorphisms and survival in patients with cutaneous melanoma: A population-based study. Carcinogenesis 2016, 37, 30–38. [Google Scholar] [CrossRef]
- Santonocito, C.; Capizzi, R.; Concolino, P.; Lavieri, M.M.; Paradisi, A.; Gentileschi, S.; Torti, E.; Rutella, S.; Rocchetti, S.; Di Carlo, A.; et al. Association between cutaneous melanoma, Breslow thickness and vitamin D receptor BsmI polymorphism. Br. J. Dermatol. 2007, 156, 277–282. [Google Scholar] [CrossRef]
- Carless, M.A.; Kraska, T.; Lintell, N.; Neale, R.E.; Green, A.C.; Griffiths, L.R. Polymorphisms of the VDR gene are associated with presence of solar keratoses on the skin. Br. J. Dermatol. 2008, 159, 804–810. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Colditz, G.A.; Hunter, D.J. Polymorphisms in the MTHFR and VDR genes and skin cancer risk. Carcinogenesis 2007, 28, 390–397. [Google Scholar] [CrossRef] [PubMed]
- Kostner, K.; Denzer, N.; Koreng, M.; Reichrath, S.; Graber, S.; Klein, R.; Tilgen, W.; Vogt, T.; Reichrath, J. Association of genetic variants of the vitamin D receptor (VDR) with cutaneous squamous cell carcinomas (SCC) and basal cell carcinomas (BCC): A pilot study in a German population. Anticancer Res. 2012, 32, 327–333. [Google Scholar] [PubMed]
- Lesiak, A.; Norval, M.; Wodz-Naskiewicz, K.; Pawliczak, R.; Rogowski-Tylman, M.; Sysa-Jedrzejowska, A.; Sobjanek, M.; Wlodarkiewicz, A.; Narbutt, J. An enhanced risk of basal cell carcinoma is associated with particular polymorphisms in the VDR and MTHFR genes. Exp. Dermatol. 2011, 20, 800–804. [Google Scholar] [CrossRef] [PubMed]
- Von Schuckmann, L.; Law, M.H.; Montgomery, G.W.; Green, A.C.; JC, V.D.P. Vitamin D Pathway Gene Polymorphisms and Keratinocyte Cancers: A Nested Case-Control Study and Meta-Analysis. Anticancer Res. 2016, 36, 2145–2152. [Google Scholar]
- Lin, Y.; Chahal, H.S.; Wu, W.; Cho, H.G.; Ransohoff, K.J.; Dai, H.; Tang, J.Y.; Sarin, K.Y.; Han, J. Association between genetic variation within vitamin D receptor-DNA binding sites and risk of basal cell carcinoma. Int. J. Cancer 2017, 140, 2085–2091. [Google Scholar] [CrossRef]
- Carmena-Ramon, R.; Mateu-Puchades, A.; Santos-Alarcon, S.; Lucas-Truyols, S. Actinic keratosis: New concept and therapeutic update. Aten Primaria 2017, 49, 492–497. [Google Scholar] [CrossRef]
- Burns, E.M.; Guroji, P.; Ahmad, I.; Nasr, H.M.; Wang, Y.; Tamimi, I.A.; Stiefel, E.; Abdelgawwad, M.S.; Shaheen, A.; Muzaffar, A.F.; et al. Association of Vitamin D Receptor Polymorphisms with the Risk of Nonmelanoma Skin Cancer in Adults. JAMA Dermatol. 2017, 153, 983–989. [Google Scholar] [CrossRef]
- Jorgensen, T.J.; Ruczinski, I.; Yao Shugart, Y.; Wheless, L.; Berthier Schaad, Y.; Kessing, B.; Hoffman-Bolton, J.; Helzlsouer, K.J.; Kao, W.H.; Francis, L.; et al. A population-based study of hedgehog pathway gene variants in relation to the dual risk of basal cell carcinoma plus another cancer. Cancer Epidemiol. 2012, 36, e288–e293. [Google Scholar] [CrossRef]
| Factors | Controls (%) | BCC | SCC | ||||
|---|---|---|---|---|---|---|---|
| Cases (%) | OR 1 | p-Value | Cases (%) | OR 1 | p-Value | ||
| BsmI | |||||||
| GG | 22 (30.10) | 23 (37.70) | - | 0.57 2 | 10 (50) | - | 0.22 3; 0.61 4 |
| AA | 11 (15.10) | 10 (16.40) | - | 3 (15) | - | ||
| AG | 40 (54.80) | 28 (45.90) | - | 7 (35) | - | ||
| BsmI homozygotes vs. heterozygotes | |||||||
| GG/AA | 33 (45.20) | 33 (54.10) | - | 0.30 2 | 13 (65) | - | 0.12 3; 0.39 4 |
| AG | 40 (54.80) | 28 (45.90) | - | 7 (35) | - | ||
| ApaI | - | ||||||
| TT | 17 (23.30) | 16 (26.20) | - | 0.26 2 | 6 (30) | - | 0.76 3; 0.78 4 |
| GG | 13 (17.80) | 17 (27.90) | - | 4 (20) | - | ||
| GT | 43 (58.90) | 28 (45.90) | - | 10 (50) | - | ||
| ApaI homozygotes vs. heterozygotes | |||||||
| TT/GG | 30 (41.10) | 33 (54.10) | - | 0.13 2 | 10 (50) | - | 0.48 3; 0.75 4 |
| GT | 43 (58.90) | 28 (45.90) | - | 10 (50) | - | ||
| Gender | - | ||||||
| Male | 22 (30.10) | 41 (67.20) | 4.38 (1.93–9.90) | 0.00 1; 0.00 2 | 9 (45) | - | 0.21 3; 0.08 4 |
| Female | 51 (69.90) | 20 (32.80) | - | 11 (55) | - | ||
| Age (68.22 ± 14.53) | |||||||
| ≤70 age | 57 (78.10) | 18 (29.50) | 0.12 (0.05–0.28) | 0.00 1; 0.00 2 | 4 (20) | 0.07 (0.02–0.24) | 0.00 1; 0.00 3; 0.41 4 |
| >70 age | 16 (21.90) | 43 (70.50) | - | 16 (80) | - | ||
| Genotype | Controls (%) | BCC | SCC | ||||
|---|---|---|---|---|---|---|---|
| Cases (%) | OR 1 | p-Value | Cases (%) | OR 1 | p-Value | ||
| BsmI * Gender | |||||||
| GG | |||||||
| Male | 7 (31.80) | 19 (82.60) | 7.01 (2.35–20.92) | 0.00 1 | 5 (50) | - | - |
| Female | 15 (68.20) | 4 (17.40) | - | 5 (50) | - | ||
| AA | |||||||
| Male | 6 (54.40) | 6 (60) | - | - | 3 (100) | - | - |
| Female | 5 (45.50) | 4 (40) | - | 0 (0) | - | ||
| AG | |||||||
| Male | 9 (22.50) | 16 (57.10) | 4.59 (1.60–13.18) | 0.01 1 | 1 (14.3) | - | - |
| Female | 31 (77.50) | 12 (42.90) | - | 6 (85.7) | - | ||
| ApaI * Gender | |||||||
| TT | |||||||
| Male | 9 (52.90) | 9 (56.30) | 3.40 (1.06–10.87) | 0.04 1 | 5 (83.3) | - | - |
| Female | 8 (47.10) | 7 (43.80) | - | 1 (16.7) | - | ||
| GG | |||||||
| Male | 4 (30.80) | 14 (82.40) | 11.90 (3.19–44.37) | 0.00 1 | 2 (50) | - | - |
| Female | 9 (69.20) | 3 (17.60) | - | 2 (50) | - | ||
| GT | |||||||
| Male | 9 (20.90) | 18 (64.30) | 6.80 (2.34–19.75) | 0.00 1 | 2 (20) | - | - |
| Female | 34 (79.10) | 10 (35.70) | - | 8 (80) | - | ||
| Age | Gender | Controls (%) | BCC | SCC | ||||
|---|---|---|---|---|---|---|---|---|
| Cases (%) | OR 1 | p-Value | Cases (%) | OR 1 | p-Value | |||
| ≤70 age | Male | 15 (26) | 11 (61) | - | 0.00 1; 0.01 2 | 4 (100) | - | 0.00 3; 0.13 4 |
| Female | 42 (73.70) | 7 (38.90) | 0.12 (0.04–0.37) | 0 (0) | - | |||
| >70 age | Male | 7 (43.80) | 30 (69.80) | - | 0.07 2 | 5 (31.30) | - | 0.46 3; 0.01 4 |
| Female | 9 (56.30) | 13 (30.20) | - | 11 (68.80) | - | |||
| Genotype | Controls (%) | BCC | SCC | ||||
|---|---|---|---|---|---|---|---|
| Cases (%) | OR 1 | p-Value | Cases (%) | OR 1 | p-Value | ||
| BsmI * Age | |||||||
| GG | |||||||
| ≤70 age | 17 (77.30) | 7 (30.40) | 0.22 (0.07–0.70) | 0.01 1 | 2 (20) | - | - |
| >70 age | 5 (22.70) | 16 (69.60) | - | 8 (80) | - | ||
| AA | |||||||
| ≤70 age | 10 (90.90) | 2 (20) | 0.11 (0.02–0.58) | 0.01 1 | 1 (33.30) | - | - |
| >70 age | 1 (9.10) | 8 (80) | - | 2 (66.70) | - | ||
| AG | |||||||
| ≤70 age | 30 (75) | 9 (32.10) | 0.16 (0.05–0.46) | 0.00 1 | 1 (14.30) | 0.06 (0.01–0.52) | 0.01 1 |
| >70 age | 10 (25) | 19 (67.90) | - | 6 (85.70) | - | ||
| ApaI * Age | |||||||
| TT | |||||||
| ≤70 age | 13 (76.50) | 3 (18.80) | 0.14 (0.03–0.60) | 0.00 1 | 3 (50) | - | - |
| >70 age | 4 (23.50) | 13 (81.30) | - | 3 (50) | - | ||
| GG | |||||||
| ≤70 age | 11 (84.60) | 4 (23.50) | 0.21 (0.05–0.86) | 0.03 1 | 0 (0) | - | - |
| >70 age | 2 (15.40) | 13 (76.50) | - | 4 (100) | - | ||
| GT | |||||||
| ≤70 age | 33 (76.70) | 11 (39.30) | - | - | 1 (10) | 0.03 (0.00–0.30) | 0.00 1 |
| >70 age | 10 (23.30) | 17 (60.70) | - | 9 (90) | - | ||
| Genotype | Controls (%) | BCC (%) | SCC (%) | Total (%) |
|---|---|---|---|---|
| AAGG | 1 (1.40) | 0 (0) | 0 (0) | 1 (6) |
| AAGT | 2 (2.70) | 0 (0) | 0 (0) | 2 (1.30) |
| AATT | 8 (11) | 10 (16.40) | 3 (15) | 21 (13.60) |
| AGGG | 1 (1.40) | 0 (0) | 0 (0) | 1 (0.60) |
| AGGT | 31 (42.50) | 22 (36.10) | 6 (30) | 59 (38.30) |
| AGTT | 8 (11) | 6 (9.80) | 1 (5) | 15 (9.70) |
| GGGG | 11 (15.10) | 17 (27.90) | 4 (20) | 32 (20.80) |
| GGGT | 10 (13.70) | 6 (9.80) | 4 (20) | 20 (13) |
| GGTT | 1 (1.40) | 0 (0) | 2 (10) | 3 (1.90) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morgado-Águila, C.; Rey-Sánchez, P.; Gil-Fernández, G.; Costa-Fernández, M.C.; Rodríguez-Velasco, F.J. Vitamin D Receptor Polymorphisms and Non-Melanoma Skin Cancer Risk: A Case-Control Study. J. Clin. Med. 2020, 9, 3819. https://doi.org/10.3390/jcm9123819
Morgado-Águila C, Rey-Sánchez P, Gil-Fernández G, Costa-Fernández MC, Rodríguez-Velasco FJ. Vitamin D Receptor Polymorphisms and Non-Melanoma Skin Cancer Risk: A Case-Control Study. Journal of Clinical Medicine. 2020; 9(12):3819. https://doi.org/10.3390/jcm9123819
Chicago/Turabian StyleMorgado-Águila, Carolina, Purificación Rey-Sánchez, Guadalupe Gil-Fernández, María Carmen Costa-Fernández, and Francisco José Rodríguez-Velasco. 2020. "Vitamin D Receptor Polymorphisms and Non-Melanoma Skin Cancer Risk: A Case-Control Study" Journal of Clinical Medicine 9, no. 12: 3819. https://doi.org/10.3390/jcm9123819
APA StyleMorgado-Águila, C., Rey-Sánchez, P., Gil-Fernández, G., Costa-Fernández, M. C., & Rodríguez-Velasco, F. J. (2020). Vitamin D Receptor Polymorphisms and Non-Melanoma Skin Cancer Risk: A Case-Control Study. Journal of Clinical Medicine, 9(12), 3819. https://doi.org/10.3390/jcm9123819






