Investigation of Vestibular Function in Adult Patients with Gitelman Syndrome: Results of an Observational Study
Abstract
1. Introduction
2. Patients and Methods
2.1. Study Population
2.2. Vestibular Evaluations
2.2.1. Neuro-Otological Clinical Exam
2.2.2. Paraclinical Vestibular Explorations
2.2.3. Definition of Vestibular Syndrome
2.3. Auditory Investigation
2.4. Treatment and Follow-Up
2.5. Statistical Analyses
3. Results
3.1. Clinical and Paraclinical Ear, Nose and Throat Investigations
3.1.1. Characteristics of Patients Referred for Investigations
3.1.2. Neuro-Otological Clinical Exam
3.1.3. Paraclinical Vestibular Tests
3.1.4. Diagnosis of Vestibular Syndrome
3.2. Comparative Results
3.3. Treatment
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Fava, C.; Montagnana, M.; Rosberg, L.; Burri, P.; Almgren, P.; Jönsson, A.; Wanby, P.; Lippi, G.; Minuz, P.; Hulthen, L.U.; et al. Subjects heterozygous for genetic loss of function of the thiazide-sensitive cotransporter have reduced blood pressure. Hum. Mol. Genet. 2008, 17, 413–418. [Google Scholar] [CrossRef] [PubMed]
- Knoers, N.V.A.M.; Levtchenko, E.N. Gitelman syndrome. Orphanet J. Rare Dis. 2008, 30, 3–22. [Google Scholar] [CrossRef] [PubMed]
- Vargas-Poussou, R.; Dahan, K.; Kahila, D.; Venisse, A.; Riveira-Munoz, E.; Debaix, H.; Grisart, B.; Bridoux, F.; Unwin, R.; Moulin, B.; et al. Spectrum of mutations in Gitelman syndrome. J. Am. Soc. Nephrol. 2011, 22, 693–703. [Google Scholar] [CrossRef] [PubMed]
- Blanchard, A.; Bockenhauer, D.; Bolignano, D.; Calò, L.A.; Cosyns, E.; Devuyst, O.; Ellison, D.H.; Karet Frankl, F.E.; Knoers, N.V.A.M.; Konrad, M.; et al. Gitelman syndrome: Consensus and guidance from a Kidney Disease: Improving Global Outcomes Controversies Conference. Kidney Int. 2017, 91, 24–33. [Google Scholar] [CrossRef] [PubMed]
- Blanchard, A.; Vargas-Poussou, R.; Vallet, M.; Caumont-Prim, A.; Allard, J.; Desport, E.; Dubourg, L.; Monge, M.; Bergerot, D.; Baron, S.; et al. Indomethacin, amiloride, or eplerenone for treating hypokalemia in Gitelman syndrome. J. Am. Soc. Nephrol. 2015, 26, 468–475. [Google Scholar] [CrossRef] [PubMed]
- Dvorak, M.M.; De Joussineau, C.; Carter, D.H.; Pisitkun, T.; Knepper, M.A.; Gamba, G.; Kemp, P.J.; Riccardi, D. Thiazide diuretics directly induce osteoblast differentiation and mineralized nodule formation by interacting with a sodium chloride co-transporter in bone. J. Am. Soc. Nephrol. 2007, 18, 2509–2516. [Google Scholar] [CrossRef]
- Møller, M.N.; Kirkeby, S.; Vikeså, J.; Nielsen, F.C.; Cayé-Thomasen, P. Gene expression in the human endolymphatic sac: The solute carrier molecules in endolymphatic fluid homeostasis. Otol. Neurotol. 2015, 36, 915–922. [Google Scholar] [CrossRef]
- Akiyama, K.; Miyashita, T.; Mori, T.; Inamoto, R.; Mori, N. Expression of thiazide-sensitive Na+-Cl- cotransporter in the rat endolymphatic sac. Biochem. Biophys. Res. Commun. 2008, 11, 649–653. [Google Scholar] [CrossRef]
- Bisdorff, A.R.; Staab, J.P.; Newman-Toker, D.E. Overview of the International Classification of Vestibular Disorders. Neurol. Clin. 2015, 33, 541–550. [Google Scholar] [CrossRef]
- Schatz, I.J.; Bannister, R.; Freeman, R.L.; Jankovic, J.; Koller, W.C.; Low, P.A.; Streeten, D.H. Consensus statement on the definition of orthostatic hypotension, pure autonomic failure and multiple system atrophy. Neurology 1996, 46, 1470. [Google Scholar]
- Richards, S.; Aziz, N.; Bale, S.; Bick, D.; Das, S.; Gastier-Foster, J.; Grody, W.W.; Hedge, M.; Lyon, E.; Spector, E.; et al. Standards and guidelines for the interpretation of sequence variants: A joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Off. J. Am. Coll. Med. Genet. 2015, 17, 405–424. [Google Scholar] [CrossRef] [PubMed]
- Ji, W.; Foo, J.N.; O’Roak, B.J.; Zhao, H.; Larson, M.G.; Simon, D.B.; Newto-Cheh, C.; State, M.W.; Levy, D.; Lifton, R.P. Rare independent mutations in renal salt handling genes contribute to blood pressure variation. Nat. Genet. 2008, 40, 592–599. [Google Scholar] [CrossRef] [PubMed]
- Mastroianni, N.; Bettinelli, A.; Bianchetti, M.; Colussi, G.; De Fusco, M.; Sereni, F.; Ballabio, A.; Casari, G. Novel molecular variants of the Na-Cl cotransporter gene are responsible for Gitelman syndrome. Am. J. Hum. Genet. 1996, 59, 1019–1026. [Google Scholar] [PubMed]
- Simon, D.B.; Nelson-Williams, C.; Bia, M.J.; Ellison, D.; Karet, F.E.; Molina, A.M.; Vaara, I.; Iwata, F.; Cushner, H.M.; Koolen, M.; et al. Gitelman’s variant of Bartter’s syndrome, inherited hypokalaemic alkalosis, is caused by mutations in the thiazide-sensitive Na-Cl cotransporter. Nat. Genet. 1996, 12, 24–30. [Google Scholar] [CrossRef]
- Cruz, D.N.; Simon, D.B.; Nelson-Williams, C.; Farhi, A.; Finberg, K.; Burleson, L.; Gill, J.R.; Lifton, R.P. Mutations in the Na-Cl cotransporter reduce blood pressure in humans. Hypertens 2001, 37, 1458–1464. [Google Scholar] [CrossRef]
- Kunchaparty, S.; Palcso, M.; Berkman, J.; Velázquez, H.; Desir, G.V.; Bernstein, P.; Reilly, R.F.; Ellison, D.H. Defective processing and expression of thiazide-sensitive Na-Cl cotransporter as a cause of Gitelman’s syndrome. Am. J. Physiol. 1999, 277, F643–F649. [Google Scholar] [CrossRef]
- Syrén, M.L.; Tedeschi, S.; Cesareo, L.; Bellantuono, R.; Colussi, G.; Procaccio, M.; Ali, A.; Domenici, R.; Malberti, F.; Sprocati, M.; et al. Identification of fifteen novel mutations in the SLC12A3 gene encoding the Na-Cl Co-transporter in Italian patients with Gitelman syndrome. Hum. Mutat. 2002, 20, 78. [Google Scholar] [CrossRef]
- De Jong, J.C.; Van Der Vliet, W.A.; Van Den Heuvel, L.P.W.J.; Willems, P.H.G.M.; Knoers, N.V.A.M.; Bindels, R.J.M. Functional expression of mutations in the human NaCl cotransporter: Evidence for impaired routing mechanisms in Gitelman’s syndrome. J. Am. Soc. Nephrol. 2002, 13, 1442–1448. [Google Scholar] [CrossRef]
- Valdez-Flores, M.A.; Vargas-Poussou, R.; Verkaart, S.; Tutakhel, O.A.Z.; Valdez-Ortiz, A.; Blanchard, A.; Treard, C.; Hoenderop, J.G.J.; Bindels, R.J.M.; Jelen, S. Functionomics of NCC mutations in Gitelman syndrome using a novel mammalian cell-based activity assay. Am. J. Physiol. Renal. Physiol. 2016, 311, F1159–F1167. [Google Scholar] [CrossRef]
- Blanchard, A.; Vallet, M.; Dubourg, L.; Hureaux, M.; Allard, J.; Haymann, J.P.; De la Faille, R.; Arnoux, A.; Dinut, A.; Bergerot, D.; et al. Resistance to Insulin in Patients with Gitelman Syndrome and a Subtle Intermediate Phenotype in Heterozygous Carriers: A Cross-Sectional Study. J. Am. Soc. Nephrol. 2019, 30, 1534–1545. [Google Scholar] [CrossRef]
- Fukuyama, S.; Okudaira, S.; Yamazato, S.; Yamazato, M.; Ohta, T. Analysis of renal tubular electrolyte transporter genes in seven patients with hypokalemic metabolic alkalosis. Kidney Int. 2003, 64, 808–816. [Google Scholar] [CrossRef]
- Lemmink, H.H.; Knoers, N.V.; Károlyi, L.; van Dijk, H.; Niaudet, P.; Antignac, C.; Guay-Woodford, L.M.; Goodyer, P.R.; Carel, J.C.; Hermes An Seyberth, H.W.; et al. Novel mutations in the thiazide-sensitive NaCl cotransporter gene in patients with Gitelman syndrome with predominant localization to the C-terminal domain. Kidney Int. 1998, 54, 720–730. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Escamez, J.A.; Carey, J.; Chung, W.H.; Goebel, J.A.; Magnusson, M.; Mandalà, M.; Newman-Toker, D.E.; Strupp, M.; Suzuki, M.; Trabalzini, F.; et al. Diagnostic criteria for Menière’s disease. J. Vestib. Res. Equilib. Orientat 2015, 25, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Lempert, T.; Olesen, J.; Furman, J.; Waterston, J.; Seemungal, B.; Carey, J.; Bisdorff, A.; Versino, M.; Evers, S.; Newman-Toker, D. Vestibular migraine: Diagnostic criteria. J. Vestib. Res. Equilib. Orientat. 2012, 22, 167–172. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, T. The stepping test: Two phases of the labyrinthine reflex. Acta Otolaryngol. 1959, 50, 95–108. [Google Scholar] [CrossRef] [PubMed]
- Asawavichiangianda, S.; Fujimoto, M.; Mai, M.; Desroches, H.; Rutka, J. Significance of head-shaking nystagmus in the evaluation of the dizzy patient. Acta Oto-Laryngol. Suppl. 1999, 540, 27–33. [Google Scholar] [CrossRef]
- Mori, N.; Miyashita, T.; Inamoto, R.; Matsubara, A.; Mori, T.; Akiyama, K.; Hoshikawa, H. Ion transport its regulation in the endolymphatic sac: Suggestions for clinical aspects of Meniere’s disease. Eur. Arch. Otorhinolaryngol. 2017, 274, 1813–1820. [Google Scholar] [CrossRef]
- Halmagyi, G.M.; Chen, L.; MacDougall, H.G.; Weber, K.P.; McGarvie, L.A.; Curthoys, I.S. The Video Head Impulse Test. Front. Neurol. 2017, 8, 258. [Google Scholar] [CrossRef]
- Eza-Nuñez, P.; Fariñas-Alvarez, C.; Fernandez, N.P. Comparison of three diagnostic tests in detecting vestibular deficit in patients with peripheral vestibulopathy. J. Laryngol. Otol. 2016, 130, 145–150. [Google Scholar] [CrossRef]
- Bhansali, S.A.; Honrubia, V. Current status of electronystagmography testing. Off. J. Am. Acad. Otolaryngol.-Head Neck Surg. 1999, 120, 419–426. [Google Scholar] [CrossRef]
- Cohen, H.S.; Sangi-Haghpeykar, H.; Ricci, N.A.; Kampangkaew, J.; Williamson, R.A. Utility of Stepping, Walking, and Head Impulses for Screening Patients for Vestibular Impairments. Off. J. Am. Acad. Otolaryngol.-Head Neck Surg. 2014, 151, 131–136. [Google Scholar] [CrossRef] [PubMed]
- Committee on Hearing and Equilibrium guidelines for the evaluation of results of treatment of conductive hearing loss. Off. J. Am. Acad. Otolaryngol.-Head Neck Surg. 1995, 113, 186–187. [CrossRef]
- Lacour, M.; Van de Heyning, P.H.; Novotny, M.; Tighilet, B. Betahistine in the treatment of Ménière’s disease. Neuropsychiatr. Dis. Treat. 2007, 3, 429–440. [Google Scholar] [PubMed]
- Ferber-Viart, C.; Dubreuil, C.; Vidal, P.P. Effects of acetyl-DL-leucine in vestibular patients: A clinical study following neurotomy and labyrinthectomy. Audiol. Neurotol. 2009, 14, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Strupp, M.; Brandt, T. Peripheral vestibular disorders. Curr. Opin. Neurol. 2013, 26, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Rajguru, S.M.; Ifediba, M.A.; Rabbitt, R.D. Biomechanics of horizontal canal benign paroxysmal positional vertigo. J. Vestib. Res. Equilib. Orientat. 2005, 15, 203–214. [Google Scholar]
- Rosenthal, A.K.; Ryan, L.M. Calcium Pyrophosphate Deposition Disease. N. Engl. J. Med. 2016, 374, 2575–2584. [Google Scholar] [CrossRef]
- Skarp, S.; Kanervo, L.; Kotimäki, J.; Sorri, M.; Männikkö, M.; Hietikko, E. Whole-exome sequencing suggests multiallelic inheritance for childhood-onset Ménière’s disease. Ann. Hum. Genet. 2019, 83, 389–396. [Google Scholar] [CrossRef]
- Eckhard, A.H.; Zhu, M.; O’Malley, J.T.; Williams, G.H.; Loffing, J.; Rauch, S.D.; Nadol, J.B.; Liberman, M.C.; Adams, J.C. Inner ear pathologies impair sodium-regulated ion transport in Meniere’s disease. Acta Neuropathol. 2018, 137, 343–357. [Google Scholar] [CrossRef]
- Zhang, S.; Leng, Y.; Liu, B.; Shi, H.; Lu, M.; Kong, W. Diagnostic Value of Vestibular Evoked Myogenic Potentials in Endolymphatic Hydrops: A Meta-Analysis. Sci. Rep. 2015, 5, 14951. [Google Scholar] [CrossRef]

| Patient | Age | Sex a | [K+] b mM [3.50–4.50] | [Mg2+] mM [0.64–0.90] b | CCA c | SBP/DBP (HR) f Standing Seated | Associated Symptoms g | Triggers h | Vertigo Duration | Frequency i | Age of First Symptoms | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 40 | M | 2.2 | 0.65 | + d | 137/88 (76) | 142/91 (93) | a | a,b | >1 day | c | 30–50 y |
| 2 | 38 | F | 3.1 | 0.60 | + d | 109/70 (88) | 109/74 (100) | a,c,d | a | >1 day | a | 30–50 y |
| 3 | 46 | M | 3.1 | 0.82 | - | 118/66 (74) | 119/73 (85) | - | b | <1 min | c | 20–30 y |
| 4 | 41 | F | 3.6 | 0.80 | + e | 114/73 (87) | 101/73 (107) | - | c | <1 min | c | 20–30 y |
| 5 | 51 | M | 2.9 | 0.53 | - | 103/57 (77) | 103/61 (79) | d | - | - | c | >50 y |
| 6 | 47 | F | 3.3 | 0.52 | + e | 125/79 (86) | 125/79 (86) | d | a,b | <1 min | a | 30–50 y |
| 7 | 41 | F | 2.2 | 0.53 | + e | 108/67 (81) | 100/67 (88) | - | a,b | <1 min | c | 20–30 y |
| 8 | 44 | M | 2.6 | 0.40 | + d | 114/76 (61) | 117/76 (78) | c | a | <1 min | b | >50 y |
| 9 | 41 | F | 2.9 | 0.53 | - | 94/64 (80) | 95/70 (86) | c | c | <20 min | c | 30–50 y |
| 10 | 28 | F | 3.2 | 0.70 | - | 117/72 (100) | 109/76 (117) | - | b | <1 min | c | <20 y |
| 11 | 37 | F | 3.4 | 0.78 | - | 124/79 (80) | 132/82 (89) | a,b,c | a | <1 min | a | 20–30 y |
| 12 | 31 | F | 3.5 | 0.70 | - | 127/71 (84) | 123/76 (99) | c,d | a | <1 min | a | <20 y |
| 13 | 47 | F | 2.7 | 0.61 | + d | 106/77 (107) | 97/77 (119) | - | c | <1 min | a | 30–50 y |
| 14 | 54 | F | 2.9 | 0.50 | + e | 103/64 (60) | 98/80 (70) | a,b,c,d | b | <20 min | b | >50 y |
| 15 | 53 | M | 2.7 | 0.49 | - | 116/65 (107) | 110/69 (111) | d | a,b | <1 min | b | <20 y |
| 16 | 34 | M | 2.6 | 0.57 | - | 119/81 (73) | 123/79 (94) | - | c | <1 min | c | 30–50 y |
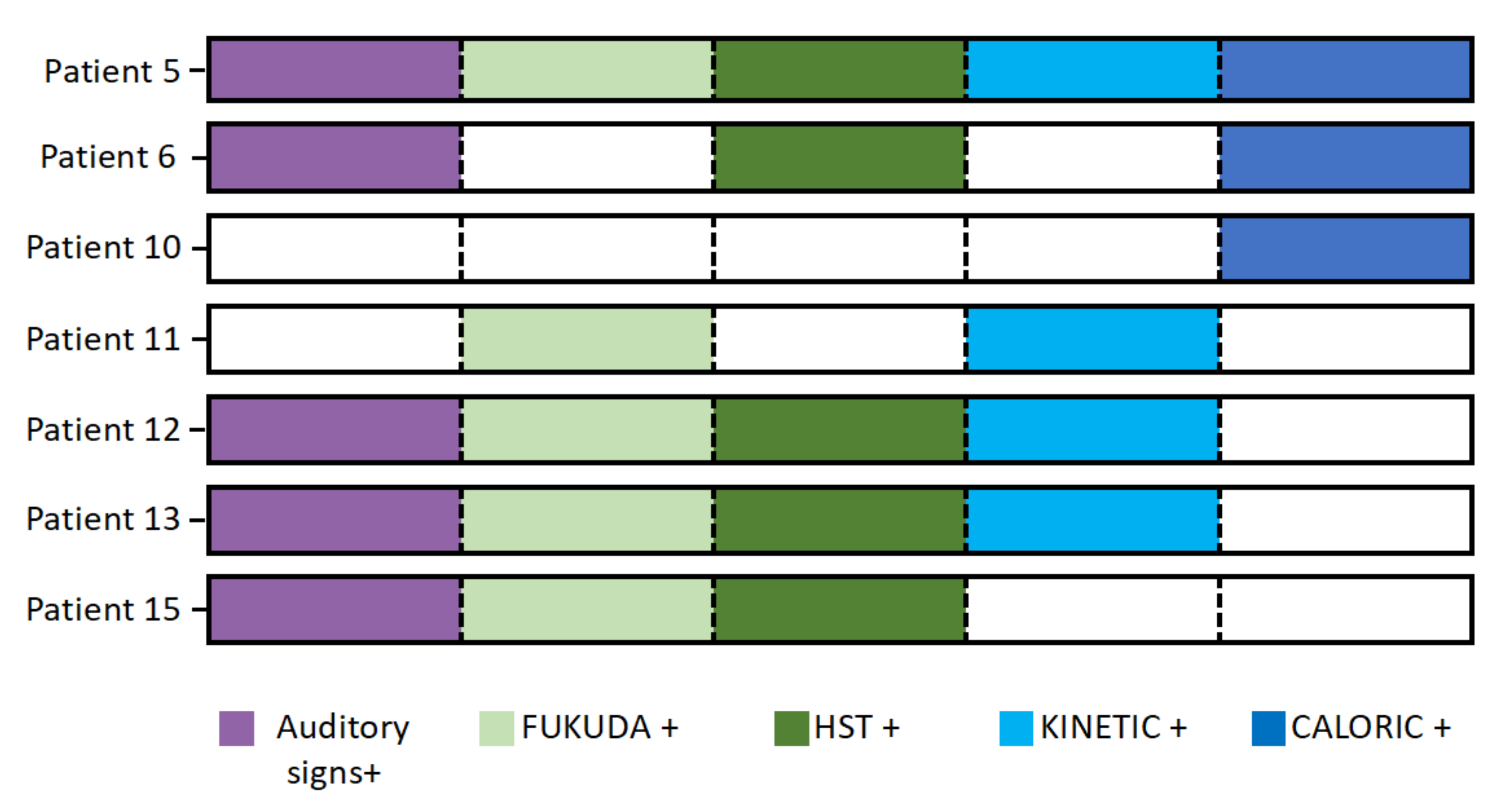
| Patient | Aural Signs a | Hearing Loss b | Fukuda Deviation | HST c | VHIT d | Kinetic e | Caloric f | Vestibular Syndrome | Drug g | Drug Efficacy |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 25 dB 4000 Hz | - | - | - | - | - | - | +(a) | - | |
| 2 | a | - | - | - | - | - | - | - | +(a,b) | +(b)/−(a) |
| 3 | - | - | - | - | - | - | - | - | - | |
| 4 | - | - | - | - | - | - | - | - | - | |
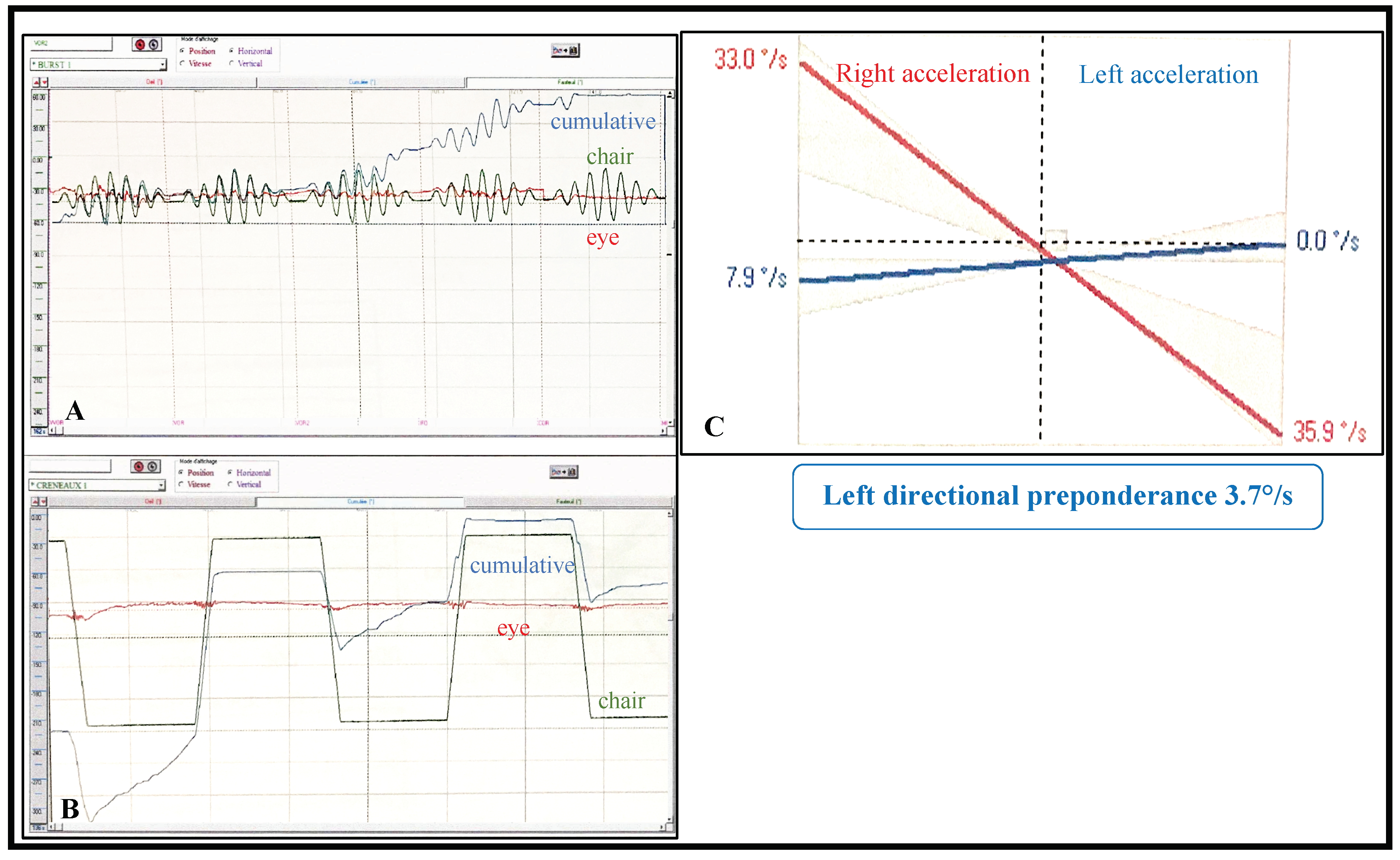
| 5 | b | 35 dB 4000 Hz 20 dB 8000 Hz | + | nystagmus | - | 3.2% | 30% | + | - | - |
| 6 | b | 25 dB 500 Hz 40 dB 4000 Hz 60 dB 8000 Hz left 35 dB 8000 Hz right | - | nystagmus | - | - | 42% | + | - | - |
| 7 | - | - | - | - | - | - | - | - | - | |
| 8 | 35 dB 4000 Hz | - | - | - | - | - | - | +(b) | + | |
| 9 | - | - | - | - | - | - | - | - | - | |
| 10 | - | - | - | - | - | 43% | + | - | - | |
| 11 | - | + | - | - | 3.5% | - | + | +(b) | + | |
| 12 | a | - | + | nystagmus | - | 2.3% | - | + | - | - |
| 13 | 20 dB 150 Hz | + | nystagmus | - | 3.7% | - | + | - | - | |
| 14 | b | 30 dB 500 Hz right | + | - | - | - | - | - | - | - |
| 15 | a | - | + | nystagmus | - | - | - | + | +(b) | + |
| 16 | - | + | - | - | - | - | - | - | - | |
| Total n (%) | 6 (37.5) | 6 (37.5) | 7 (44) | 5 (31.2) | 0 (0) | 4 (25) | 5 (31.2) | 7 (44) | 5 (31.2) | 4 (25) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alexandru, M.; Courbebaisse, M.; Le Pajolec, C.; Ménage, A.; Papon, J.-F.; Vargas-Poussou, R.; Nevoux, J.; Blanchard, A. Investigation of Vestibular Function in Adult Patients with Gitelman Syndrome: Results of an Observational Study. J. Clin. Med. 2020, 9, 3790. https://doi.org/10.3390/jcm9113790
Alexandru M, Courbebaisse M, Le Pajolec C, Ménage A, Papon J-F, Vargas-Poussou R, Nevoux J, Blanchard A. Investigation of Vestibular Function in Adult Patients with Gitelman Syndrome: Results of an Observational Study. Journal of Clinical Medicine. 2020; 9(11):3790. https://doi.org/10.3390/jcm9113790
Chicago/Turabian StyleAlexandru, Mihaela, Marie Courbebaisse, Christine Le Pajolec, Adeline Ménage, Jean-François Papon, Rosa Vargas-Poussou, Jérôme Nevoux, and Anne Blanchard. 2020. "Investigation of Vestibular Function in Adult Patients with Gitelman Syndrome: Results of an Observational Study" Journal of Clinical Medicine 9, no. 11: 3790. https://doi.org/10.3390/jcm9113790
APA StyleAlexandru, M., Courbebaisse, M., Le Pajolec, C., Ménage, A., Papon, J.-F., Vargas-Poussou, R., Nevoux, J., & Blanchard, A. (2020). Investigation of Vestibular Function in Adult Patients with Gitelman Syndrome: Results of an Observational Study. Journal of Clinical Medicine, 9(11), 3790. https://doi.org/10.3390/jcm9113790





