Reticulated Platelets in Medicine: Current Evidence and Further Perspectives
Abstract
1. Introduction
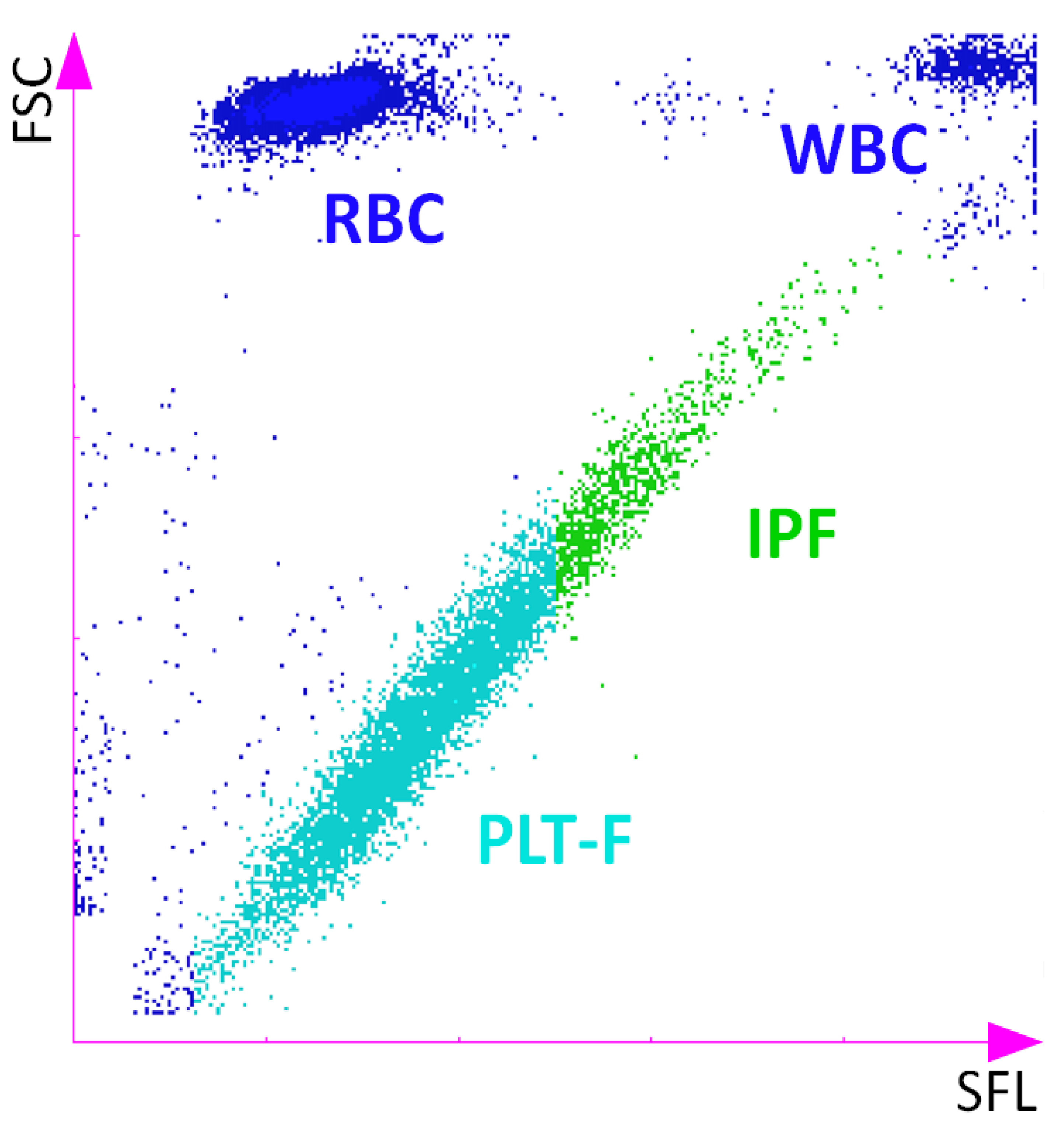
2. Detection and Quantification of Reticulated Platelets
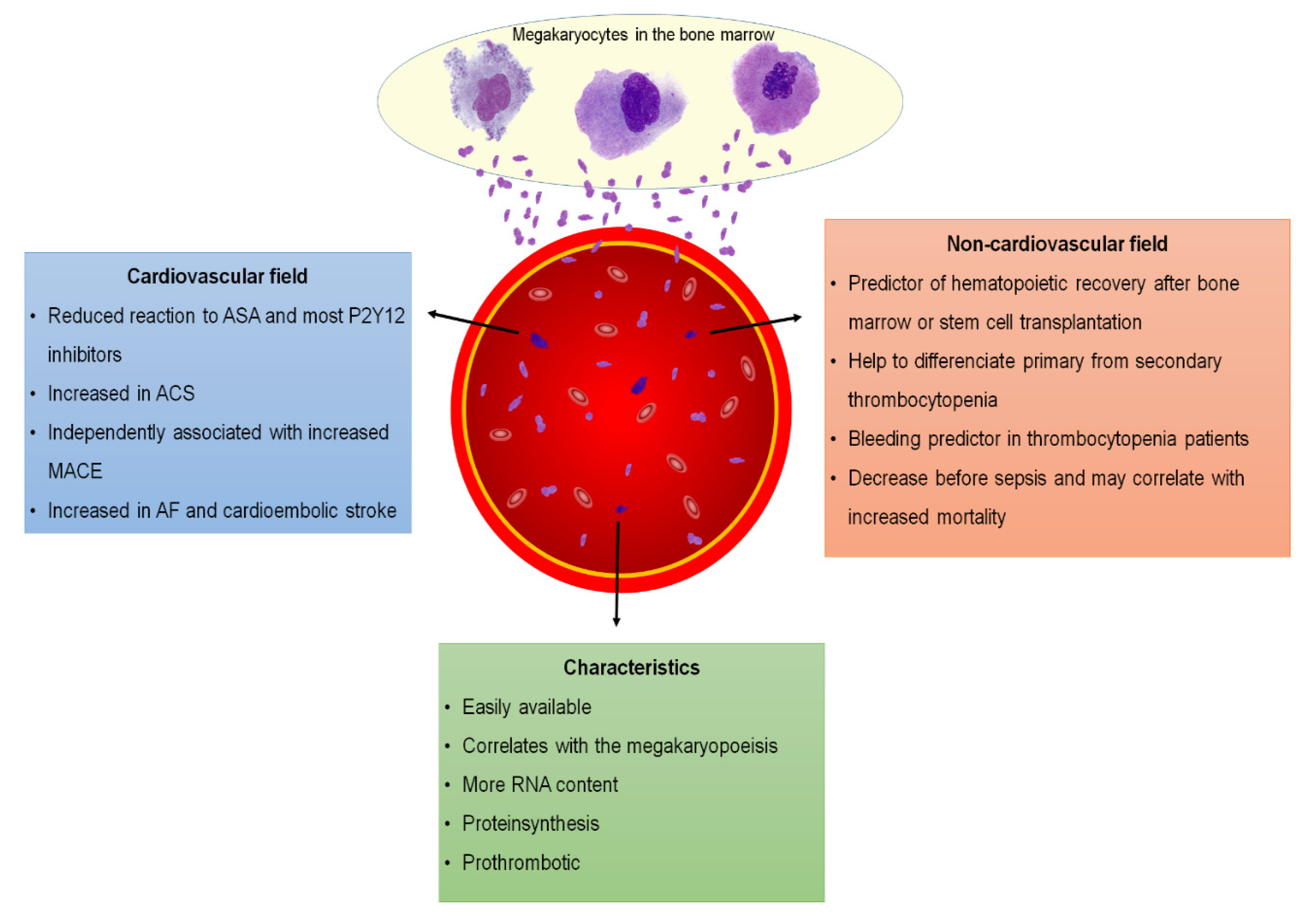
3. Immature Platelets and Noncardiovascular Diseases
4. Immature Platelets and Cardiovascular Disease
5. Future Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ault, K.A.; Rinder, H.M.; Mitchell, J.; Carmody, M.B.; Vary, C.P.H.; Hillman, R.S. The significance of platelets with increased RNA content (reticulated platelets). A measure of the rate of thrombopoiesis. Am. J. Clin. Pathol. 1992, 98, 637–646. [Google Scholar] [CrossRef] [PubMed]
- Ingram, M.; Coopersmith, A. Reticulated Platelets following Acute Blood Loss. Br. J. Haematol. 1969, 17, 225–229. [Google Scholar] [CrossRef] [PubMed]
- Bernlochner, I.; Goedel, A.; Plischke, C.; Schüpke, S.; Haller, B.; Schulz, C.; Mayer, K.; Morath, T.; Braun, S.; Schunkert, H.; et al. Impact of immature platelets on platelet response to ticagrelor and prasugrel in patients with acute coronary syndrome. Eur. Hear. J. 2015, 36, 3202–3210. [Google Scholar] [CrossRef] [PubMed]
- Guthikonda, S.; Lev, E.I.; Patel, R.; Delao, T.; Bergeron, A.L.; Dong, J.-F.; Kleiman, N.S. Reticulated platelets and uninhibited COX-1 and COX-2 decrease the antiplatelet effects of aspirin. J. Thromb. Haemost. 2007, 5, 490–496. [Google Scholar] [CrossRef] [PubMed]
- Harrison, P.; Goodall, A.H. “Message in the platelet”—More than just vestigial mRNA! Platelets 2008, 19, 395–404. [Google Scholar] [CrossRef] [PubMed]
- Robinson, M.; Machin, S.; Mackie, I.; Harrison, P. In vivo biotinylation studies: Specificity of labelling of reticulated platelets by thiazole orange and mepacrine. Br. J. Haematol. 2000, 108, 859–864. [Google Scholar] [CrossRef]
- Deutsch, V.; Tomer, A. Megakaryocyte development and platelet production. Br. J. Haematol. 2006, 134, 453–466. [Google Scholar] [CrossRef]
- Ault, K.A.; Knowles, C. In vivo biotinylation demonstrates that reticulated platelets are the youngest platelets in circulation. Exp. Hematol. 1995, 23, 996–1001. [Google Scholar]
- Hoffmann, J.J.M.L. Reticulated platelets: Analytical aspects and clinical utility. Clin. Chem. Lab. Med. 2014, 52, 1107–1117. [Google Scholar] [CrossRef]
- Kienast, J.; Schmitz, G. Flow cytometric analysis of thiazole orange uptake by platelets: A diagnostic aid in the evaluation of thrombocytopenic disorders. Blood 1990, 75, 116–121. [Google Scholar] [CrossRef]
- Dale, G.; Friese, P.; Hynes, L.; Burstein, S. Demonstration that thiazole-orange-positive platelets in the dog are less than 24 hours old. Blood 1995, 85, 1822–1825. [Google Scholar] [CrossRef] [PubMed]
- Buttarello, M.; Mezzapelle, G.; Freguglia, F.; Plebani, M. Reticulated platelets and immature platelet fraction: Clinical applications and method limitations. Int. J. Lab. Hematol. 2020, 42, 363–370. [Google Scholar] [CrossRef] [PubMed]
- Hannawi, B.; Hannawi, Y.; Kleiman, N.S. Reticulated Platelets: Changing Focus from Basics to Outcomes. Thromb. Haemost. 2018, 118, 1517–1527. [Google Scholar] [CrossRef] [PubMed]
- Stratz, C.; Bömicke, T.; Younas, I.; Kittel, A.; Amann, M.; Valina, C.M.; Nührenberg, T.; Trenk, D.; Neumann, F.-J.; Hochholzer, W. Comparison of Immature Platelet Count to Established Predictors of Platelet Reactivity During Thienopyridine Therapy. J. Am. Coll. Cardiol. 2016, 68, 286–293. [Google Scholar] [CrossRef] [PubMed]
- Kurahashi, H.; Takami, A.; Murotani, K.; Numoto, S.; Okumura, A. Decreased platelet count in children with epilepsy treated with valproate and its relationship to the immature platelet fraction. Int. J. Hematol. 2017, 107, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Xu, K.; Chan, N.C.; Hirsh, J.; Ginsberg, J.S.; Bhagirath, V.; Krüger, P.; Dale, B.J.; Crowther, M.; Whitlock, R.P.; Li, C.; et al. Quantifying immature platelets as markers of increased platelet production after coronary artery bypass grafting surgery. Eur. J. Haematol. 2018, 101, 362–367. [Google Scholar] [CrossRef] [PubMed]
- Cohen, A.; Harari, E.; Cipok, M.; Laish-Farkash, A.; Bryk, G.; Yahud, E.; Sela, Y.; Lador, N.K.; Mann, T.; Mayo, A.; et al. Immature platelets in patients hospitalized with Covid-19. J. Thromb. Thrombolysis 2020, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Hille, L.; Cederqvist, M.; Hromek, J.; Stratz, C.; Trenk, D.; Nührenberg, T.G. Evaluation of an Alternative Staining Method Using SYTO 13 to Determine Reticulated Platelets. Thromb. Haemost. 2019, 119, 779–785. [Google Scholar] [CrossRef]
- Bongiovanni, D.; Santamaria, G.; Klug, M.; Santovito, D.; Felicetta, A.; Hristov, M.; Von Scheidt, M.; Aslani, M.; Cibella, J.; Weber, C.; et al. Transcriptome Analysis of Reticulated Platelets Reveals a Prothrombotic Profile. Thromb. Haemost. 2019, 119, 1795–1806. [Google Scholar] [CrossRef]
- Strauss, G.; Vollert, C.; Von Stackelberg, A.; Weimann, A.; Gaedicke, G.; Schulze, H. Immature platelet count: A simple parameter for distinguishing thrombocytopenia in pediatric acute lymphocytic leukemia from immune thrombocytopenia. Pediatr. Blood Cancer 2011, 57, 641–647. [Google Scholar] [CrossRef]
- Hille, L.; Lenz, M.; Vlachos, A.; Grüning, B.; Hein, L.; Neumann, F.; Nührenberg, T.G.; Trenk, D. Ultrastructural, transcriptional, and functional differences between human reticulated and non-reticulated platelets. J. Thromb. Haemost. 2020, 18, 2034–2046. [Google Scholar] [CrossRef] [PubMed]
- Briggs, C.; Kunka, S.; Hart, D.; Oguni, S.; Machin, S.J. Assessment of an immature platelet fraction (IPF) in peripheral thrombocytopenia. Br. J. Haematol. 2004, 126, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Sakuragi, M.; Hayashi, S.; Maruyama, M.; Kabutomori, O.; Kiyokawa, T.; Nagamine, K.; Kato, H.; Kashiwagi, H.; Kanakura, Y.; Tomiyama, Y. Clinical significance of IPF% or RP% measurement in distinguishing primary immune thrombocytopenia from aplastic thrombocytopenic disorders. Int. J. Hematol. 2015, 101, 369–375. [Google Scholar] [CrossRef] [PubMed]
- Jung, H.; Jeon, H.-K.; Kim, H.-J.; Kim, S.-H. Immature Platelet Fraction: Establishment of a Reference Interval and Diagnostic Measure for Thrombocytopenia. Ann. Lab. Med. 2010, 30, 451–459. [Google Scholar] [CrossRef]
- McDonnell, A.; Bride, K.L.; Lim, D.; Paessler, M.; Witmer, C.M.; Lambert, M.P. Utility of the immature platelet fraction in pediatric immune thrombocytopenia: Differentiating from bone marrow failure and predicting bleeding risk. Pediatr. Blood Cancer 2018, 65, e26812. [Google Scholar] [CrossRef]
- Estcourt, L.; Stanworth, S.; Harrison, P.; Powter, G.; McClure, M.; Murphy, M.F.; Mumford, A.D. Prospective observational cohort study of the association between thromboelastometry, coagulation and platelet parameters and bleeding in patients with haematological malignancies—The ATHENA study. Br. J. Haematol. 2014, 166, 581–591. [Google Scholar] [CrossRef]
- El-Gamal, R.A.; Mekawy, M.A.; Abd Elkader, A.M.; Abdelbary, H.M.; Fayek, M.Z. Combined Immature Platelet Fraction and Schistocyte Count to Differentiate Pregnancy-Associated Thrombotic Thrombocytopenic Purpura from Severe Preeclampsia/Haemolysis, Elevated Liver Enzymes, and Low Platelet Syndrome (SPE/HELLP). Indian J. Hematol. Blood Transfus. 2020, 36, 316–323. [Google Scholar] [CrossRef]
- Zucker, M.; Murphy, C.; Rachel, J.; Martinez, G.; Abhyankar, S.; McGuirk, J.; Reid, K.; Plapp, F. Immature Platelet Fraction as a Predictor of Platelet Recovery Following Hematopoietic Progenitor Cell Transplantation. Lab. Hematol. 2006, 12, 125–130. [Google Scholar] [CrossRef]
- Takami, A.; Shibayama, M.; Orito, M.; Omote, M.; Okumura, H.; Yamashita, T.; Shimadoi, S.; Yoshida, T.; Nakao, S.; Asakura, H. Immature platelet fraction for prediction of platelet engraftment after allogeneic stem cell transplantation. Bone Marrow Transplant. 2007, 39, 501–507. [Google Scholar] [CrossRef]
- Van Der Linden, N.; Klinkenberg, L.J.J.; Meex, S.J.R.; Beckers, E.A.M.; De Wit, N.C.J.; Prinzen, L. Immature platelet fraction measured on the S ysmex XN hemocytometer predicts thrombopoietic recovery after autologous stem cell transplantation. Eur. J. Haematol. 2014, 93, 150–156. [Google Scholar] [CrossRef]
- Parco, S.; Vascotto, F. Application of reticulated platelets to transfusion management during autologous stem cell transplantation. OncoTargets Ther. 2012, 5, 1–5. [Google Scholar] [CrossRef] [PubMed]
- De Blasi, R.A.; Cardelli, P.; Costante, A.; Sandri, M.; Mercieri, M.; Arcioni, R. Immature platelet fraction in predicting sepsis in critically ill patients. Intensive Care Med. 2013, 39, 636–643. [Google Scholar] [CrossRef] [PubMed]
- Muronoi, T.; Koyama, K.; Nunomiya, S.; Lefor, A.K.; Wada, M.; Koinuma, T.; Shima, J.; Suzukawa, M. Immature platelet fraction predicts coagulopathy-related platelet consumption and mortality in patients with sepsis. Thromb. Res. 2016, 144, 169–175. [Google Scholar] [CrossRef] [PubMed]
- Hubert, R.M.E.; Rodrigues, M.V.; Andreguetto, B.D.; Santos, T.M.; Gilberti, M.D.F.P.; De Castro, V.; Annichino-Bizzacchi, J.M.; Dragosavac, D.; Carvalho-Filho, M.A.; De Paula, E.V. Association of the immature platelet fraction with sepsis diagnosis and severity. Sci. Rep. 2015, 5, 8019. [Google Scholar] [CrossRef] [PubMed]
- Koyama, K.; Katayama, S.; Muronoi, T.; Tonai, K.; Goto, Y.; Koinuma, T.; Shima, J.; Nunomiya, S. Time course of immature platelet count and its relation to thrombocytopenia and mortality in patients with sepsis. PLoS ONE 2018, 13, e0192064. [Google Scholar] [CrossRef] [PubMed]
- Thorup, C.V.; Christensen, S.; Hvas, A.M. Immature Platelets as a Predictor of Disease Severity and Mortality in Sepsis and Septic Shock: A Systematic Review. Semin. Thromb. Hemost. 2020, 46, 320–327. [Google Scholar] [CrossRef] [PubMed]
- Sugimori, N.; Kondo, Y.; Shibayama, M.; Omote, M.; Takami, A.; Sugimori, C.; Ishiyama, K.; Yamazaki, H.; Nakao, S. Aberrant increase in the immature platelet fraction in patients with myelodysplastic syndrome: A marker of karyotypic abnormalities associated with poor prognosis. Eur. J. Haematol. 2009, 82, 54–60. [Google Scholar] [CrossRef]
- Jeon, M.J.; Yu, E.S.; Kang, K.-W.; Lee, B.-H.; Park, Y.; Lee, S.R.; Sung, H.J.; Yoon, S.Y.; Choi, C.W.; Kim, B.S.; et al. Immature platelet fraction based diagnostic predictive scoring model for immune thrombocytopenia. Korean J. Intern. Med. 2020, 35, 970–978. [Google Scholar] [CrossRef]
- Lakkis, N.; Dokainish, H.; Abuzahra, M.; Tsyboulev, V.; Jorgensen, J.; De Leon, A.P.; Saleem, A. Reticulated platelets in acute coronary syndrome: A marker of platelet activity. J. Am. Coll. Cardiol. 2004, 44, 2091–2093. [Google Scholar] [CrossRef]
- Hochholzer, W.; Ruff, C.T.; Mesa, R.A.; Mattimore, J.F.; Cyr, J.F.; Lei, L.; Frelinger, A.L.; Michelson, A.D.; Berg, D.D.; Angiolillo, D.J.; et al. Variability of Individual Platelet Reactivity Over Time in Patients Treated With Clopidogrel. J. Am. Coll. Cardiol. 2014, 64, 361–368. [Google Scholar] [CrossRef]
- Chen, W.-H.; Lee, P.-Y.; Ng, W.; Tse, H.-F.; Lau, C.-P. Aspirin resistance is associated with a high incidence of myonecrosis after non-urgent percutaneous coronary intervention despite clopidogrel pretreatment. J. Am. Coll. Cardiol. 2004, 43, 1122–1126. [Google Scholar] [CrossRef] [PubMed]
- Lev, E.I.; Patel, R.T.; Maresh, K.J.; Guthikonda, S.; Granada, J.; Delao, T.; Bray, P.F.; Kleiman, N.S. Aspirin and Clopidogrel Drug Response in Patients Undergoing Percutaneous Coronary Intervention. J. Am. Coll. Cardiol. 2006, 47, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Guthikonda, S.; Alviar, C.L.; Vaduganathan, M.; Arikan, M.; Tellez, A.; Delao, T.; Granada, J.F.; Dong, J.-F.; Kleiman, N.S.; Lev, E.I. Role of Reticulated Platelets and Platelet Size Heterogeneity on Platelet Activity after Dual Antiplatelet Therapy with Aspirin and Clopidogrel in Patients with Stable Coronary Artery Disease. J. Am. Coll. Cardiol. 2008, 52, 743–749. [Google Scholar] [CrossRef] [PubMed]
- Perl, L.; Lerman-Shivek, H.; Rechavia, E.; Vaduganathan, M.; Leshem-Lev, D.; Zemer-Wassercug, N.; Dadush, O.; Codner, P.; Bental, T.; Battler, A.; et al. Response to Prasugrel and Levels of Circulating Reticulated Platelets in Patients With ST-Segment Elevation Myocardial Infarction. J. Am. Coll. Cardiol. 2014, 63, 513–517. [Google Scholar] [CrossRef]
- Marcucci, R.; Caporale, R.; Paniccia, R.; Romano, E.; Gensini, G.F.; Abbate, R.; Gori, A.M.; Cesari, F. Relationship between high platelet turnover and platelet function in high-risk patients with coronary artery disease on dual antiplatelet therapy. Thromb. Haemost. 2008, 99, 930–935. [Google Scholar] [CrossRef]
- Nührenberg, T.; Amann, M.; Cederqvist, M.; Kleiner, P.; Valina, C.M.; Trenk, D.; Neumann, F.-J.; Hochholzer, W.; Stratz, C. Impact of reticulated platelets on antiplatelet response to thienopyridines is independent of platelet turnover. Thromb. Haemost. 2016, 116, 941–948. [Google Scholar] [CrossRef]
- Stratz, C.; Nührenberg, T.; Valina, C.M.; Löffelhardt, N.; Mashayekhi, K.; Ferenc, M.; Trenk, D.; Neumann, F.-J.; Hochholzer, W. Impact of Reticulated Platelets on the Antiplatelet Effect of the Intravenous P2Y12-Receptor Inhibitor Cangrelor. Thromb. Haemost. 2018, 118, 362–368. [Google Scholar] [CrossRef]
- Verdoia, M.; Nardin, M.; Rolla, R.; Marino, P.; Bellomo, G.; Suryapranata, H.; De Luca, G. Immature platelet fraction and the extent of coronary artery disease: A single centre study. Atherosclerosis 2017, 260, 110–115. [Google Scholar] [CrossRef]
- Verdoia, M.; Nardin, M.; Negro, F.; Tonon, F.; Gioscia, R.; Rolla, R.; De Luca, G. Impact of aging on immature platelet count and its relationship with coronary artery disease. Platelets 2020, 31, 1060–1068. [Google Scholar] [CrossRef]
- Negro, F.; The Novara Atherosclerosis Study Group (NAS); Verdoia, M.; Tonon, F.; Nardin, M.; Kedhi, E.; De Luca, G. Impact of gender on immature platelet count and its relationship with coronary artery disease. J. Thromb. Thrombolysis 2020, 49, 511–521. [Google Scholar] [CrossRef]
- Verdoia, M.; Nardin, M.; Rolla, R.; Pergolini, P.; Suryapranata, H.; Kedhi, E.; Carriero, A.; De Luca, G.; on behalf of the Novara Atherosclerosis Study Group (NAS). Impact of diabetes mellitus on immature platelet fraction and its association with coronary artery disease. Diabetes Metab. Res. Rev. 2020, 36, e3290. [Google Scholar] [CrossRef] [PubMed]
- Perl, L.; Matatov, Y.; Koronowski, R.; Lev, E.I.; Solodky, A. Prognostic significance of reticulated platelet levels in diabetic patients with stable coronary artery disease. Platelets 2019, 31, 1012–1018. [Google Scholar] [CrossRef] [PubMed]
- Grove, E.L.; Hvas, A.-M.; Kristensen, S.D. Immature platelets in patients with acute coronary syndromes. Thromb. Haemost. 2009, 101, 151–153. [Google Scholar] [CrossRef] [PubMed]
- López-Jiménez, R.A.; Martin-Herrero, F.; González-Porras, J.R.; Sanchez-Barba, M.; Martín-Luengo, C.; Pabon-Osuna, P. Immature Platelet Fraction: A New Prognostic Marker in Acute Coronary Syndrome. Rev. Esp. Cardiol. (Engl. Ed.) 2013, 66, 147–148. [Google Scholar] [CrossRef]
- Berny-Lang, M.A.; Darling, C.E.; Frelinger, A.L.; Barnard, M.R.; Smith, C.S.; Michelson, A.D. Do immature platelet levels in chest pain patients presenting to the emergency department aid in the diagnosis of acute coronary syndrome? Int. J. Lab. Hematol. 2015, 37, 112–119. [Google Scholar] [CrossRef]
- Cesari, F.; Gori, A.M.; Caporale, R.; Fanelli, A.; Casola, G.; Balzi, D.; Barchielli, A.; Valente, S.; Giglioli, C.; Gensini, G.F.; et al. Reticulated platelets predict cardiovascular death in acute coronary syndrome patients. Thromb. Haemost. 2013, 109, 846–853. [Google Scholar] [CrossRef]
- Ibrahim, H.; Schutt, R.C.; Hannawi, B.; Delao, T.; Barker, C.M.; Kleiman, N.S. Association of Immature Platelets with Adverse Cardiovascular Outcomes. J. Am. Coll. Cardiol. 2014, 64, 2122–2129. [Google Scholar] [CrossRef]
- Tscharre, M.; Farhan, S.; Bruno, V.; Rohla, M.; Egger, F.; Weiss, T.W.; Hübl, W.; Willheim, M.; Wojta, J.; Geppert, A.; et al. Impact of platelet turnover on long-term adverse cardiovascular outcomes in patients undergoing percutaneous coronary intervention. Eur. J. Clin. Investig. 2019, 49, e13157. [Google Scholar] [CrossRef]
- Meershoek, A.J.; Leunissen, T.C.; Van Waes, J.A.; Klei, W.A.; Huisman, A.; De Groot, M.C.; Hoefer, I.E.; Van Solinge, W.W.; Moll, F.L.; De Borst, G.J. Reticulated Platelets as Predictor of Myocardial Injury and 30 Day Mortality After Non-cardiac Surgery. Eur. J. Vasc. Endovasc. Surg. 2020, 59, 309–318. [Google Scholar] [CrossRef]
- Kirchhof, P.; Benussi, S.; Kotecha, D.; Ahlsson, A.; Atar, D.; Casadei, B.; Castella, M.; Diener, M.H.; Heidbuchel, H.; Hendriks, J.M.; et al. 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur. Hear. J. 2016, 37, 2893–2962. [Google Scholar] [CrossRef]
- Tafur, A.J.; McBane, R.D.; Ammash, N.; Asirvatham, S.J.; Miller, R.D.; Janczak, D.; Slusser, J.P.; Grill, D.E.; Whelan, S.L.; Wysokinski, W.E. Impact of Atrial Fibrillation and Sinus Rhythm Restoration on Reticulated Platelets. Mayo Clin. Proc. 2015, 90, 1650–1658. [Google Scholar] [CrossRef] [PubMed]
- Wysokinski, W.E.; Tafur, A.; Wu, Y.; Ammash, N.; Asirvatham, S.J.; Gosk-Bierska, I.; Ms, D.E.G.; Bs, J.P.S.; Mruk, J.; McBane, R.D. Platelet-predominate gene expression and reticulated platelets in nonvalvular atrial fibrillation: Effect of pulmonary veins isolation. J. Cardiovasc. Electrophysiol. 2018, 29, 412–420. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, T.; Uchiyama, S.; Yamazaki, M.; Okubo, K.; Takakuwa, Y.; Iwata, M. Flow cytometric analysis of reticulated platelets in patients with ischemic stroke. Thromb. Res. 2002, 106, 171–177. [Google Scholar] [CrossRef]
- McCabe, D.J.; Harrison, P.; Sidhu, P.S.; Brown, M.M.; Machin, S.J. Circulating reticulated platelets in the early and late phases after ischaemic stroke and transient ischaemic attack. Br. J. Haematol. 2004, 126, 861–869. [Google Scholar] [CrossRef] [PubMed]
- Verdoia, M.; Pergolini, P.; Rolla, R.; Barbieri, L.; Schaffer, A.; Marino, P.; Bellomo, G.; Suryapranata, H.; De Luca, G. Impact of Long-Term Dual Antiplatelet Therapy on Immature Platelet Count and Platelet Reactivity. Angiology 2018, 69, 490–496. [Google Scholar] [CrossRef] [PubMed]
- Verdoia, M.; Nardin, M.; Negro, F.; Rolla, R.; Carriero, A.; De Luca, G. Novara Atherosclerosis Study Group (NAS) Impact of long-term therapy with acetylsalicylic acid on immature platelet count. J. Cardiovasc. Med. 2019, 20, 306–312. [Google Scholar] [CrossRef] [PubMed]
| Authors | Year of Publication | No. of Patients | Study Setting Summary |
|---|---|---|---|
| Reticulated platelets in hematological disorders | |||
| C. Briggs et al. [22] | 2004 | Control group: 50 | Assessment of RPs in peripheral thrombocytopenia |
| Study group: 22 | |||
| M.L. Zucker et al. [28] | 2006 | 50 | RPs used to predict platelet recovery after hematopoietic progenitor cell transplantation |
| A. Takami et al. [29] | 2007 | 25 | RPs for the prediction of platelet engraftment after allogenic stem cell transplantation |
| N. Sugimori et al. [37] | 2009 | Control group: 170 | RPs in patients with myelodysplastic syndrome |
| Study group: 71 | |||
| H. Jung et al. [24] | 2010 | Control group: 1837 | Determining the reference intervals of RPs and the optimal cutoff value to differentiate ITP from AA |
| Study group: 202 | |||
| G. Strauss et al. [20] | 2010 | 87 | RPs in distinguishing ITP from ALL in pediatric patients |
| N. van der Linden et al. [30] | 2014 | 18 | RPs used to predict platelet recovery after autologous stem cell transplantation |
| M. Sakuragi et al. [23] | 2015 | Control group: 80 | RPs for distinguishing ITP from aplastic thrombocytopenic disorders |
| Study group: 75 | |||
| A. McDonnell et al. [25] | 2018 | 272 | RPs in pediatric patients to differentiate ITP from bone marrow failure and predict bleeding score |
| R.A. El-Gamal Fayek et al. [27] | 2019 | 73 | RP and schistocyte count in pregnant women with ITP and SPE/HELLP |
| M.J. Jeon et al. [38] | 2020 | 568 | RP predictive scoring model for ITP |
| Reticulated platelets in sepsis | |||
| De Blasi, R.A., et al. [32] | 2013 | 64 | RPs in predicting sepsis in critically ill patients |
| Enz Hubert RM et al. [34] | 2015 | 41 | Association of RPs with sepsis diagnosis and severity |
| T. Muronoi et al. [33] | 2016 | 149 | RPs in predicting mortality in patients with sepsis |
| Koyama K. et al. [35] | 2018 | 205 | RPs and their relation to thrombocytopenia and mortality in patients with sepsis |
| Reticulated platelets in SARS-CoV-2 infections | |||
| Cohen A. [17] | 2020 | Control group: 164 Study group: 47 | RPs in patients hospitalized with COVID-19 |
| Authors | Year of Publication | No. of Patients | RP Count Method | Study Setting Summary |
|---|---|---|---|---|
| Reticulated platelets and coronary artery disease | ||||
| N. Lakkis et al. [39] | 2004 | Control group: 13 | Nonautomated | RPs in ACS |
| Study group: 79 | ||||
| F. Cesari et al. [56] | 2013 | 229 | Automated (Sysmex) | RPs predicting cardiovascular death in ACS |
| L. Perl et al. [52] | 2019 | 104 | Automated (NA) | Prognostic significance of RP levels in DM patients with stable CAD |
| NAS [48,49,50,51] | 2017 | 1789 | Automated (Sysmex) | Assessing the relationship between RPs and the prevalence and extent of CAD |
| 2020 | 2236 | Automated (Sysmex) | Impact of aging on RP count and its relationship with CAD | |
| 2020 | 1781 | Automated (Sysmex) | Impact of DM on RPs and its association with CAD | |
| 2020 | 2550 | Automated (Sysmex) | Impact of gender on RP count and its relationship with CAD | |
| Reticulated platelets and antiplatelet therapy | ||||
| S. Guthikonda et al. [4] | 2007 | 60 | Nonautomated | RPs and the effects of ASA |
| S. Guthikonda et al. [43] | 2008 | 90 | Nonautomated | Role of RPs in platelet activity after DAPT with clopidogrel and ASA |
| F. Cesari et al. [45,56] | 2008 | 372 | Automated (Sysmex) | Role of RPs in platelet function of high-risk patients under DAPT |
| L. Perl et al. [44,52] | 2014 | 62 | Nonautomated | Association between RPs and platelet aggregation under prasugrel |
| I. Bernlochner et al. [3] | 2015 | 124 | Automated (Sysmex) | Association between RPs and platelet aggregation under prasugrel or ticagrelor |
| C. Stratz et al. [46] | 2016 | 199 | Automated (Sysmex) | Impact of RPs on antiplatelet response to thienopyridines |
| C. Stratz et al. [47] | 2018 | 110 | Automated (Sysmex) | Impact of RPs on antiplatelet effect of cangrelor |
| NAS [65,66] | 2018 | 286 | Automated (Sysmex) | Impact of long-term DAPT on RP count and platelet reactivity |
| 2019 | 1475 | Automated (Sysmex) | Impact of long-term ASA on RP count | |
| Reticulated platelets and cardiovascular outcomes | ||||
| H. Ibrahim et al. [57] | 2014 | 89 | Automated (Sysmex) | Association of RPs with adverse cardiovascular outcomes |
| M. Tscharre et al. [58] | 2019 | 477 | Automated (Sysmex) | Impact of RPs on long-term adverse cardiovascular outcomes |
| A.J.A. Meershoek et al. [59] | 2020 | 2971 | Automated (Abbott) | RPs as predictors of MI and mortality after noncardiac surgery |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Corpataux, N.; Franke, K.; Kille, A.; Valina, C.M.; Neumann, F.-J.; Nührenberg, T.; Hochholzer, W. Reticulated Platelets in Medicine: Current Evidence and Further Perspectives. J. Clin. Med. 2020, 9, 3737. https://doi.org/10.3390/jcm9113737
Corpataux N, Franke K, Kille A, Valina CM, Neumann F-J, Nührenberg T, Hochholzer W. Reticulated Platelets in Medicine: Current Evidence and Further Perspectives. Journal of Clinical Medicine. 2020; 9(11):3737. https://doi.org/10.3390/jcm9113737
Chicago/Turabian StyleCorpataux, Noé, Kilian Franke, Alexander Kille, Christian Marc Valina, Franz-Josef Neumann, Thomas Nührenberg, and Willibald Hochholzer. 2020. "Reticulated Platelets in Medicine: Current Evidence and Further Perspectives" Journal of Clinical Medicine 9, no. 11: 3737. https://doi.org/10.3390/jcm9113737
APA StyleCorpataux, N., Franke, K., Kille, A., Valina, C. M., Neumann, F.-J., Nührenberg, T., & Hochholzer, W. (2020). Reticulated Platelets in Medicine: Current Evidence and Further Perspectives. Journal of Clinical Medicine, 9(11), 3737. https://doi.org/10.3390/jcm9113737




