Low Levels of Low-Density Lipoprotein Cholesterol and Endothelial Function in Subjects without Lipid-Lowering Therapy
Abstract
1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Measurement of FMD
2.3. Statistical Analysis
3. Results
3.1. Baseline Characteristics
3.2. Relationship of LDL-C with Endothelial Function
3.3. Relationship of Extremely Low LDL-C with Endothelial Function
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mendis, S.; Davis, S.; Norrving, B. Organizational update: The world health organization global status report on noncommunicable diseases 2014; one more landmark step in the combat against stroke and vascular disease. Stroke 2015, 46, e121–e122. [Google Scholar] [CrossRef]
- Lloyd-Jones, D.M.; Huffman, M.D.; Karmali, K.N.; Sanghavi, D.M.; Wright, J.S.; Pelser, C.; Gulati, M.; Masoudi, F.A.; Goff, D.C., Jr. Estimating Longitudinal Risks and Benefits from Cardiovascular Preventive Therapies Among Medicare Patients: The Million Hearts Longitudinal ASCVD Risk Assessment Tool: A Special Report from the American Heart Association and American College of Cardiology. Circulation 2017, 135, e793–e813. [Google Scholar] [CrossRef]
- Di Angelantonio, E.; Gao, P.; Pennells, L.; Kaptoge, S.; Caslake, M.; Thompson, A.; Butterworth, A.S.; Sarwar, N.; Wormser, D.; Saleheen, D.; et al. Lipid-related markers and cardiovascular disease prediction. JAMA 2012, 307, 2499–2506. [Google Scholar] [CrossRef] [PubMed]
- Abdullah, S.M.; Defina, L.F.; Leonard, D.; Barlow, C.E.; Radford, N.B.; Willis, B.L.; Rohatgi, A.; McGuire, D.K.; de Lemos, J.A.; Grundy, S.M.; et al. Long-Term Association of Low-Density Lipoprotein Cholesterol with Cardiovascular Mortality in Individuals at Low 10-Year Risk of Atherosclerotic Cardiovascular Disease. Circulation 2018, 138, 2315–2325. [Google Scholar] [CrossRef]
- Mihaylova, B.; Emberson, J.; Blackwell, L.; Keech, A.; Simes, J.; Barnes, E.H.; Voysey, M.; Gray, A.; Collins, R.; Baigent, C. The effects of lowering LDL cholesterol with statin therapy in people at low risk of vascular disease: Meta-analysis of individual data from 27 randomised trials. Lancet 2012, 380, 581–590. [Google Scholar] [CrossRef] [PubMed]
- Collins, R.; Reith, C.; Emberson, J.; Armitage, J.; Baigent, C.; Blackwell, L.; Blumenthal, R.; Danesh, J.; Smith, G.D.; DeMets, D.; et al. Interpretation of the evidence for the efficacy and safety of statin therapy. Lancet 2016, 388, 2532–2561. [Google Scholar] [CrossRef]
- Cholesterol Treatment Trialists’ Collaboration. Efficacy and safety of statin therapy in older people: A meta-analysis of individual participant data from 28 randomised controlled trials. Lancet 2019, 393, 407–415. [Google Scholar] [CrossRef]
- Amarenco, P.; Kim, J.S.; Labreuche, J.; Charles, H.; Abtan, J.; Bejot, Y.; Cabrejo, L.; Cha, J.K.; Ducrocq, G.; Giroud, M.; et al. A Comparison of Two LDL Cholesterol Targets after Ischemic Stroke. N. Engl. J. Med. 2019. [Google Scholar] [CrossRef]
- Ference, B.A.; Ginsberg, H.N.; Graham, I.; Ray, K.K.; Packard, C.J.; Bruckert, E.; Hegele, R.A.; Krauss, R.M.; Raal, F.J.; Schunkert, H.; et al. Low-density lipoproteins cause atherosclerotic cardiovascular disease. 1. Evidence from genetic, epidemiologic, and clinical studies. A consensus statement from the European Atherosclerosis Society Consensus Panel. Eur. Heart J. 2017, 38, 2459–2472. [Google Scholar] [CrossRef]
- Oesterle, A.; Laufs, U.; Liao, J.K. Pleiotropic Effects of Statins on the Cardiovascular System. Circ. Res. 2017, 120, 229–243. [Google Scholar] [CrossRef]
- Grundy, S.M.; Stone, N.J.; Bailey, A.L.; Beam, C.; Birtcher, K.K.; Blumenthal, R.S.; Braun, L.T.; de Ferranti, S.; Faiella-Tommasino, J.; Forman, D.E.; et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the Management of Blood Cholesterol: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation 2019, 139, e1082–e1143. [Google Scholar] [CrossRef] [PubMed]
- Cannon, C.P.; Blazing, M.A.; Giugliano, R.P.; McCagg, A.; White, J.A.; Theroux, P.; Darius, H.; Lewis, B.S.; Ophuis, T.O.; Jukema, J.W.; et al. Ezetimibe Added to Statin Therapy after Acute Coronary Syndromes. N. Engl. J. Med. 2015, 372, 2387–2397. [Google Scholar] [CrossRef] [PubMed]
- Sabatine, M.S.; Giugliano, R.P.; Keech, A.C.; Honarpour, N.; Wiviott, S.D.; Murphy, S.A.; Kuder, J.F.; Wang, H.; Liu, T.; Wasserman, S.M.; et al. Evolocumab and Clinical Outcomes in Patients with Cardiovascular Disease. N. Engl. J. Med. 2017, 376, 1713–1722. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, G.G.; Steg, P.G.; Szarek, M.; Bhatt, D.L.; Bittner, V.A.; Diaz, R.; Edelberg, J.M.; Goodman, S.G.; Hanotin, C.; Harrington, R.A.; et al. Alirocumab and Cardiovascular Outcomes after Acute Coronary Syndrome. N. Engl. J. Med. 2018, 379, 2097–2107. [Google Scholar] [CrossRef]
- Petursson, H.; Sigurdsson, J.A.; Bengtsson, C.; Nilsen, T.I.; Getz, L. Is the use of cholesterol in mortality risk algorithms in clinical guidelines valid? Ten years prospective data from the Norwegian HUNT 2 study. J. Eval. Clin. Pract. 2012, 18, 159–168. [Google Scholar] [CrossRef]
- Nago, N.; Ishikawa, S.; Goto, T.; Kayaba, K. Low cholesterol is associated with mortality from stroke, heart disease, and cancer: The Jichi Medical School Cohort Study. J. Epidemiol. 2011, 21, 67–74. [Google Scholar] [CrossRef]
- Liang, Y.; Vetrano, D.L.; Qiu, C. Serum total cholesterol and risk of cardiovascular and non-cardiovascular mortality in old age: A population-based study. BMC Geriatr. 2017, 17, 294. [Google Scholar] [CrossRef]
- Sung, K.C.; Huh, J.H.; Ryu, S.; Lee, J.Y.; Scorletti, E.; Byrne, C.D.; Kim, J.Y.; Hyun, D.S.; Ko, S.B. Low Levels of Low-Density Lipoprotein Cholesterol and Mortality Outcomes in Non-Statin Users. J. Clin. Med. 2019, 8, 1571. [Google Scholar] [CrossRef]
- Ma, C.; Gurol, M.E.; Huang, Z.; Lichtenstein, A.H.; Wang, X.; Wang, Y.; Neumann, S.; Wu, S.; Gao, X. Low-density lipoprotein cholesterol and risk of intracerebral hemorrhage: A prospective study. Neurology 2019, 93, e445–e457. [Google Scholar] [CrossRef]
- Vallejo-Vaz, A.J.; Robertson, M.; Catapano, A.L.; Watts, G.F.; Kastelein, J.J.; Packard, C.J.; Ford, I.; Ray, K.K. Low-Density Lipoprotein Cholesterol Lowering for the Primary Prevention of Cardiovascular Disease Among Men with Primary Elevations of Low-Density Lipoprotein Cholesterol Levels of 190 mg/dL or Above: Analyses from the WOSCOPS (West of Scotland Coronary Prevention Study) 5-Year Randomized Trial and 20-Year Observational Follow-Up. Circulation 2017, 136, 1878–1891. [Google Scholar] [CrossRef]
- Pi, X.; Xie, L.; Patterson, C. Emerging Roles of Vascular Endothelium in Metabolic Homeostasis. Circ. Res. 2018, 123, 477–494. [Google Scholar] [CrossRef] [PubMed]
- Vanhoutte, P.M.; Zhao, Y.; Xu, A.; Leung, S.W. Thirty Years of Saying NO: Sources, Fate, Actions, and Misfortunes of the Endothelium-Derived Vasodilator Mediator. Circ. Res. 2016, 119, 375–396. [Google Scholar] [CrossRef] [PubMed]
- Corretti, M.C.; Anderson, T.J.; Benjamin, E.J.; Celermajer, D.; Charbonneau, F.; Creager, M.A.; Deanfield, J.; Drexler, H.; Gerhard-Herman, M.; Herrington, D.; et al. Guidelines for the ultrasound assessment of endothelial-dependent flow-mediated vasodilation of the brachial artery: A report of the International Brachial Artery Reactivity Task Force. J. Am. Coll. Cardiol. 2002, 39, 257–265. [Google Scholar] [CrossRef]
- Ross, R. Atherosclerosis—An inflammatory disease. N. Engl. J. Med. 1999, 340, 115–126. [Google Scholar] [CrossRef] [PubMed]
- Higashi, Y.; Noma, K.; Yoshizumi, M.; Kihara, Y. Endothelial function and oxidative stress in cardiovascular diseases. Circ. J. 2009, 73, 411–418. [Google Scholar] [CrossRef] [PubMed]
- Matsuzawa, Y.; Kwon, T.G.; Lennon, R.J.; Lerman, L.O.; Lerman, A. Prognostic Value of Flow-Mediated Vasodilation in Brachial Artery and Fingertip Artery for Cardiovascular Events: A Systematic Review and Meta-Analysis. J. Am. Heart Assoc. 2015, 4. [Google Scholar] [CrossRef]
- Maruhashi, T.; Soga, J.; Fujimura, N.; Idei, N.; Mikami, S.; Iwamoto, Y.; Iwamoto, A.; Kajikawa, M.; Matsumoto, T.; Oda, N.; et al. Endothelial Dysfunction, Increased Arterial Stiffness, and Cardiovascular Risk Prediction in Patients with Coronary Artery Disease: FMD-J (Flow-Mediated Dilation Japan) Study A. J. Am. Heart Assoc. 2018, 7. [Google Scholar] [CrossRef]
- Matsui, S.; Kajikawa, M.; Hida, E.; Maruhashi, T.; Iwamoto, Y.; Iwamoto, A.; Oda, N.; Kishimoto, S.; Hidaka, T.; Kihara, Y.; et al. Optimal Target Level of Low-density Lipoprotein Cholesterol for Vascular Function in Statin Naive Individuals. Sci. Rep. 2017, 7, 8422. [Google Scholar] [CrossRef]
- Tomiyama, H.; Kohro, T.; Higashi, Y.; Takase, B.; Suzuki, T.; Ishizu, T.; Ueda, S.; Yamazaki, T.; Furumoto, T.; Kario, K.; et al. A multicenter study design to assess the clinical usefulness of semi-automatic measurement of flow-mediated vasodilatation of the brachial artery. Int. Heart J. 2012, 53, 170–175. [Google Scholar] [CrossRef]
- Expert Committee on the Diagnosis and Clasification of Diabetes Mellitus. American Diabetes Association: Clinical practice recommendations 1999. Diabetes Care 1999, 22 (Suppl. S1), S1–S114. [Google Scholar]
- Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive Summary of the Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, And Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). JAMA 2001, 285, 2486–2497. [Google Scholar] [CrossRef] [PubMed]
- Wilson, P.W.; D’Agostino, R.B.; Levy, D.; Belanger, A.M.; Silbershatz, H.; Kannel, W.B. Prediction of coronary heart disease using risk factor categories. Circulation 1998, 97, 1837–1847. [Google Scholar] [CrossRef] [PubMed]
- Tomiyama, H.; Kohro, T.; Higashi, Y.; Takase, B.; Suzuki, T.; Ishizu, T.; Ueda, S.; Yamazaki, T.; Furumoto, T.; Kario, K.; et al. Reliability of measurement of endothelial function across multiple institutions and establishment of reference values in Japanese. Atherosclerosis 2015, 242, 433–442. [Google Scholar] [CrossRef] [PubMed]
- Penson, P.E.; Long, D.L.; Howard, G.; Toth, P.P.; Muntner, P.; Howard, V.J.; Safford, M.M.; Jones, S.R.; Martin, S.S.; Mazidi, M.; et al. Associations between very low concentrations of low density lipoprotein cholesterol, high sensitivity C-reactive protein, and health outcomes in the Reasons for Geographical and Racial Differences in Stroke (REGARDS) study. Eur. Heart J. 2018, 39, 3641–3653. [Google Scholar] [CrossRef]
- Laclaustra, M.; Frangi, A.F.; Frangi, A.G.; Casasnovas, J.A.; Cia, P. Association of endothelial function and vascular data with LDL-c and HDL-c in a homogeneous population of middle-aged, healthy military men: Evidence for a critical role of optimal lipid levels. Int. J. Cardiol. 2008, 125, 376–382. [Google Scholar] [CrossRef]
- Brunetti, N.D.; Maulucci, G.; Casavecchia, G.P.; Distaso, C.; De Gennaro, L.; Luigi Pellegrino, P.; Di Biase, M. Improvement in Endothelium Dysfunction in Diabetics Treated with Statins: A Randomized Comparison of Atorvastatin 20 mg versus Rosuvastatin 10 mg. J. Interv. Cardiol. 2007, 20, 481–487. [Google Scholar] [CrossRef]
- Frick, M.; Alber, H.F.; Hügel, H.; Schwarzacher, S.P.; Pachinger, O.; Weidinger, F. Short- and long-term changes of flow-mediated vasodilation in patients under statin therapy. Clin. Cardiol. 2002, 25, 291–294. [Google Scholar] [CrossRef]
- Karatzis, E.; Lekakis, J.; Papamichael, C.; Andreadou, I.; Cimponeriu, A.; Aznaouridis, K.; Papaioannou, T.G.; Protogerou, A.; Mavrikakis, M. Rapid effect of pravastatin on endothelial function and lipid peroxidation in unstable angina. Int. J. Cardiol. 2005, 101, 65–70. [Google Scholar] [CrossRef]
- Kabaklić, A.; Fras, Z. Moderate-dose atorvastatin improves arterial endothelial function in patients with angina pectoris and normal coronary angiogram: A pilot study. Arch. Med Sci. AMS 2017, 13, 827–836. [Google Scholar] [CrossRef]
- Gong, X.; Ma, Y.; Ruan, Y.; Fu, G.; Wu, S. Long-term atorvastatin improves age-related endothelial dysfunction by ameliorating oxidative stress and normalizing eNOS/iNOS imbalance in rat aorta. Exp. Gerontol. 2014, 52, 9–17. [Google Scholar] [CrossRef]
- Kinlay, S. Low-density lipoprotein-dependent and -independent effects of cholesterol-lowering therapies on C-reactive protein: A meta-analysis. J. Am. Coll. Cardiol. 2007, 49, 2003–2009. [Google Scholar] [CrossRef] [PubMed]
- Beishuizen, E.D.; Tamsma, J.T.; Jukema, J.W.; van de Ree, M.A.; van der Vijver, J.C.; Meinders, A.E.; Huisman, M.V. The effect of statin therapy on endothelial function in type 2 diabetes without manifest cardiovascular disease. Diabetes Care 2005, 28, 1668–1674. [Google Scholar] [CrossRef] [PubMed]
- van Venrooij, F.V.; van de Ree, M.A.; Bots, M.L.; Stolk, R.P.; Huisman, M.V.; Banga, J.D.; Group, D.S. Aggressive lipid lowering does not improve endothelial function in type 2 diabetes: The Diabetes Atorvastatin Lipid Intervention (DALI) Study: A randomized, double-blind, placebo-controlled trial. Diabetes Care 2002, 25, 1211–1216. [Google Scholar] [CrossRef]
- ter Avest, E.; Abbink, E.J.; Holewijn, S.; de Graaf, J.; Tack, C.J.; Stalenhoef, A.F.H. Effects of rosuvastatin on endothelial function in patients with familial combined hyperlipidaemia (FCH). Curr. Med. Res. Opin. 2005, 21, 1469–1476. [Google Scholar] [CrossRef] [PubMed]
- Fulcher, J.; O’Connell, R.; Voysey, M.; Emberson, J.; Blackwell, L.; Mihaylova, B.; Simes, J.; Collins, R.; Kirby, A.; Colhoun, H.; et al. Efficacy and safety of LDL-lowering therapy among men and women: Meta-analysis of individual data from 174,000 participants in 27 randomised trials. Lancet 2015, 385, 1397–1405. [Google Scholar] [CrossRef] [PubMed]
- Hooper, A.J.; Burnett, J.R. Update on primary hypobetalipoproteinemia. Curr. Atheroscler. Rep. 2014, 16, 423. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J.C.; Boerwinkle, E.; Mosley, T.H., Jr.; Hobbs, H.H. Sequence variations in PCSK9, low LDL, and protection against coronary heart disease. N. Engl. J. Med. 2006, 354, 1264–1272. [Google Scholar] [CrossRef] [PubMed]
- Hooper, A.J.; Marais, A.D.; Tanyanyiwa, D.M.; Burnett, J.R. The C679X mutation in PCSK9 is present and lowers blood cholesterol in a Southern African population. Atherosclerosis 2007, 193, 445–448. [Google Scholar] [CrossRef] [PubMed]
- Musunuru, K.; Pirruccello, J.P.; Do, R.; Peloso, G.M.; Guiducci, C.; Sougnez, C.; Garimella, K.V.; Fisher, S.; Abreu, J.; Barry, A.J.; et al. Exome sequencing, ANGPTL3 mutations, and familial combined hypolipidemia. N. Engl. J. Med. 2010, 363, 2220–2227. [Google Scholar] [CrossRef]
- Dewey, F.E.; Gusarova, V.; Dunbar, R.L.; O’Dushlaine, C.; Schurmann, C.; Gottesman, O.; McCarthy, S.; Van Hout, C.V.; Bruse, S.; Dansky, H.M.; et al. Genetic and Pharmacologic Inactivation of ANGPTL3 and Cardiovascular Disease. N. Engl. J. Med. 2017, 377, 211–221. [Google Scholar] [CrossRef]
- Minicocci, I.; Cantisani, V.; Poggiogalle, E.; Favari, E.; Zimetti, F.; Montali, A.; Labbadia, G.; Pigna, G.; Pannozzo, F.; Zannella, A.; et al. Functional and morphological vascular changes in subjects with familial combined hypolipidemia: An exploratory analysis. Int. J. Cardiol. 2013, 168, 4375–4378. [Google Scholar] [CrossRef] [PubMed]
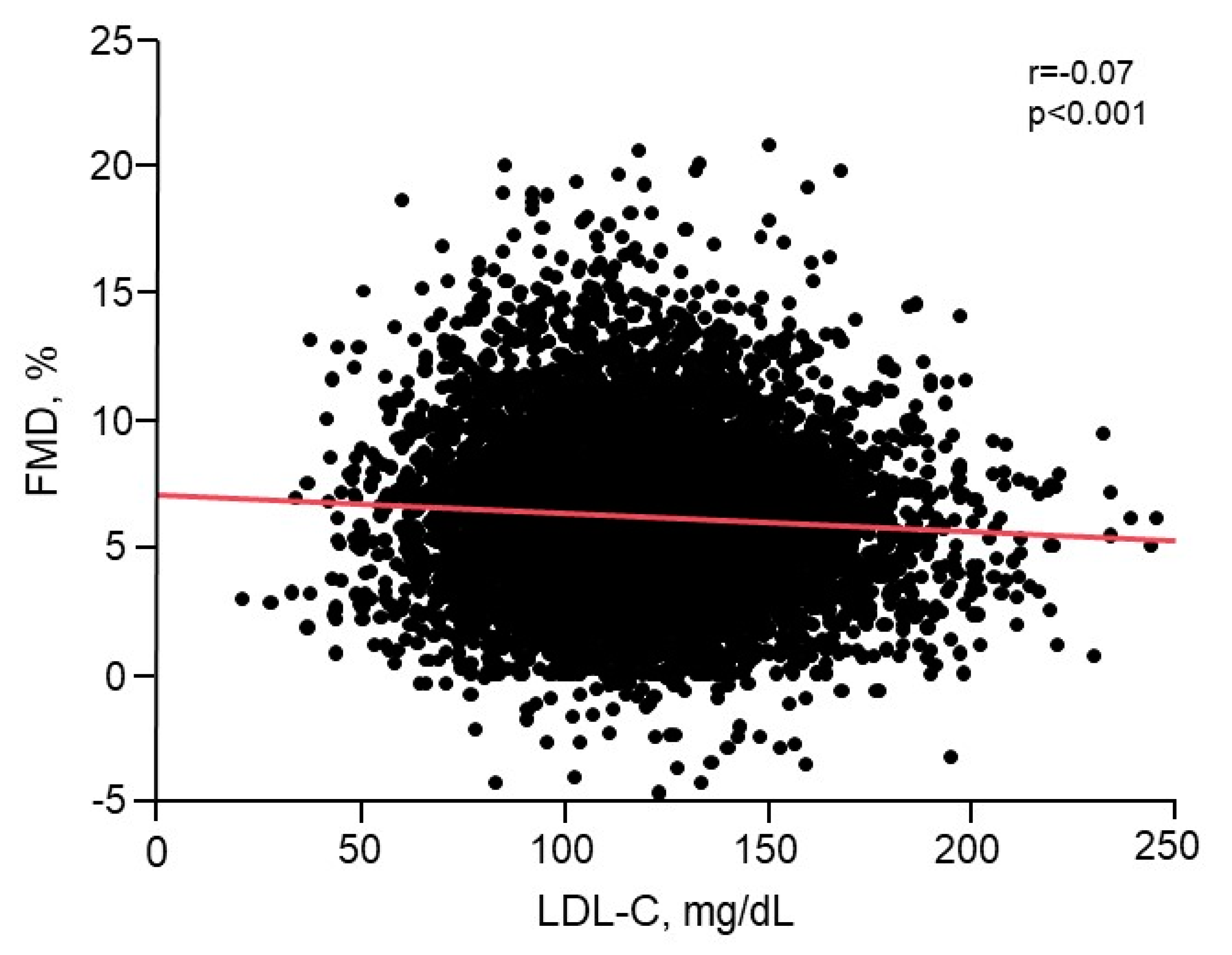
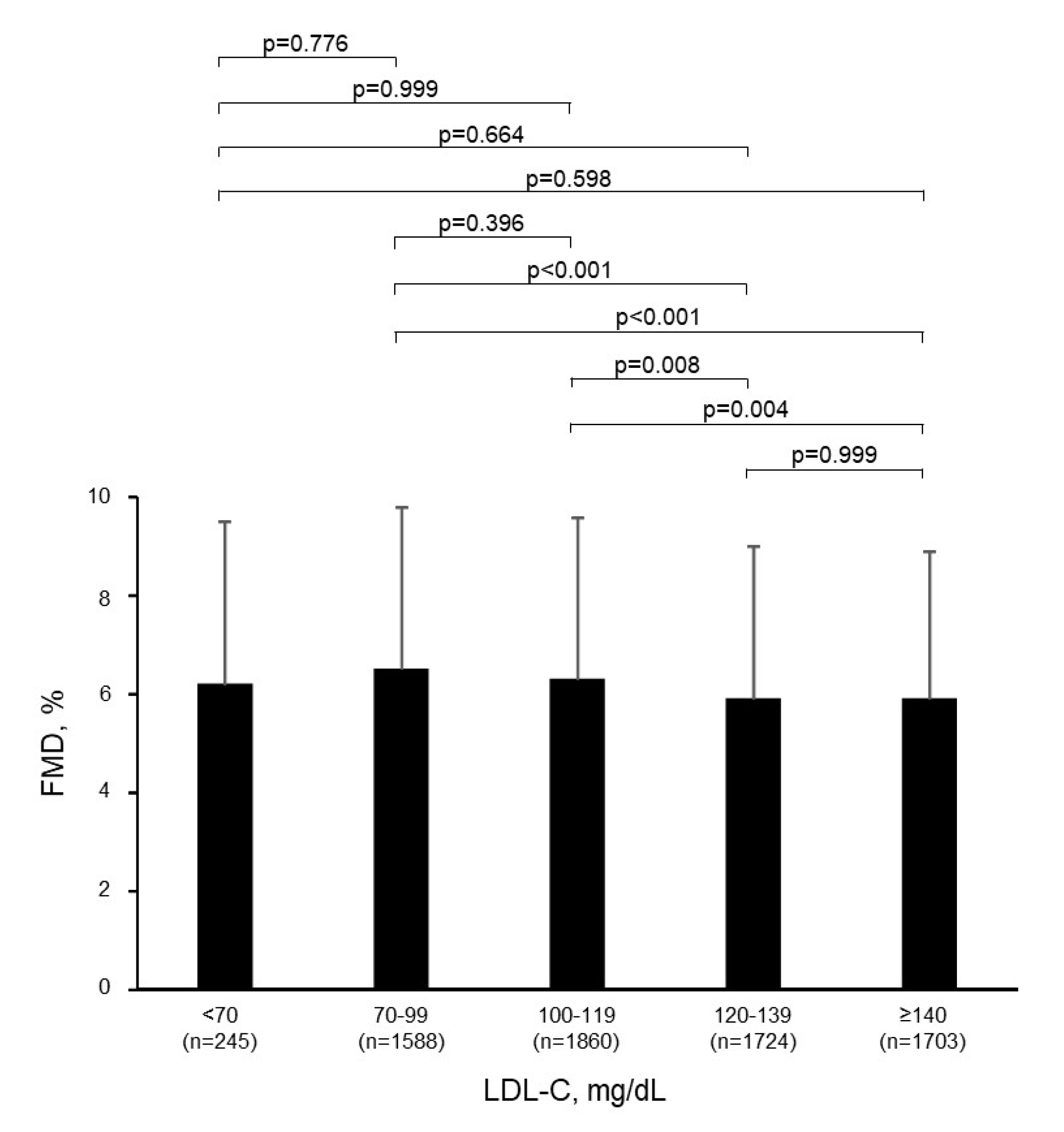
| Variables | Total (n = 7120) | <70 mg/dL (n = 245) | 70–99 mg/dL (n = 1588) | 100–119 mg/dL (n = 1860) | 120–139 mg/dL (n = 1724) | ≥140 mg/dL (n = 1703) | p-Value |
|---|---|---|---|---|---|---|---|
| Age, year | 50.2 ± 12 | 47.5 ± 14 | 47.1 ± 13 | 50.4 ± 12 | 51.2 ± 11 | 52.3 ± 10 | <0.001 |
| Body mass index, kg/m2 | 23.3 ± 3.4 | 22.2 ± 3.4 | 22.3 ± 3.3 | 23.1 ± 3.3 | 23.8 ± 3.3 | 24.1 ± 3.4 | <0.001 |
| Gender, men/women | 5465/1655 | 182/63 | 1222/366 | 1428/432 | 1361/363 | 1272/431 | 0.050 |
| Systolic blood pressure, mmHg | 127 ± 17 | 125 ± 19 | 124 ± 17 | 127 ± 17 | 129 ± 16 | 130 ± 17 | <0.001 |
| Diastolic blood pressure, mmHg | 79 ± 12 | 78 ± 13 | 77 ± 12 | 79 ± 12 | 81 ± 12 | 81 ± 12 | <0.001 |
| Heart rate, bpm | 65 ± 11 | 66 ± 13 | 64 ± 11 | 65 ± 11 | 64 ± 10 | 66 ± 11 | <0.001 |
| Total cholesterol, mg/dL | 203 ± 33 | 146 ± 23 | 172 ± 19 | 193 ± 16 | 211 ± 16 | 242 ± 23 | <0.001 |
| Triglycerides, mg/dL | 104 (72, 149) | 87 (57, 145) | 85 (61, 130) | 95 (67, 136) | 111 (79, 155) | 123 (90, 162) | <0.001 |
| HDL-C, mg/dL | 60 ± 16 | 64 ± 20 | 63 ± 17 | 61 ± 15 | 58 ± 15 | 57 ± 14 | <0.001 |
| LDL-C, mg/dL | 120 ± 30 | 60 ± 9 | 88 ± 8 | 110 ± 6 | 129 ± 6 | 159 ± 18 | - |
| Glucose, mg/dL | 100 ± 20 | 100 ± 21 | 97 ± 18 | 100 ± 21 | 100 ± 19 | 102 ± 22 | <0.001 |
| Medications, n (%) | |||||||
| Anti-hypertensive therapy | 1669 (23.5) | 59 (24.0) | 312 (19.7) | 443 (23.8) | 460 (26.7) | 395 (23.2) | <0.001 |
| Anti-hyperglycemic therapy | 311 (4.4) | 16 (6.5) | 70 (4.4) | 80 (4.3) | 70 (4.1) | 75 (4.4) | 0.544 |
| Framingham risk score, % | 8.1 ± 7.2 | 2.7 ± 2.2 | 3.3 ± 2.9 | 7.9 ± 6.4 | 9.4 ± 7.0 | 12.3 ± 8.5 | <0.001 |
| Medical history, n (%) | |||||||
| Hypertension | 2924 (41.1) | 90 (36.6) | 543 (34.2) | 767 (41.2) | 769 (44.6) | 755 (44.3) | <0.001 |
| Dyslipidemia | 2986 (41.9) | 63 (25.7) | 320 (20.2) | 392 (21.1) | 508 (29.5) | 1703 (100.0) | <0.001 |
| Diabetes mellitus | 511 (7.2) | 22 (9.0) | 96 (6.1) | 132 (7.1) | 117 (6.8) | 144 (8.5) | 0.066 |
| Smokers | 2165 (30.5) | 82 (33.5) | 530 (33.4) | 542 (29.2) | 524 (30.5) | 487 (28.7) | 0.021 |
| FMD, % | 6.1 ± 3.2 | 6.2 ± 3.3 | 6.5 ± 3.3 | 6.3 ± 3.3 | 5.9 ± 3.1 | 5.9 ± 3.0 | <0.001 |
| Variables | β | VIF | Parameter Estimate | Standard Error | p-Value |
|---|---|---|---|---|---|
| Age, year | −0.284 | 1.45 | −0.077 | 0.004 | <0.001 |
| Body mass index, kg/m2 | −0.114 | 1.30 | −0.109 | 0.012 | <0.001 |
| Systolic blood pressure, mmHg | −0.112 | 2.85 | −0.021 | 0.004 | <0.001 |
| Diastolic blood pressure, mmHg | 0.074 | 2.66 | 0.020 | 0.005 | <0.001 |
| Heart rate, bpm | 0.048 | 1.11 | 0.014 | 0.003 | <0.001 |
| Total cholesterol, mg/dL | 0.860 | 70.45 | 0.083 | 0.009 | <0.001 |
| Triglycerides, mg/dL | −0.386 | 10.69 | −0.019 | 0.002 | <0.001 |
| HDL-C, mg/dL | −0.420 | 16.78 | −0.085 | 0.009 | <0.001 |
| LDL-C, mg/dL | −0.744 | 58.13 | −0.080 | 0.009 | <0.001 |
| Glucose, mg/dL | −0.035 | 1.21 | −0.006 | 0.002 | 0.004 |
| Framingham risk score, % | −0.036 | 2.40 | −0.016 | 0.008 | 0.037 |
| Variables | <70 mg/dL (n = 244) | ≥70 mg/dL (n = 244) | p-Value |
|---|---|---|---|
| Age, year | 47.5 ± 14 | 47.5 ± 13 | 0.997 |
| Body mass index, kg/m2 | 22.2 ± 3.4 | 22.2 ± 3.5 | 0.856 |
| Gender, men/women | 182/61 | 196/47 | 0.127 |
| Systolic blood pressure, mmHg | 125 ± 19 | 126 ± 17 | 0.354 |
| Diastolic blood pressure, mmHg | 78 ± 13 | 78 ± 12 | 0.795 |
| Heart rate, bpm | 66 ± 13 | 66 ± 11 | 0.619 |
| Total cholesterol, mg/dL | 147 ± 22 | 203 ± 32 | <0.001 |
| Triglycerides, mg/dL | 87 (56, 145) | 95 (67, 144) | 0.826 |
| HDL-C, mg/dL | 65 ± 20 | 65 ± 19 | 0.998 |
| LDL-C, mg/dL | 60 ± 9 | 117 ± 28 | <0.001 |
| Glucose, mg/dL | 100 ± 22 | 99 ± 23 | 0.759 |
| Medications, n (%) | |||
| Anti-hypertensive therapy | 59 (24.3) | 59 (24.3) | 1.000 |
| Anti-hyperglycemic therapy | 16 (6.6) | 17 (7.0) | 0.857 |
| Framingham risk score, % | 2.7 ± 2.1 | 7.2 ± 6.9 | <0.001 |
| Medical history, n (%) | |||
| Hypertension | 90 (37.0) | 82 (33.7) | 0.448 |
| Dyslipidemia | 62 (25.5) | 84 (34.6) | 0.030 |
| Diabetes mellitus | 22 (9.1) | 24 (9.9) | 0.757 |
| Smokers | 82 (33.7) | 79 (32.5) | 0.773 |
| FMD, % | 6.2 ± 3.2 | 6.4 ± 3.3 | 0.386 |
| Variables | Total (n = 7120) | <50 mg/dL (n = 35) | 50–69 mg/dL (n = 210) | 70–99 mg/dL (n = 1588) | 100–119 mg/dL (n = 1860) | 120–139 mg/dL (n = 1724) | ≥140 mg/dL (n = 1703) | p-Value |
|---|---|---|---|---|---|---|---|---|
| Age, year | 50.2 ± 12 | 49.1 ± 13 | 47.2 ± 14 | 47.1 ± 13 | 50.4 ± 12 | 51.2 ± 11 | 52.3 ± 10 | <0.001 |
| Body mass index, kg/m2 | 23.3 ± 3.4 | 22.5 ± 4.0 | 22.1 ± 3.3 | 22.3 ± 3.3 | 23.1 ± 3.3 | 23.8 ± 3.3 | 24.1 ± 3.4 | <0.001 |
| Gender, men/women | 5465/1655 | 28/7 | 154/56 | 1222/366 | 1428/432 | 1361/363 | 1272/431 | 0.067 |
| Systolic blood pressure, mmHg | 127 ± 17 | 126 ± 21 | 124 ± 18 | 124 ± 17 | 127 ± 17 | 129 ± 16 | 130 ± 17 | <0.001 |
| Diastolic blood pressure, mmHg | 79 ± 12 | 78 ± 13 | 78 ± 13 | 77 ± 12 | 79 ± 12 | 81 ± 12 | 81 ± 12 | <0.001 |
| Heart rate, bpm | 65 ± 11 | 66 ± 11 | 66 ± 13 | 64 ± 11 | 65 ± 11 | 64 ± 10 | 66 ± 11 | <0.001 |
| Total cholesterol, mg/dL | 203 ± 33 | 132 ± 22 | 149 ± 22 | 172 ± 19 | 193 ± 16 | 211 ± 16 | 242 ± 23 | <0.001 |
| Triglycerides, mg/dL | 104 (72, 149) | 87 (65, 168) | 87 (56, 141) | 85 (61, 130) | 95 (67, 136) | 111 (79, 155) | 123 (90, 162) | <0.001 |
| HDL-C, mg/dL | 60 ± 16 | 65 ± 21 | 64 ± 20 | 63 ± 17 | 61 ± 15 | 58 ± 15 | 57 ± 14 | <0.001 |
| LDL-C, mg/dL | 120 ± 30 | 43 ± 6 | 62 ± 5 | 88 ± 8 | 110 ± 6 | 129 ± 6 | 159 ± 18 | - |
| Glucose, mg/dL | 100 ± 20 | 100 ± 12 | 100 ± 23 | 97 ± 18 | 100 ± 21 | 100 ± 19 | 102 ± 22 | <0.001 |
| Medications, n (%) | ||||||||
| Anti-hypertensive therapy | 1669 (23.5) | 10 (28.6) | 49 (23.3) | 312 (19.7) | 443 (23.8) | 460 (26.7) | 395 (23.2) | <0.001 |
| Anti-hyperglycemic therapy | 311 (4.4) | 3 (8.6) | 13 (6.2) | 70 (4.4) | 80 (4.3) | 70 (4.1) | 75 (4.4) | 0.617 |
| Framingham risk score, % | 8.1 ± 7.2 | 2.6 ± 2.0 | 2.7 ± 2.2 | 3.3 ± 2.9 | 7.9 ± 6.4 | 9.4 ± 7.0 | 12.3 ± 8.5 | <0.001 |
| Medical history, n (%) | ||||||||
| Hypertension | 2924 (41.1) | 13 (37.1) | 77 (36.7) | 543 (34.2) | 767 (41.2) | 769 (44.6) | 755 (44.3) | <0.001 |
| Dyslipidemia | 2986 (41.9) | 12 (34.3) | 51 (24.3) | 320 (20.2) | 392 (21.1) | 508 (29.5) | 1703 (100.0) | <0.001 |
| Diabetes mellitus | 511 (7.2) | 3 (8.6) | 19 (9.1) | 96 (6.1) | 132 (7.1) | 117 (6.8) | 144 (8.5) | 0.115 |
| Smokers | 2165 (30.5) | 12 (34.3) | 70 (33.3) | 530 (33.4) | 542 (29.2) | 524 (30.5) | 487 (28.7) | 0.040 |
| FMD, % | 6.1 ± 3.2 | 6.2 ± 3.5 | 6.2 ± 3.2 | 6.5 ± 3.3 | 6.3 ± 3.3 | 5.9 ± 3.1 | 5.9 ± 3.0 | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Takaeko, Y.; Kajikawa, M.; Kishimoto, S.; Yamaji, T.; Harada, T.; Han, Y.; Kihara, Y.; Hida, E.; Chayama, K.; Goto, C.; et al. Low Levels of Low-Density Lipoprotein Cholesterol and Endothelial Function in Subjects without Lipid-Lowering Therapy. J. Clin. Med. 2020, 9, 3796. https://doi.org/10.3390/jcm9123796
Takaeko Y, Kajikawa M, Kishimoto S, Yamaji T, Harada T, Han Y, Kihara Y, Hida E, Chayama K, Goto C, et al. Low Levels of Low-Density Lipoprotein Cholesterol and Endothelial Function in Subjects without Lipid-Lowering Therapy. Journal of Clinical Medicine. 2020; 9(12):3796. https://doi.org/10.3390/jcm9123796
Chicago/Turabian StyleTakaeko, Yuji, Masato Kajikawa, Shinji Kishimoto, Takayuki Yamaji, Takahiro Harada, Yiming Han, Yasuki Kihara, Eisuke Hida, Kazuaki Chayama, Chikara Goto, and et al. 2020. "Low Levels of Low-Density Lipoprotein Cholesterol and Endothelial Function in Subjects without Lipid-Lowering Therapy" Journal of Clinical Medicine 9, no. 12: 3796. https://doi.org/10.3390/jcm9123796
APA StyleTakaeko, Y., Kajikawa, M., Kishimoto, S., Yamaji, T., Harada, T., Han, Y., Kihara, Y., Hida, E., Chayama, K., Goto, C., Aibara, Y., Yusoff, F. M., Maruhashi, T., Nakashima, A., & Higashi, Y. (2020). Low Levels of Low-Density Lipoprotein Cholesterol and Endothelial Function in Subjects without Lipid-Lowering Therapy. Journal of Clinical Medicine, 9(12), 3796. https://doi.org/10.3390/jcm9123796





