Drug Repurposing Approaches to Combating Viral Infections
Abstract
1. Introduction
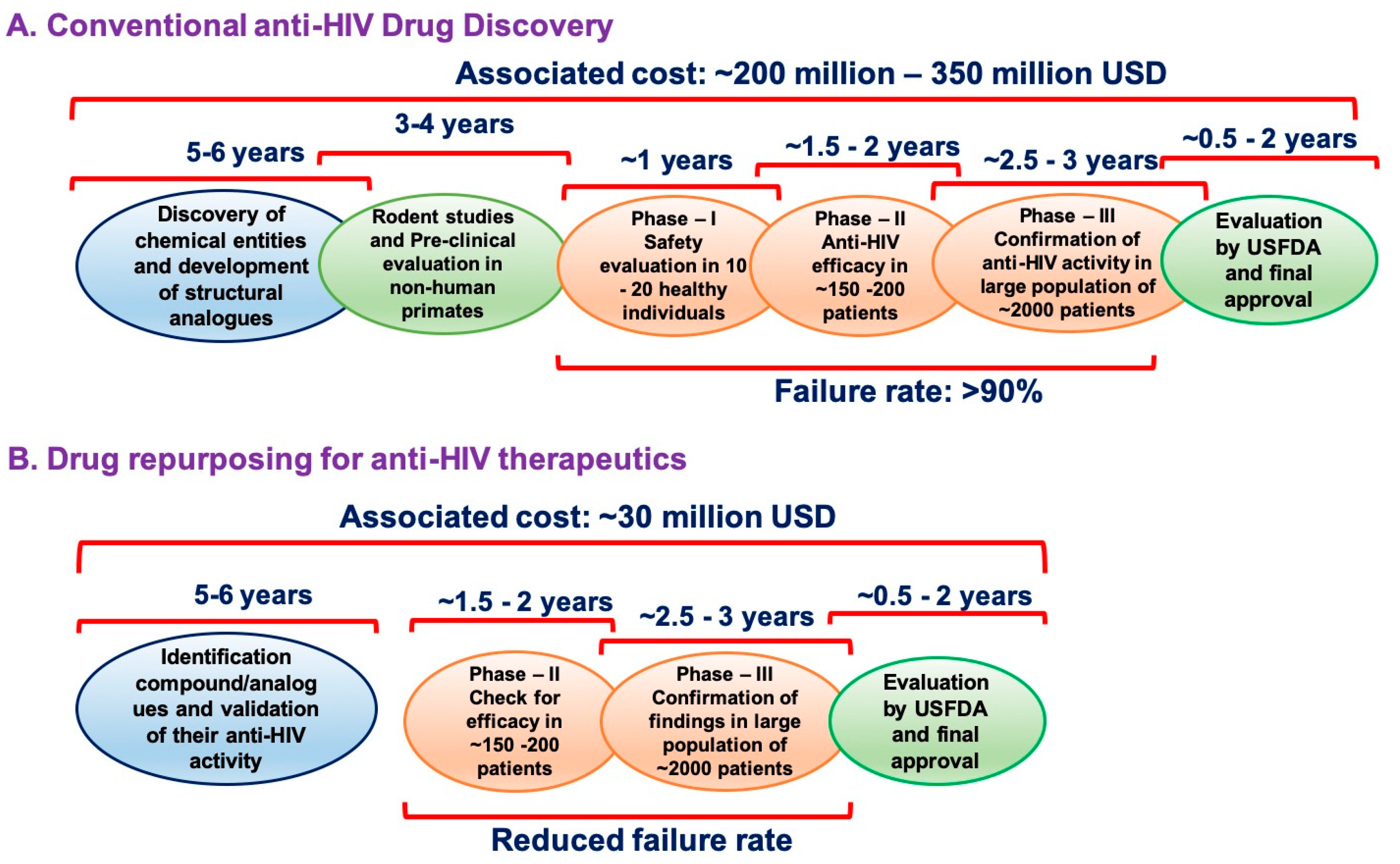
2. The Origin of Drug Repurposing Approach: Zidovudine as an Example
3. Anti-HIV Therapeutics as Drug Repurposing Candidates
4. Drug Repurposing Approach at Present: Against COVID-19
5. Drawbacks of Drug Repurposing
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Martorana, A.; Perricone, U.; Lauria, A. The Repurposing of Old Drugs or Unsuccessful Lead Compounds by in Silico Approaches: New Advances and Perspectives. Curr. Top. Med. Chem. 2016, 16, 1. [Google Scholar] [CrossRef]
- Pushpakom, S.; Iorio, F.; Eyers, P.A.; Escott, K.J.; Hopper, S.; Wells, A.; Doig, A.; Guilliams, T.; Latimer, J.; McNamee, C.; et al. Drug repurposing: Progress, challenges and recommendations. Nat. Rev. Drug Discov. 2019, 18, 41–58. [Google Scholar] [CrossRef] [PubMed]
- Gouveia, M.J.; Brindley, P.J.; Gärtner, F.; da Costa, J.M.C.; Vale, N. Drug Repurposing for Schistosomiasis: Combinations of Drugs or Biomolecules. Pharmaceuticals 2018, 11, 15. [Google Scholar] [CrossRef]
- Lv, B.-M.; Tong, X.-Y.; Quan, Y.; Liu, M.-Y.; Zhang, Q.; Song, Y.; Zhang, H.-Y. Drug Repurposing for Japanese Encephalitis Virus Infection by Systems Biology Methods. Molecules 2018, 23, 3346. [Google Scholar] [CrossRef]
- Seliger, C.; Hau, P. Drug Repurposing of Metabolic Agents in Malignant Glioma. Int. J. Mol. Sci. 2018, 19, 2768. [Google Scholar] [CrossRef]
- Cha, Y.; Erez, T.; Reynolds, I.J.; Kumar, D.; Ross, J.; Koytiger, G.; Kusko, R.; Zeskind, B.; Risso, S.; Kagan, E.; et al. Drug repurposing from the perspective of pharmaceutical companies. Br. J. Pharmacol. 2017, 175, 168–180. [Google Scholar] [CrossRef]
- Giovannoni, G.; Baker, D.; Schmierer, K. The problem with repurposing: Is there really an alternative to Big Pharma for developing new drugs for multiple sclerosis? Mult. Scler. Relat. Disord. 2015, 4, 3–5. [Google Scholar] [CrossRef]
- Fischl, M.A.; Richman, D.D.; Grieco, M.H.; Gottlieb, M.S.; Volberding, P.A.; Laskin, O.L.; Leedom, J.M.; Groopman, J.E.; Mildvan, D.; Schooley, R.T.; et al. The Efficacy of Azidothymidine (AZT) in the Treatment of Patients with AIDS and AIDS-Related Complex. N. Engl. J. Med. 1987, 317, 185–191. [Google Scholar] [CrossRef]
- Topical minoxidil approved by FDA. Clin. Pharm. 1988, 7, 858–862.
- Goldstein, I.; Lue, T.F.; Padma-Nathan, H.; Rosen, R.C.; Steers, W.D.; Wicker, P.A. Oral sildenafil in the treatment of erectile dysfunction. Sildenafil Study Group. N Engl. J. Med. 1998, 338, 1397–1404. [Google Scholar]
- Calabrese, L.; Resztak, K. Thalidomide revisited: Pharmacology and clinical applications. Expert Opin. Investig. Drugs 1998, 7, 2043–2060. [Google Scholar] [CrossRef]
- Glasmacher, A.; Hahn, C.; Hoffmann, F.; Naumann, R.; Goldschmidt, H.; Lilienfeld-Toal, M.; Orlopp, K.; Schmidt-Wolf, I.; Gorschlüter, M. A systematic review of phase-II trials of thalidomide monotherapy in patients with relapsed or refractory multiple myeloma. Br. J. Haematol. 2006, 132, 584–593. [Google Scholar] [CrossRef]
- Steinbach, G.; Lynch, P.M.; Phillips, R.K.; Wallace, M.H.; Hawk, E.; Gordon, G.B.; Wakabayashi, N.; Saunders, B.; Shen, Y.; Fujimura, T.; et al. The Effect of Celecoxib, a Cyclooxygenase-2 Inhibitor, in Familial Adenomatous Polyposis. New Engl. J. Med. 2000, 342, 1946–1952. [Google Scholar] [CrossRef]
- Michelson, D.; Allen, A.J.; Busner, J.; Casat, C.; Dunn, D.; Kratochvil, C.; Newcorn, J.; Sallee, F.R.; Sangal, R.B.; Saylor, K.; et al. Once-Daily Atomoxetine Treatment for Children and Adolescents with Attention Deficit Hyperactivity Disorder: A Randomized, Placebo-Controlled Study. Am. J. Psychiatry 2002, 159, 1896–1901. [Google Scholar] [CrossRef] [PubMed]
- Jost, W.H.; Marsalek, P. Duloxetine: Mechanism of action at the lower urinary tract and Onufs nucleus. Clin. Auton. Res. 2004, 14, 220–227. [Google Scholar] [CrossRef]
- Emery, P.; Fleischmann, R.; Filipowicz-Sosnowska, A.; Schechtman, J.; Szczepanski, L.; Kavanaugh, A.; Racewicz, A.J.; van Vollenhoven, R.F.; Li, N.F.; Agarwal, S.; et al. The efficacy and safety of rituximab in patients with active rheumatoid arthritis despite methotrexate treatment: Results of a phase IIB randomized, double-blind, placebo-controlled, dose-ranging trial. Arthritis Rheum. 2006, 54, 1390–1400. [Google Scholar] [CrossRef] [PubMed]
- Fabian, C.J. Tamoxifen or raloxifene in postmenopausal women for prevention of breast cancer: A tale of two choices-counterpoint. Cancer Epidemiol. Biomar. Prev. 2007, 16, 2210–2212. [Google Scholar] [CrossRef][Green Version]
- Chun, J.; Hartung, H.-P. Mechanism of Action of Oral Fingolimod (FTY720) in Multiple Sclerosis. Clin. Neuropharmacol. 2010, 33, 91–101. [Google Scholar] [CrossRef]
- McMahon, C.G. Dapoxetine: A new option in the medical management of premature ejaculation. Ther. Adv. Urol. 2012, 4, 233–251. [Google Scholar] [CrossRef] [PubMed]
- Garvey, W.T.; Ryan, D.H.; Look, M.; Gadde, K.M.; Allison, D.B.; Peterson, C.A.; Schwiers, M.; Day, W.W.; Bowden, C.H. Two-year sustained weight loss and metabolic benefits with controlled-release phentermine/topiramate in obese and overweight adults (SEQUEL): A randomized, placebo-controlled, phase 3 extension study. Am. J. Clin. Nutr. 2012, 95, 297–308. [Google Scholar] [CrossRef] [PubMed]
- Castinetti, F.; Guignat, L.; Giraud, P.; Muller, M.; Kamenicky, P.; Drui, D.; Bihan, H. Ketoconazole in Cushing’s disease: Is it worth a try? J. Clin. Endocrinol. Metab. 2014, 99, 1623–1630. [Google Scholar] [PubMed]
- Drew, D.A.; Cao, Y.; Chan, A.T. Aspirin and colorectal cancer: The promise of precision chemoprevention. Nat. Rev. Cancer 2016, 16, 173–186. [Google Scholar] [CrossRef] [PubMed]
- Pammolli, F.; Magazzini, L.; Riccaboni, M. The productivity crisis in pharmaceutical R&D. Nat. Rev. Drug Discov. 2011, 10, 428–438. [Google Scholar] [CrossRef] [PubMed]
- Scannell, J.W.; Blanckley, A.; Boldon, H.; Warrington, B. Diagnosing the decline in pharmaceutical R&D efficiency. Nat. Rev. Drug Discov. 2012, 11, 191–200. [Google Scholar] [CrossRef]
- Paul, S.M.; Mytelka, D.S.; Dunwiddie, C.T.; Persinger, C.C.; Munos, B.H.; Lindborg, S.R.; Schacht, A.L. How to improve R&D productivity: The pharmaceutical industry’s grand challenge. Nat. Rev. Drug Discov. 2010, 9, 203–214. [Google Scholar] [CrossRef]
- Moreno, S.G.; Epstein, D. The price of innovation—The role of drug pricing in financing pharmaceutical innovation. A conceptual framework. J. Mark. Access Heal. Policy 2019, 7, 1583536. [Google Scholar] [CrossRef]
- Schuhmacher, A.; Gassmann, O.; Hinder, M. Changing R&D models in research-based pharmaceutical companies. J. Transl. Med. 2016, 14, 1–11. [Google Scholar] [CrossRef]
- Shim, J.S.; Liu, J.O. Recent Advances in Drug Repositioning for the Discovery of New Anticancer Drugs. Int. J. Biol. Sci. 2014, 10, 654–663. [Google Scholar] [CrossRef]
- Bedair, A. Insights into the FDA 2018 new drug approvals. Curr. Drug Discov. Technol. 2019, 16, 1. [Google Scholar] [CrossRef]
- Broder, S. The development of antiretroviral therapy and its impact on the HIV-1/AIDS pandemic. Antiv. Res. 2010, 85, 1–18. [Google Scholar]
- Ostertag, W.; Roesler, G.; Krieg, C.J.; Kind, J.; Cole, T.; Crozier, T.; Gaedicke, G.; Steinheider, G.; Kluge, N.; Dube, S. Induction of Endogenous Virus and of Thymidine Kinase by Bromodeoxyuridine in Cell Cultures Transformed by Friend Virus. Proc. Natl. Acad. Sci. USA 1974, 71, 4980–4985. [Google Scholar] [CrossRef] [PubMed]
- Mitsuya, H.; Weinhold, K.J.; Furman, P.A.; Clair, M.H.S.; Lehrman, S.N.; Gallo, R.C.; Bolognesi, D.; Barry, D.W.; Broder, S. 3’-Azido-3’-deoxythymidine (BW A509U): An antiviral agent that inhibits the infectivity and cytopathic effect of human T-lymphotropic virus type III/lymphadenopathy-associated virus in vitro. Proc. Natl. Acad. Sci. USA 1985, 82, 7096–7100. [Google Scholar] [CrossRef] [PubMed]
- Nanfack, A.J.; Redd, A.D.; Bimela, J.S.; Ncham, G.; Achem, E.; Banin, A.N.; Kirkpatrick, A.R.; Porcella, S.F.; Agyingi, L.A.; Meli, J.; et al. Multimethod Longitudinal HIV Drug Resistance Analysis in Antiretroviral-Therapy-Naive Patients. J. Clin. Microbiol. 2017, 55, 2785–2800. [Google Scholar] [CrossRef] [PubMed]
- Schott, K.; König, R. Picking the Survivor! CRISPR Reveals HIV Dependency Factors. Trends Microbiol. 2017, 25, 243–245. [Google Scholar] [CrossRef]
- Yamashita, M.; Engelman, A.N. Capsid-Dependent Host Factors in HIV-1 Infection. Trends Microbiol. 2017, 25, 741–755. [Google Scholar] [CrossRef]
- Buffalo, C.Z.; Iwamoto, Y.; Hurley, J.H.; Ren, X. How HIV Nef Proteins Hijack Membrane Traffic to Promote Infection. J. Virol. 2019, 93. [Google Scholar] [CrossRef]
- Cohen, O.J.; Kinter, A.; Fauci, A.S. Host factors in the pathogenesis of HIV disease. Immunol. Rev. 1997, 159, 31–48. [Google Scholar] [CrossRef]
- Evans, J.P.; Liu, S.-L. Multifaceted Roles of TIM-Family Proteins in Virus–Host Interactions. Trends Microbiol. 2020, 28, 224–235. [Google Scholar] [CrossRef]
- Fabryova, H.; Strebel, K. Vpr and Its Cellular Interaction Partners: R We There Yet? Cells 2019, 8, 1310. [Google Scholar] [CrossRef]
- Fauci, A.S. Host factors and the pathogenesis of HIV-induced disease. Nat. Cell Biol. 1996, 384, 529–534. [Google Scholar] [CrossRef]
- Friedrich, B.M.; Dziuba, N.; Li, G.; Endsley, M.A.; Murray, J.L.; Ferguson, M.R. Host factors mediating HIV-1 replication. Virus Res. 2011, 161, 101–114. [Google Scholar] [CrossRef] [PubMed]
- Harwig, A.; Landick, R.; Berkhout, B. The Battle of RNA Synthesis: Virus versus Host. Viruses 2017, 9, 309. [Google Scholar] [CrossRef]
- Lama, J.; Planelles, V. Host factors influencing susceptibility to HIV infection and AIDS progression. Retrovirology 2007, 4, 52. [Google Scholar] [CrossRef] [PubMed]
- Rowland-Jones, S.; Pinheiro, S.; Kaul, R. New insights into host factors in HIV-1 pathogenesis. Cell 2001, 104, 473–476. [Google Scholar] [CrossRef] [PubMed]
- Santa-Marta, M.; de Brito, P.M.; Godinho-Santos, A.; Gonçalves, J. Host Factors and HIV-1 Replication: Clinical Evidence and Potential Therapeutic Approaches. Front. Immunol. 2013, 4, 343. [Google Scholar] [CrossRef] [PubMed]
- Yue, L.; Prentice, H.A.; Farmer, P.; Song, W.; He, D.; Lakhi, S.; Goepfert, P.; Gilmour, J.; Allen, S.; Tang, J.; et al. Cumulative Impact of Host and Viral Factors on HIV-1 Viral-Load Control during Early Infection. J. Virol. 2012, 87, 708–715. [Google Scholar] [CrossRef] [PubMed]
- Asiimwe, F.; Moore, D.M.; Were, W.; Nakityo, R.; Campbell, J.; Barasa, A.; Kaharuza, F. Clinical outcomes of HIV-infected patients with Kaposi’s sarcoma receiving nonnucleoside reverse transcriptase inhibitor-based antiretroviral therapy in Uganda. HIV Med. 2012, 13, 166–171. [Google Scholar] [PubMed]
- Chemaly, R.F.; Hill, J.A.; Voigt, S.; Peggs, K.S. In vitro comparison of currently available and investigational antiviral agents against pathogenic human double-stranded DNA viruses: A systematic literature review. Antivir. Res. 2019, 163, 50–58. [Google Scholar] [CrossRef]
- Hadaczek, P.; Ozawa, T.; Soroceanu, L.; Yoshida, Y.; Matlaf, L.; Singer, E.; Fiallos, E.; James, C.D.; Cobbs, C.S. Cidofovir: A Novel Antitumor Agent for Glioblastoma. Clin. Cancer Res. 2013, 19, 6473–6483. [Google Scholar] [CrossRef]
- Neyts, J.; Sadler, R.; de Clercq, E.; Raab-Traub, N.; Pagano, J.S. The antiviral agent cidofovir [(S)-1-(3-hydroxy-2-phosphonyl-methoxypropyl)cytosine] has pronounced activity against nasopharyngeal carcinoma grown in nude mice. Cancer Res. 1998, 58, 384–388. [Google Scholar]
- Hecht, M.; Erber, S.; Harrer, T.; Klinker, H.; Roth, T.; Parsch, H.; Fiebig, N.; Fietkau, R.; Distel, L.V. Efavirenz Has the Highest Anti-Proliferative Effect of Non-Nucleoside Reverse Transcriptase Inhibitors against Pancreatic Cancer Cells. PLoS ONE 2015, 10, e0130277. [Google Scholar] [CrossRef]
- Sariyer, I.K.; Gordon, J.; Burdo, T.H.; Wollebo, H.S.; Gianti, E.; Donadoni, M.; Bellizzi, A.; Cicalese, S.; Loomis, R.; Robinson, J.A.; et al. Suppression of Zika Virus Infection in the Brain by the Antiretroviral Drug Rilpivirine. Mol. Ther. 2019, 27, 2067–2079. [Google Scholar] [CrossRef]
- Dunn, L.A.; Andrews, K.T.; McCarthy, J.S.; Wright, J.M.; Skinner-Adams, T.S.; Upcroft, P.; Upcroft, J.A. The activity of protease inhibitors against Giardia duodenalis and metronidazole-resistant Trichomonas vaginalis. Int. J. Antimicrob. Agents 2007, 29, 98–102. [Google Scholar] [CrossRef] [PubMed]
- Andrews, K.T.; Fairlie, D.P.; Madala, P.K.; Ray, J.; Wyatt, D.M.; Hilton, P.M.; Melville, L.A.; Beattie, L.; Gardiner, D.L.; Reid, R.C.; et al. Potencies of Human Immunodeficiency Virus Protease Inhibitors In Vitro against Plasmodium falciparum and In Vivo against Murine Malaria. Antimicrob. Agents Chemother. 2006, 50, 639–648. [Google Scholar] [CrossRef] [PubMed]
- Doyle, P.S.; Zhou, Y.M.; Engel, J.C.; McKerrow, J.H. A Cysteine Protease Inhibitor Cures Chagas’ Disease in an Immunodeficient-Mouse Model of Infection. Antimicrob. Agents Chemother. 2007, 51, 3932–3939. [Google Scholar] [CrossRef] [PubMed]
- Zumla, A.; Chan, J.F.W.; Azhar, E.I.; Hui, D.S.C.; Yuen, K.-Y. Coronaviruses—Drug discovery and therapeutic options. Nat. Rev. Drug Discov. 2016, 15, 327–347. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Cao, R.; Zhang, L.; Yang, X.; Liu, J.; Xu, M.; Shi, Z.; Hu, Z.; Zhong, W.; Xiao, G. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020, 30, 269–271. [Google Scholar] [CrossRef]
- de Wit, E.; Feldmann, F.; Cronin, J.; Jordan, R.; Okumura, A.; Thomas, T.; Scott, D.; Cihlar, T.; Feldmann, H. Prophylactic and therapeutic remdesivir (GS-5734) treatment in the rhesus macaque model of MERS-CoV infection. Proc. Natl. Acad. Sci. USA 2020, 117, 6771–6776. [Google Scholar] [CrossRef]
- Gordon, C.J.; Tchesnokov, E.P.; Feng, J.Y.; Porter, D.P.; Götte, M. The antiviral compound remdesivir potently inhibits RNA-dependent RNA polymerase from Middle East respiratory syndrome coronavirus. J. Biol. Chem. 2020, 295, 4773–4779. [Google Scholar] [CrossRef]
- Tchesnokov, E.P.; Feng, J.Y.; Porter, D.P.; Götte, M. Mechanism of Inhibition of Ebola Virus RNA-Dependent RNA Polymerase by Remdesivir. Viruses 2019, 11, 326. [Google Scholar] [CrossRef]
- Liu, J.; Cao, R.; Xu, M.; Wang, X.; Zhang, H.; Hu, H.; Li, Y.; Hu, Z.; Zhong, W.; Wang, M. Hydroxychloroquine, a less toxic derivative of chloroquine, is effective in inhibiting SARS-CoV-2 infection in vitro. Cell Discov. 2020, 6, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Yao, X.; Ye, F.; Zhang, M.; Cui, C.; Huang, B.; Niu, P.; Liu, X.; Zhao, L.; Dong, E.; Song, C.; et al. In Vitro Antiviral Activity and Projection of Optimized Dosing Design of Hydroxychloroquine for the Treatment of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2). Clin. Infect. Dis. 2020, 71, 732–739. [Google Scholar] [CrossRef] [PubMed]
- Cao, B.; Wang, Y.; Wen, D.; Liu, W.; Wang, J.; Fan, G.; Ruan, L.; Song, B.; Cai, Y.; Wei, M.; et al. A Trial of Lopinavir–Ritonavir in Adults Hospitalized with Severe Covid-19. N. Engl. J. Med. 2020, 382, 1787–1799. [Google Scholar] [CrossRef] [PubMed]
- ElFiky, A.A. Anti-HCV, nucleotide inhibitors, repurposing against COVID-19. Life Sci. 2020, 248, 117477. [Google Scholar] [CrossRef]
- Kumar, S.; Zhi, K.; Mukherjee, A.; Gerth, K. Repurposing Antiviral Protease Inhibitors Using Extracellular Vesicles for Potential Therapy of COVID-19. Viruses 2020, 12, 486. [Google Scholar] [CrossRef]
- Zhang, W.; Zhao, Y.; Zhang, F.; Wang, Q.; Li, T.; Liu, Z.; Wang, J.; Qin, Y.; Zhang, X.; Yan, X.; et al. The use of anti-inflammatory drugs in the treatment of people with severe coronavirus disease 2019 (COVID-19): The Perspectives of clinical immunologists from China. Clin. Immunol. 2020, 214, 108393. [Google Scholar] [CrossRef]
- Sargiacomo, C.; Sotgia, F.; Lisanti, M.P. COVID-19 and chronological aging: Senolytics and other anti-aging drugs for the treatment or prevention of corona virus infection? Aging 2020, 12, 6511–6517. [Google Scholar] [CrossRef]
- Keyaerts, E.; Vijgen, L.; Maes, P.; Neyts, J.; van Ranst, M. In vitro inhibition of severe acute respiratory syndrome coronavirus by chloroquine. Biochem. Biophys. Res. Commun. 2004, 323, 264–268. [Google Scholar] [CrossRef]
- Mehra, M.R.; Desai, S.S.; Ruschitzka, F.; Patel, A.N. Hydroxychloroquine or chloroquine with or without a macrolide for treatment of COVID-19: A multinational registry analysis. Lancet 2020, S0140. [Google Scholar]
- Boulware, D.R.; Pullen, M.F.; Bangdiwala, A.S.; Pastick, K.A.; Lofgren, S.M.; Okafor, E.C.; Skipper, C.P.; Nascene, A.A.; Nicol, M.R.; Abassi, M.; et al. A Randomized Trial of Hydroxychloroquine as Postexposure Prophylaxis for Covid-19. New Engl. J. Med. 2020, 383, 517–525. [Google Scholar] [CrossRef]
- Geleris, J.; Sun, Y.; Platt, J.; Zucker, J.; Baldwin, M.; Hripcsak, G.; Labella, A.; Manson, D.K.; Kubin, C.; Barr, R.G.; et al. Observational Study of Hydroxychloroquine in Hospitalized Patients with Covid-19. N. Engl. J. Med. 2020, 382, 2411–2418. [Google Scholar] [CrossRef] [PubMed]
- Clausen, T.M.; Sandoval, D.R.; Spliid, C.B.; Pihl, J.; Painter, C.D.; Thacker, B.E.; Glass, C.A.; Narayanan, A.; Majowicz, S.A.; Zhang, Y.; et al. SARS-CoV-2 Infection Depends on Cellular Heparan Sulfate and ACE2. Cell 2020. [Google Scholar] [CrossRef]
- Zhang, Q.; Chen, C.Z.; Swaroop, M.; Xu, M.; Wang, L.; Lee, J.; Pradhan, M.; Shen, M.; Luo, Z.; Xu, Y.; et al. Targeting heparan sulfate proteoglycan-assisted endocytosis as a COVID-19 therapeutic option. bioRxiv 2020. [Google Scholar] [CrossRef]
- Chang, R.; Ng, T.B.; Sun, W.-Z. Lactoferrin as potential preventative and adjunct treatment for COVID-19. Int. J. Antimicrob. Agents 2020, 56, 106118. [Google Scholar] [CrossRef] [PubMed]
- Biancatelli, R.M.L.C.; Berrill, M.; Catravas, J.D.; Marik, P.E. Quercetin and Vitamin C: An Experimental, Synergistic Therapy for the Prevention and Treatment of SARS-CoV-2 Related Disease (COVID-19). Front. Immunol. 2020, 11, 1451. [Google Scholar] [CrossRef]
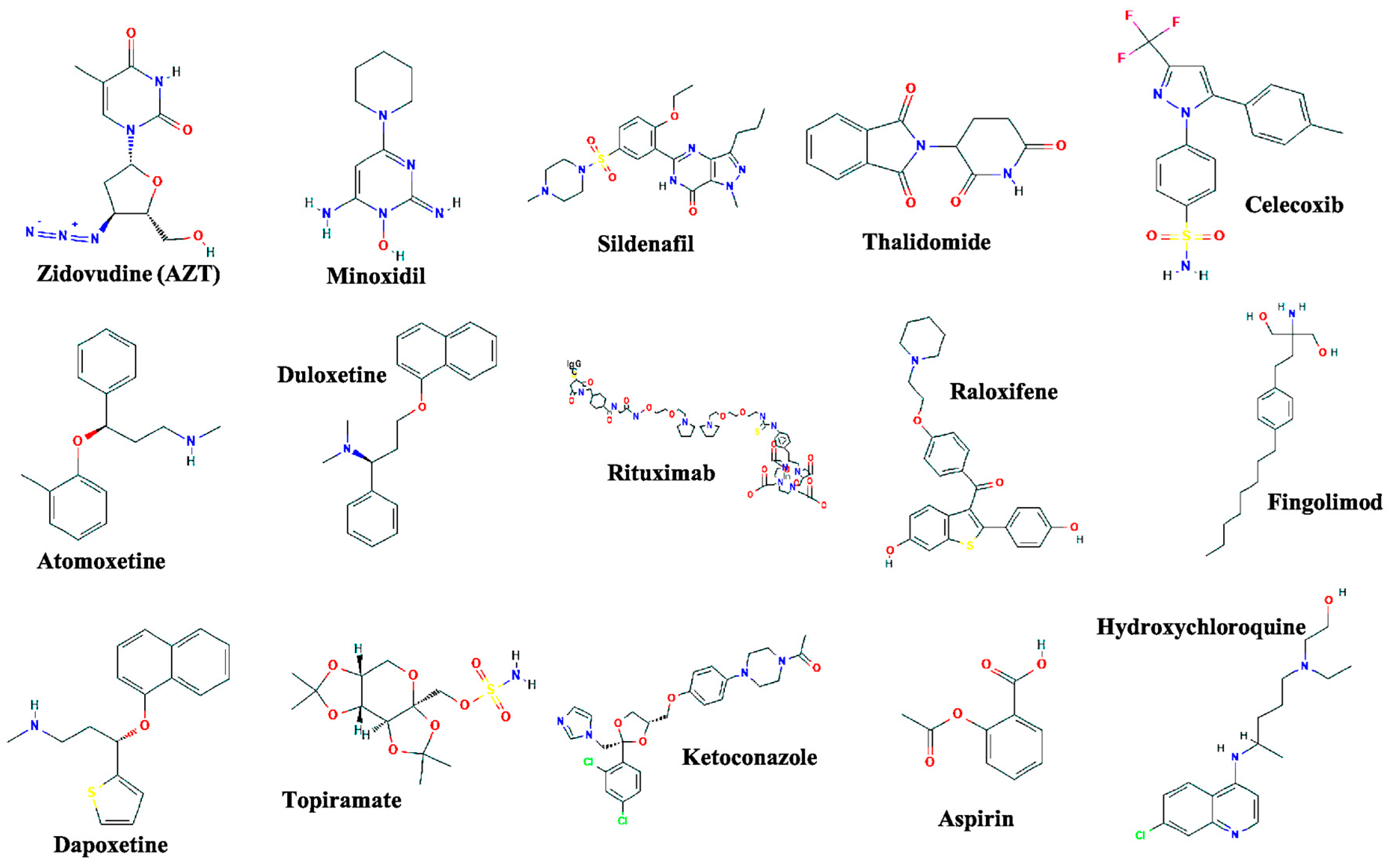
| Drug Name | Original Indication | New Indication | Year of Approval |
|---|---|---|---|
| Zidovudine | Cancer | AIDS | 1987 [8] |
| Minoxidil | Hypertension | Hair loss | 1988 [9] |
| Sildenafil | Angina | Erectile dysfunction | 1998 [10] |
| Thalidomide | Morning sickness | Erythema nodosum leprosum and multiple myeloma | 1998 [11] and 2006 [12] |
| Celecoxib | Pain and inflammation | Familial adenomatous polyps | 2000 [13] |
| Atomoxetine | Parkinson disease | ADHD | 2002 [14] |
| Duloxetine | Depression | SUI | 2004 [15] |
| Rituximab | Various cancers | Rheumatoid arthritis | 2006 [16] |
| Raloxifene | Osteoporosis | Breast cancer | 2007 [17] |
| Fingolimod | Transplant rejection | MS | 2010 [18] |
| Dapoxetine | Analgesia and depression | Premature ejaculation | 2012 [19] |
| Topiramate | Epilepsy | Obesity | 2012 [20] |
| Ketoconazole | Fungal infections | Cushing syndrome | 2014 [21] |
| Aspirin | Analgesia | Colorectal cancer | 2015 [22] |
| Approach | Advantages | Disadvantages |
|---|---|---|
| Activity-based approach | No limitation for in vitro cell-based as well as cell-free target-based screening assays | Time and labor-consuming and required highly skilled individuals |
| Easy to validate screening hits | Requires a large collection of existing drugs | |
| Reduced chances of false-positive hits during the screening | Requires the development and optimization of efficient screening assays | |
| Molecules with activities due to primary and secondary metabolites are also obtained | ||
| In silico approach | Not time and labor efficient | Requires detailed structural insight of target proteins both in normal as well as diseased conditions |
| No need for an entire collection of existing drugs | Increased rates of false-positive hits during the screening | |
| No need to develop a screening assay |
| Therapeutic Intervention | Class of the Drug/s | Clinical Condition/s of the Participants of the Trial | Trial Identification Number * | Phase |
|---|---|---|---|---|
| Hydroxychloroquine | Antimalarial and amebicide | 30 patients suffering from pneumonia due to COVID-19 | NCT04261517 | 3 |
| Chloroquine | Antimalarial and amebicide | 10,000 patients in a prophylaxis study for COVID-19 | NCT04303507 | N/A |
| Human immunoglobulin | Antibody | 80 patients suffering from pneumonia due to COVID-19 | NCT04261426 | 2 and 3 |
| Remdesivir | Nucleotide reverse transcriptase inhibitor | 452 patients suffering from a severe respiratory infection due to COVID-19 | NCT04257656 | 3 |
| Remdesivir | Nucleotide reverse transcriptase inhibitor | 308 patients with mild or moderate respiratory tract infection caused by COVID-19 | NCT04252664 | 3 |
| Arbidol (umifenovir) | Virus entry (Fusion) inhibitor | 380 patients suffering from Pneumonia caused by COVID-19 | NCT04260594 | 4 |
| Arbidol or lopinavir-ritonavir or oseltamivir | Combination of virus entry (Fusion) inhibitor and protease inhibitor | 400 patients infected with COVID-19 | NCT04255017 | 4 |
| Arbidol or lopinavir + ritonavir | Combination of virus entry (Fusion) inhibitor and protease inhibitor | 125 patients infected with COVID-19 | NCT04252885 | 4 |
| Darunavir + cobicistat | Protease inhibitor (Darunavir) in combination with Booster (cobicistat, a CYP3A inhibitor) | 30 patients suffering from Pneumonia caused by COVID-19 | NCT04252274 | 3 |
| TCM combination with lopinavir + ritonavir, α-interferon via aerosol | Cytokine in combination with protease inhibitor | 150 patients infected with COVID-19 | NCT04251871 | N/A |
| Recombinant human interferon α2β | Cytokine | 328 patients infected with COVID-19 | NCT04293887 | 1 |
| Carrimycin or lopinavir + ritonavir or arbidol or chloroquine phosphate | Antibiotic in combination with booster (arbidol) or antimalarial/ amebicide | 520 patients infected with COVID-19 | NCT04286503 | 4 |
| Danoprevir + ritonavir + interferon inhalation or lopinavir + ritonavir or TCM plus interferon inhalation | Protease inhibitors with cytokine as aerosol | 50 patients suffering from pneumonia caused by COVID-19 | NCT04291729 | 4 |
| Xiyanping or lopinavir-ritonavir-interferon inhalation | Anti-inflammatory (Xiyanping) or Protease inhibitors with cytokine | 384 patients with pneumonia caused by COVID-19 | NCT04275388 | N/A |
| Xiyanping combined with lopinavir + ritonavir | Anti-inflammatory (Xiyanping) in combination with Protease inhibitors | 80 patients infected with COVID-19 | NCT04295551 | N/A |
| Combinations of oseltamivir, favipiravir, and chloroquine | Neuraminidase (Oseltamivir) in combination with antimalarial/ amebicide | 80 patients infected with COVID-19 | NCT04303299 | 3 |
| Thalidomide | Angiogenesis inhibitor and immunomodulator | 40 patients infected with COVID-19 | NCT04273581 | 2 |
| Thalidomide | Angiogenesis inhibitor and immunomodulator | 100 patients suffering from pneumonia caused by COVID-19 | NCT04273529 | 2 |
| Vitamin C | Vitamin (Ascorbic acis) | 140 patients with severe pneumonia caused by COVID-19 | NCT04264533 | 2 |
| Methylprednisolone | Corticosteroid | 80 patients infected with COVID-19 | NCT04244591 | 2 |
| Pirfenidone | Pyridone | 294 patients with severe pneumonia caused by COVID-19 | NCT04282902 | 3 |
| Bromhexine hydrochloride | Mucolytics | 60 patients with suspected and mild pneumonia caused by COVID-19 | NCT04273763 | N/A |
| Bevacizumab | Monoclonal antibody | 20 patients with COVID-19 associated with severe pneumonia | NCT04275414 | 2 and 3 |
| Fingolimod | Sphingosine 1-phosphate receptor modulator | 30 patients infected with COVID-19 | NCT04280588 | N/A |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Trivedi, J.; Mohan, M.; Byrareddy, S.N. Drug Repurposing Approaches to Combating Viral Infections. J. Clin. Med. 2020, 9, 3777. https://doi.org/10.3390/jcm9113777
Trivedi J, Mohan M, Byrareddy SN. Drug Repurposing Approaches to Combating Viral Infections. Journal of Clinical Medicine. 2020; 9(11):3777. https://doi.org/10.3390/jcm9113777
Chicago/Turabian StyleTrivedi, Jay, Mahesh Mohan, and Siddappa N. Byrareddy. 2020. "Drug Repurposing Approaches to Combating Viral Infections" Journal of Clinical Medicine 9, no. 11: 3777. https://doi.org/10.3390/jcm9113777
APA StyleTrivedi, J., Mohan, M., & Byrareddy, S. N. (2020). Drug Repurposing Approaches to Combating Viral Infections. Journal of Clinical Medicine, 9(11), 3777. https://doi.org/10.3390/jcm9113777





