Is the Oxidative Stress in Obstructive Sleep Apnea Associated with Cardiovascular Complications?—Systematic Review
Abstract
1. Introduction
1.1. Oxidative Stress in Obstructive Sleep Apnea
1.2. Cardiovascular Complications in Obstructive Sleep Apnea
1.3. Objective of This Study
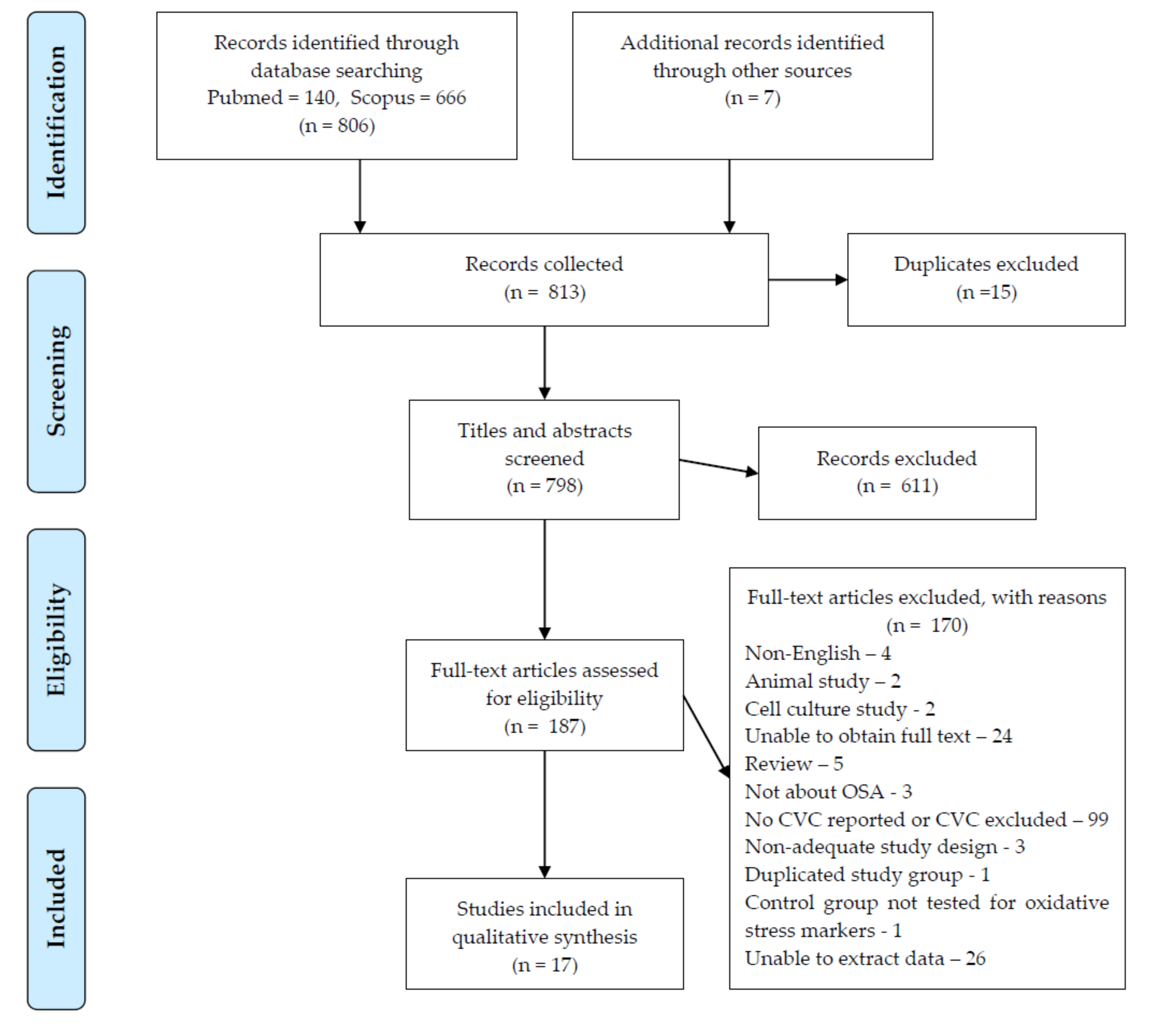
2. Methods
3. Results
- OSA vs. non-OSA control;
- severe OSA vs. non-severe OSA;
- OSA with CVC vs. OSA without CVC.
3.1. OSA Group vs. Non-OSA Control Group
3.2. Severe OSA vs. Non-Severe OSA
3.3. OSA with CVC vs. OSA without CVC
4. Discussion
Study Limitations
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Lavie, L. Oxidative stress in obstructive sleep apnea and intermittent hypoxia—Revisited—The bad ugly and good: Implications to the heart and brain. Sleep Med. Rev. 2015, 20, 27–45. [Google Scholar] [CrossRef] [PubMed]
- Kapur, V.K.; Auckley, D.H.; Chowdhuri, S.; Kuhlmann, D.C.; Mehra, R.; Ramar, K.; Harrod, C.G. Clinical Practice Guideline for Diagnostic Testing for Adult Obstructive Sleep Apnea. J. Clin. Sleep Med. 2017, 1313, 479–504. [Google Scholar] [CrossRef] [PubMed]
- Tingting, X.; Danming, Y.; Xin, C. Non-surgical treatment of obstructive sleep apnea syndrome. Eur. Arch. Oto-Rhino-Laryngol. 2018, 275, 335–346. [Google Scholar] [CrossRef] [PubMed]
- Pang, K.P.; Oto, F.; Olszewska, E.; Baptista, P.M.; Braverman, I.; Carrasco-llatas, M.; Kishore, S.; Chandra, S.; Yang, H.C.; Mei, C.; et al. Does Drug-Induced Sleep Endoscopy Affect Surgical Outcome? A Multicenter Study of 326 Obstructive Sleep Apnea Patients. Clin. Trial 2019, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Olszewska, E.; Woodson, B.T. Palatal anatomy for sleep apnea surgery. Laryngoscope Investig. Otolaryngol. 2019, 4, 181–187. [Google Scholar] [CrossRef] [PubMed]
- Senaratna, C.V.; Perret, J.L.; Lodge, C.J.; Lowe, A.J.; Campbell, B.E.; Matheson, M.C.; Hamilton, G.S.; Dharmage, S.C. Prevalence of obstructive sleep apnea in the general population: A systematic review. Sleep Med. Rev. 2017, 34, 70–81. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Niu, L.; Li, S.; Le, W. Pathological impacts of chronic hypoxia on alzheimer’s disease. ACS Chem. Neurosci. 2019, 10, 902–909. [Google Scholar] [CrossRef]
- Chiang, C.L.; Chen, Y.T.; Wang, K.L.; Su, V.Y.F.; Wu, L.A.; Perng, D.W.; Chang, S.C.; Chen, Y.M.; Chen, T.J.; Chou, K.T. Comorbidities and risk of mortality in patients with sleep apnea. Ann. Med. 2017, 49, 377–383. [Google Scholar] [CrossRef]
- Redline, S.; Yenokyan, G.; Gottlieb, D.J.; Shahar, E.; O’Connor, G.T.; Resnick, H.E.; Diener-West, M.; Sanders, M.H.; Wolf, P.A.; Geraghty, E.M.; et al. Obstructive sleep apnea-hypopnea and incident stroke: The sleep heart health study. Am. J. Respir. Crit. Care Med. 2010. [Google Scholar] [CrossRef]
- Olszewska, E.; Panek, J.; O’Day, J.; Rogowski, M. Usefulness of snoreplasty in the treatment of simple snoring and mild obstructive sleep apnea/hypopnea syndrome—Preliminary report. Otolaryngol. Pol. 2014, 68, 184–188. [Google Scholar] [CrossRef]
- McNicholas, W.T.; Bonsignore, M.R. Sleep apnoea as an independent risk for cardiovascular disease: Current evidence, basic mechanisms and research priorities. Eur. Respir. J. 2007, 29, 156–178. [Google Scholar] [CrossRef] [PubMed]
- Nita, M.; Grzybowski, A. The Role of the Reactive Oxygen Species and Oxidative Stress in the Pathomechanism of the Age-Related Ocular Diseases and Other Pathologies of the Anterior and Posterior Eye Segments in Adults. Oxid. Med. Cell. Longev. 2016, 2016. [Google Scholar] [CrossRef] [PubMed]
- Yoshikawa, T.; Naito, Y. What is oxidative stress? Jpn. Med. Assoc. J. 2002, 271–276. [Google Scholar] [CrossRef]
- McCord, J.M. Oxygen-Derived Free Radicals in Postischemic Tissue Injury. N. Engl. J. Med. 1985, 312, 159–163. [Google Scholar] [PubMed]
- Erel, O. A new automated colorimetric method for measuring total oxidant status. Clin. Biochem. 2005, 38, 1103–1111. [Google Scholar] [CrossRef] [PubMed]
- Miller, N.J.; Rice-Evans, C.; Davies, M.J.; Gopinathan, V.; Milner, A. A novel method for measuring antioxidant capacity and its application to monitoring the antioxidant status in premature neonates. Clin. Sci. 1993, 84, 407–412. [Google Scholar] [CrossRef] [PubMed]
- Förstermann, U.; Sessa, W.C. Nitric oxide synthases: Regulation and function. Eur. Heart J. 2012, 33, 1–13. [Google Scholar] [CrossRef]
- Zhao, Y.; Vanhoutte, P.M.; Leung, S.W.S. Vascular nitric oxide: Beyond eNOS. J. Pharmacol. Sci. 2015, 129, 83–94. [Google Scholar] [CrossRef]
- Caimi, G.; Hopps, E.; Montana, M.; Carollo, C.; Calandrino, V.; Incalcaterra, E.; Canino, B.; Lo Presti, R. Nitric oxide metabolites (nitrite and nitrate) in several clinical condition. Clin. Hemorheol. Microcirc. 2014, 56, 359–369. [Google Scholar] [CrossRef]
- Tsikas, D. Assessment of lipid peroxidation by measuring malondialdehyde (MDA) and relatives in biological samples: Analytical and biological challenges. Anal. Biochem. 2017, 524, 13–30. [Google Scholar] [CrossRef]
- Grisham, M.B.; Granger, D.N. Neutrophil-mediated mucosal injury—Role of reactive oxygen metabolites. Dig. Dis. Sci. 1988, 33. [Google Scholar] [CrossRef] [PubMed]
- Ghani, M.A.; Barril, C.; Bedgood, D.R.; Prenzler, P.D. Measurement of antioxidant activity with the thiobarbituric acid reactive substances assay. Food Chem. 2017, 230, 195–207. [Google Scholar] [CrossRef] [PubMed]
- Di Minno, A.; Turnu, L.; Porro, B.; Squellerio, I.; Cavalca, V.; Tremoli, E.; Di Minno, M.N.D. 8-Hydroxy-2-deoxyguanosine levels and heart failure: A systematic review and meta-analysis of the literature. Nutr. Metab. Cardiovasc. Dis. 2017, 27, 201–208. [Google Scholar] [CrossRef] [PubMed]
- Yen, H.C.; Wei, H.J.; Lin, C.L. Unresolved issues in the analysis of F2-isoprostanes, F4-neuroprostanes, isofurans, neurofurans, and F2-dihomo-isoprostanes in body fluids and tissue using gas chromatography/negative-ion chemical-ionization mass spectrometry. Free Radic. Res. 2015, 49, 861–880. [Google Scholar] [CrossRef] [PubMed]
- Miao, L.; Clair, D.K.S. Regulation of superoxide dismutase genes: Implications in disease. Free Radic. Biol. Med. 2009, 47, 344–356. [Google Scholar] [CrossRef] [PubMed]
- Diaz-Vivancos, P.; De Simone, A.; Kiddle, G.; Foyer, C.H. Glutathione—Linking cell proliferation to oxidative stress. Free Radic. Biol. Med. 2015, 89, 1154–1164. [Google Scholar] [CrossRef] [PubMed]
- Benhar, M. Roles of mammalian glutathione peroxidase and thioredoxin reductase enzymes in the cellular response to nitrosative stress. Free Radic. Biol. Med. 2018, 127, 160–164. [Google Scholar] [CrossRef]
- Kunutsor, S.K.; Bakker, S.J.L.; James, R.W.; Dullaart, R.P.F. Serum paraoxonase-1 activity and risk of incident cardiovascular disease: The PREVEND study and meta-analysis of prospective population studies. Atherosclerosis 2016, 245, 143–154. [Google Scholar] [CrossRef]
- Deluyker, D.; Evens, L.; Bito, V. Advanced glycation end products (AGEs) and cardiovascular dysfunction: Focus on high molecular weight AGEs. Amino Acids 2017, 49, 1535–1541. [Google Scholar] [CrossRef]
- Ou, H.; Huang, Z.; Mo, Z.; Xiao, J. The Characteristics and Roles of Advanced Oxidation Protein Products in Atherosclerosis. Cardiovasc. Toxicol. 2017, 17. [Google Scholar] [CrossRef]
- Zhou, S.; Zhu, Q.; Li, X.; Chen, C.; Liu, J.; Ye, Y.; Ruan, Y.; Hei, Z. Asymmetric dimethylarginine and all-cause mortality: A systematic review and meta-analysis. Sci. Rep. 2017, 7, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Škovierová, H.; Vidomanová, E.; Mahmood, S.; Sopková, J.; Drgová, A.; Červeňová, T.; Halašová, E.; Lehotský, J. The molecular and cellular effect of homocysteine metabolism imbalance on human health. Int. J. Mol. Sci. 2016, 17, 1733. [Google Scholar] [CrossRef] [PubMed]
- Trpkovic, A.; Resanovic, I.; Stanimirovic, J.; Radak, D.; Mousa, S.A.; Cenic-Milosevic, D.; Jevremovic, D.; Isenovic, E.R. Oxidized low-density lipoprotein as a biomarker of cardiovascular diseases. Crit. Rev. Clin. Lab. Sci. 2015, 52, 70–85. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, A.; Brunssen, C.; Morawietz, H. Contribution of lectin-like oxidized low-density lipoprotein receptor-1 and LOX-1 modulating compounds to vascular diseases. Vascul. Pharmacol. 2018, 107, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Manafikhi, H.; Drummen, G.; Palmery, M.; Peluso, I. Total Antioxidant Capacity in beta-thalassemia: A systematic review and meta-analysis of case-control studies. Crit. Rev. Oncol. Hematol. 2017, 110, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Iris, F.F.; Benzie, J.J.S. The Ferric Reducing Ability of Plasma (FRAP) as a Measure of “Antioxidant Power”: The FRAP Assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef]
- Lima, W.G.; Martins-Santos, M.E.S.; Chaves, V.E. Uric acid as a modulator of glucose and lipid metabolism. Biochimie 2015, 116, 17–23. [Google Scholar] [CrossRef]
- Marzocchi, B.; Perrone, S.; Paffetti, P.; Magi, B.; Bini, L.; Tani, C.; Longini, M.; Buonocore, G. Nonprotein-bound iron and plasma protein oxidative stress at birth. Pediatr. Res. 2005, 58, 1295–1299. [Google Scholar] [CrossRef]
- Franczak, A.; Bil-Lula, I.; Sawicki, G.; Fenton, M.; Ayas, N.; Skomro, R. Matrix metalloproteinases as possible biomarkers of obstructive sleep apnea severity—A systematic review. Sleep Med. Rev. 2019, 46, 9–16. [Google Scholar] [CrossRef]
- Bradley, T.D.; Floras, J.S. Obstructive sleep apnoea and its cardiovascular consequences. Lancet 2009, 373, 82–93. [Google Scholar] [CrossRef]
- Peppard, P.E.; Young, T.; Barnet, J.H.; Palta, M.; Hagen, E.W.; Hla, K.M. Increased prevalence of sleep-disordered breathing in adults. Am. J. Epidemiol. 2013, 177, 1006–1014. [Google Scholar] [CrossRef] [PubMed]
- Marin, J.M.; Carrizo, S.J.; Vicente, E.; Agusti, A.G.N. Long-term cardiovascular outcomes in men with obstructive sleep apnoea-hypopnoea with or without treatment with continuous positive airway pressure: An observational study. Lancet 2005, 365, 1046–1053. [Google Scholar] [CrossRef]
- Gottlieb, D.J.; Yenokyan, G.; Newman, A.B.; O’Connor, G.T.; Punjabi, N.M.; Quan, S.F.; Redline, S.; Resnick, H.E.; Tong, E.K.; Diener-West, M.; et al. A Prospective Study of Obstructive Sleep Apnea and Incident Coronary Heart Disease and Heart Failure: The Sleep Heart Health Study Daniel. Circulation 2010, 122, 352–360. [Google Scholar] [CrossRef] [PubMed]
- Bonetti, P.O.; Lerman, L.O.; Lerman, A. Endothelial dysfunction: A marker of atherosclerotic risk. Arterioscler. Thromb. Vasc. Biol. 2003, 23, 168–175. [Google Scholar] [CrossRef] [PubMed]
- Lui, M.M.S.; Ip, M.S.M. OSA and atherosclerosis. J. Thorac. Dis. 2012, 4, 164–172. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Zhang, D.; Chen, B. Endothelial mechanisms of endothelial dysfunction in patients with obstructive sleep apnea. Sleep Breath. 2012, 16, 283–294. [Google Scholar] [CrossRef]
- Atkeson, A.; Jelic, S. Mechanisms of endothelial dysfunction in obstructive sleep apnea. Vasc. Health Risk Manag. 2008, 4, 1327–1335. [Google Scholar] [CrossRef] [PubMed]
- Zamarrón, C.; Valdés Cuadrado, L.; Álvarez-Sala, R. Pathophysiologic mechanisms of cardiovascular disease in obstructive sleep apnea syndrome. Pulm. Med. 2013, 2013. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; Altman, D.; Antes, G.; Atkins, D.; Barbour, V.; Barrowman, N.; Berlin, J.A.; et al. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef]
- Kwon, Y.; Lemieux, M.; McTavish, J.; Wathen, N. Identifying and removing duplicate records from systematic review searches. J. Med. Libr. Assoc. 2015, 103, 184–188. [Google Scholar] [CrossRef]
- Wells, B.G.A.; Shea, D.; O’Connell, J.; Peterson, V.; Welch, M.; Losos, P.T. Newcastle-Ottawa quality assessment form for cohort studies. Otawwa Hosp. Res. Inst. 2014, 2–4. [Google Scholar] [CrossRef]
- Stang, A.; Jonas, S.; Poole, C. Case study in major quotation errors: A critical commentary on the Newcastle–Ottawa scale. Eur. J. Epidemiol. 2018, 33, 1025–1031. [Google Scholar] [CrossRef] [PubMed]
- Anunciato, I.F.; Lobo, R.R.; Coelho, E.B.; Verri, W.A.; Eckeli, A.L.; Évora, P.R.B.; Nobre, F.; Moriguti, J.C.; Ferriolli, E.; Lima, N.K.; et al. Big Endothelin-1 and Nitric Oxide in Hypertensive Elderly Patients with with and without Obstructive Sleep Apnea-Hypopnea Syndrome. Arq. Bras. Cardiol. 2013, 101, 344–351. [Google Scholar] [CrossRef]
- Cherneva, R.V.; Cherneva, Z.V.; Georgiev, O.B.; Petrova, D.S.; Petrova, J.I. 8-isoprostanes and resistin as markers of vascular damage in non-hypersomnolent obstructive sleep apnoea patients. Clin. Physiol. Funct. Imaging 2016, 37, 695–702. [Google Scholar] [CrossRef] [PubMed]
- De Lima, A.M.J.; Franco, C.M.R.; De Castro, C.M.M.H.B.; Bezerra, A.D.A.; Ataíde, L.; Halpern, A. Effects of nasal continuous positive airway pressure treatment on oxidative stress and adiponectin levels in obese patients with obstructive sleep apnea. Respiration 2009, 79, 370–376. [Google Scholar] [CrossRef] [PubMed]
- Del Ben, M.; Fabiani, M.; Loffredo, L.; Polimeni, L.; Carnevale, R.; Baratta, F.; Brunori, M.; Albanese, F.; Augelletti, T.; Violi, F.; et al. Oxidative stress mediated arterial dysfunction in patients with obstructive sleep apnoea and the effect of continuous positive airway pressure treatment. BMC Pulm. Med. 2012, 12, 36. [Google Scholar] [CrossRef]
- Monneret, D.; Tamisier, R.; Ducros, V.; Garrel, C.; Levy, P.; Baguet, J.P.; Faure, P.; Pépin, J.L. The impact of obstructive sleep apnea on homocysteine and carotid remodeling in metabolic syndrome. Respir. Physiol. Neurobiol. 2011, 180, 298–304. [Google Scholar] [CrossRef]
- Piérola, J.; Alemany, A.; Yañez, A.; De-La-Peña, M.; Sánchez-De-La-Torre, M.; Esquinas, C.; Pérez-Gutierrez, C.; Burguera, B.; Barbé, F.; Barceló, A. NADPH oxidase p22phox polymorphisms and oxidative stress in patients with obstructive sleep apnoea. Respir. Med. 2011, 105, 1748–1754. [Google Scholar] [CrossRef]
- Wang, L.; Li, J.; Xie, Y.; Zhang, X.-G.X.G. Association between serum homocysteine and oxidative stress in elderly patients with obstructive sleep apnea/hypopnea syndrome. Biomed. Environ. Sci. 2010, 23, 42–47. [Google Scholar] [CrossRef]
- Ortaç Ersoy, E.; Firat, H.; Akaydin, S.; Özkan, Y.; Durusu, M.; Darilmaz Yüce, G.; Ergün, R.; Topeli, A.; Ardiç, S. Association of obstructive sleep apnea with homocystein, Nitric oxide and total antioxidant capacity levels in patients with or without coronary artery disease|Koroner arter hastalığı olan ve olmayan hastalarda obstrüktif uyku apnesinin homosistein, Nit. Tuberk. Toraks 2014, 62, 207–214. [Google Scholar] [CrossRef]
- Chen, C.-Y.; Chen, C.-L.; Yu, C.-C.; Chen, T.-T.; Tseng, S.-T.; Ho, C.-H. Association of inflammation and oxidative stress with obstructive sleep apnea in ischemic stroke patients. Sleep Med. 2015, 16, 113–118. [Google Scholar] [CrossRef] [PubMed]
- Gille, T.; Didier, M.; Boubaya, M.; Moya, L.; Sutton, A.; Carton, Z.; Baran-Marszak, F.; Sadoun-Danino, D.; Israël-Biet, D.; Cottin, V.; et al. Obstructive sleep apnoea and related comorbidities in incident idiopathic pulmonary fibrosis. Eur. Respir. J. 2017, 49. [Google Scholar] [CrossRef] [PubMed]
- Yadav, R.; France, M.; Aghamohammadzadeh, R.; Liu, Y.; Hama, S.; Kwok, S.; Schofield, J.; Turkington, P.; Syed, A.A.; Malik, R.; et al. Impairment of high-density lipoprotein resistance to lipid peroxidation and adipose tissue inflammation in obesity complicated by obstructive sleep apnea. J. Clin. Endocrinol. Metab. 2014, 99, 3390–3398. [Google Scholar] [CrossRef] [PubMed]
- Yamauchi, M.; Nakano, H.; Maekawa, J.; Okamoto, J.; Ohnishi, Y.; Suzuki, T.; Kimura, H. Oxidative stress in obstructive sleep apnea. Chest 2005, 127, 1674–1679. [Google Scholar] [CrossRef] [PubMed]
- Yardim-Akaydin, S.; Caliskan-Can, E.; Gökalp, F.; Firat, H.; Ardiç, S.; Simsek, B. Lipid peroxidation and DNA damage in apnea patients with or without metabolic syndrome. Sleep Biol. Rhythm. 2013, 11, 116–124. [Google Scholar] [CrossRef]
- Feres, M.C.; Fonseca, F.A.H.; Cintra, F.D.; Mello-Fujita, L.; de Souza, A.L.; De Martino, M.C.; Tufik, S.; Poyares, D. An assessment of oxidized LDL in the lipid profiles of patients with obstructive sleep apnea and its association with both hypertension and dyslipidemia, and the impact of treatment with CPAP. Atherosclerosis 2015, 241, 342–349. [Google Scholar] [CrossRef]
- In, E.; Özdemir, C.; Kaman, D.; Sökücü, S.N. Heat Shock Proteins, L-Arginine, and Asymmetric Dimethylarginine Levels in Patients With Obstructive Sleep Apnea Syndrome. Arch. Bronconeumol. 2015, 51, 544–550. [Google Scholar] [CrossRef]
- Lavie, L.; Vishnevsky, A.; Lavie, P. Evidence for lipid peroxidation in obstructive sleep apnea. Sleep 2004, 27, 123–128. [Google Scholar]
- Murri, M.; Alcázar-Ramírez, J.; Garrido-Sánchez, L.; Linde, F.; Alcaide, J.; Cardona, F.; Tinahones, F.J. Oxidative stress and metabolic changes after continuous positive airway pressure treatment according to previous metabolic disorders in sleep apnea-hypopnea syndrome patients. Transl. Res. 2009, 154, 111–121. [Google Scholar] [CrossRef]
- Frijhoff, J.; Winyard, P.G.; Zarkovic, N.; Davies, S.S.; Stocker, R.; Cheng, D.; Knight, A.R.; Taylor, E.L.; Oettrich, J.; Ruskovska, T.; et al. Clinical Relevance of Biomarkers of Oxidative Stress. Antioxid. Redox Signal. 2015, 23, 1144–1170. [Google Scholar] [CrossRef]
- Dyugovskaya, L.; Polyakov, A.; Lavie, P.; Lavie, L. Delayed neutrophil apoptosis in patients with sleep apnea. Am. J. Respir. Crit. Care Med. 2008, 177, 544–554. [Google Scholar] [CrossRef] [PubMed]
- Schulz, R.; Mahmoudi, S.; Hattar, K.; Sibelius, U.L.F.; Olschewski, H.; Mayer, K.; Seeger, W.; Grimminger, F. Enhanced release of superoxide from polymorphonuclear neutrophils in obstructive sleep apnea: Impact of continuous positive airway pressure therapy. Am. J. Respir. Crit. Care Med. 2000, 162, 566–570. [Google Scholar] [CrossRef] [PubMed]
- Badran, M.; Ayas, N.; Laher, I. Cardiovascular complications of sleep apnea: Role of oxidative stress. Oxid. Med. Cell. Longev. 2014, 2014. [Google Scholar] [CrossRef] [PubMed]
- Punjabi, N.M.; Newman, A.B.; Young, T.B.; Resnick, H.E.; Sanders, M.H. Sleep-disordered breathing and cardiovascular disease: An outcome-based definition of hypopneas. Am. J. Respir. Crit. Care Med. 2008, 177, 1150–1155. [Google Scholar] [CrossRef] [PubMed]
- Kendzerska, T.; Gershon, A.S.; Hawker, G.; Leung, R.S.; Tomlinson, G. Obstructive Sleep Apnea and Risk of Cardiovascular Events and All-Cause Mortality: A Decade-Long Historical Cohort Study. PLoS Med. 2014, 11. [Google Scholar] [CrossRef]
- Zinchuk, A.V.; Jeon, S.; Koo, B.B.; Yan, X.; Bravata, D.M.; Qin, L.; Selim, B.J.; Strohl, K.P.; Redeker, N.S.; Concato, J.; et al. Polysomnographic phenotypes and their cardiovascular implications in obstructive sleep apnoea. Thorax 2018, 73, 472–480. [Google Scholar] [CrossRef]
| Biomarker | Relation to Oxidative Stress |
|---|---|
| Reactive oxygen species | Reactive oxygen species (ROS) are oxygen molecules with unpaired electrons that interfere with nucleic acids, proteins and lipids structure due to high reactivity and giving rise to cytotoxic tissue damage. ROS are produced by fluctuations in oxygen saturation [14]. |
| Total oxidant status | Total oxidant status (TOS) is a form of presenting the total oxidation state of the sample (plasma, serum, etc.) by conducting a redox reaction. Higher generation of ROS results in higher TOS [15]. |
| Total antioxidant status | Total antioxidant status (TAS) is a form of presenting the total antioxidation state of the sample (plasma, serum, etc.) by conducting a redox reaction. Higher generation of ROS results in lower TAS [16]. |
| Endothelial nitric oxide synthase | Endothelial nitric oxide synthase (eNOS) is the main producer of nitric oxide (NO). eNOS shows a positive correlation with oxidative stress [17,18]. |
| Nitric oxide Metabolites | Nitric oxide metabolites (NOx) are the final metabolites of NO. These metabolites, including nitrites and nitrates, are considered to be involved in tissue injury and exhibit a negative correlation with oxidative stress [19]. |
| Nitric oxide | Nitric oxide (NO) is a form of free radical that plays a key role in the physiological regulation and protection of the cardiovascular system. NO is an antioxidant that reacts with ROS and produces cytotoxic metabolites such as peroxynitrite; therefore, it exhibits a negative correlation with oxidative stress [17,18]. |
| Malondialdehyde | Malondialdehyde (MDA) is a product of the reaction between polyunsaturated fatty acids (PUFAs) and reactive oxygen species (ROS) [20]. |
| Reactive oxygen metabolites | Reactive oxygen metabolites (ROMs) are partially reduced oxygen species produced by oxidases such as xanthine oxidase or NADPH oxidase. They play an important role in mediating cellular injury [21]. |
| Thiobarbituric acid reactive substances | Thiobarbituric acid reactive substances (TBARS) are the products of the reaction between thiobarbituric acid (TBA) and malondialdehyde (MDA), categorized as one of the simplest ways to measure MDA, which is the product of the reaction between PUFAs and ROS [22]. |
| 8-hydroxy-2′-deoxyguanosine | 8-hydroxy-2′-deoxyguanosine (8-OHdG) is generated during the process of repairing ROS-mediated DNA damages. Higher levels of ROS are related to increased DNA damages, and as a result, more 8-OHdG is generated [23]. |
| F2-isoprostane (8-isoprostane) | F2-isoprostanes (F2-IsoPs) are formed from arachidonic acid (AA) oxidized by free radicals in lipid peroxidation reaction [24]. |
| Superoxide dismutase | Superoxide dismutase (SOD) is an antioxidant enzyme that protects cells from the toxic effects of reactive oxygen species (ROS) [25]. |
| Glutathione | Glutathione (GSH) is involved in a diverse range of processes that include cell growth and programmed death. Serves as an antioxidant by interacting with ROS [26]. |
| Glutathione peroxidase | Glutathione peroxidase (GPx) is a family of enzymes that play an important role in hydrogen peroxide and lipid hydroperoxide detoxification [27]. |
| Serum paraoxonase 1 | Paraoxonase-1 (PON-1) is an HDL-bound esterase enzyme that hydrolyzes lipid peroxides, protecting low-density lipoproteins (LDLs) from free radicals [28]. |
| Advanced glycation end products | Advanced glycation end products (AGEs) are lipids or proteins that become glycated and oxidized after repeated contact of free radicals with reducing sugars or aldehydes [29]. |
| Advanced oxidation protein products | Advanced oxidation protein products (AOPPs) are formed from oxidation-modified albumin, which is their main source. They can promote oxidative stress and inflammation [30]. |
| Asymmetric dimethylarginine | Asymmetrical dimethylarginine (ADMA) is an inhibitor of nitric oxide synthase (NOS) and thus inhibits nitric oxide (NO) synthesis [31]. |
| Homocysteine | Homocysteine (Hcy) is an amino acid in which metabolic imbalance can lead to protein, nucleic acid and carbohydrate oxidation [32]. |
| Oxidized low-density lipoprotein | oxidized low-density lipoprotein (oxLDL) is a product of the reaction between ROS and LDL and is considered to play an important role in atherogenesis [33]. |
| Lectin-like oxidized low-density lipoprotein receptor-1 | Lectin-like oxidized low-density lipoprotein receptor-1 (LOX-1) in endothelial cells is a crucial receptor responsible for binding and uptake of oxidized low-density lipoprotein (oxLDL) [34]. |
| Total antioxidant capacity | Total antioxidant capacity (TAC) is characterized as the moles of oxidants neutralized by one liter of plasma and serves as a biomarker that measures the body fluids’ antioxidant potential, including synergistic redox interactions [35]. |
| Ferric-reducing antioxidant power | Ferric-reducing antioxidant power (FRAP) is defined as plasma ability to reduce iron complexes and reflects plasma antioxidant potential [36]. |
| Uric acid | Uric acid (UA) is an end product of purine metabolism originating from the oxidation of purine compounds. Uric acid inhibits lipid peroxidation and is considered to be a powerful antioxidant [37]. |
| Non-protein--bound iron | Non-protein-bound iron (NPBI) is a form of low molecular mass iron that interacts with superoxide and hydrogen peroxide, causing the release of hydroxyl radical (OH·), which is an immensely destructive oxidizing species [38]. |
| Catalase | Catalase is a heme protein that decomposes hydrogen superoxide and provides the first line of defense against reactive oxygen metabolites (ROMs) [21]. |
| Matrix metalloproteinases | Matrix metalloproteinases (MMPs) are enzymes that take part in proteolytic remodeling of the extracellular matrix. They interact with ROS, which can disrupt a protein conformation exposing the catalytic site of MMP and increasing MMPs activity [39]. |
| Do Patients with Obstructive Sleep Apnea Demonstrate an Extent Oxidative Stress Correlated with Cardiovascular Complications? | |
|---|---|
| Population | Patients with OSA defined as AHI > 5 in polysomnographic sleep study (PSG) |
| Indicator | Oxidative stress markers/antioxidative markers |
| Control | Groups of patients without OSA (AHI < 5 in PSG), other OSA patients |
| Outcome | Cardiovascular complications, such as hypertension. myocardial infarction, stroke, ischemic heart disease, heart failure |
| Study design | Peer-reviewed English articles Adult (>18 years) human subjects Case–control/observational studies Studies comparing oxidative stress biomarkers between OSA and control groups and providing data on cardiovascular complications |
| Author | Year | Oxidative Stress Biomarkers | AHI Scoring Criteria | OSA Case Definition | CVC Reported | Population | Newcastle–Ottawa Scale * | ||
|---|---|---|---|---|---|---|---|---|---|
| Selection | Comparability | Exposure | |||||||
| OSA vs non-OSA control | |||||||||
| Anunciato et al. [53] | 2013 | Plasma NO | PSG with no oxygen desaturation cutoff value | AHI > 5 | HT | Elderly hypertensive | ★▢★★ | ★ ★ | ★★★ |
| Cherneva et al. [54] | 2016 | Urinary 8-isoprostane | PSG apnea or hypopnea 3% ↓SpO2 or arousal >10 s | AHI > 5 | HT, CAD | ESS < 11 | ★▢▢★ | ★★ | ★★★ |
| de Lima et al. [55] | 2010 | NOx, superoxides | PSG apnea or hypopnea 4% ↓SpO2 | AHI > 20 | HT, CAD | Obese (BMI > 30) | ★▢▢★ | ★★ | ★★★ |
| Del Ben et al. [56] | 2012 | Urinary 8-isoprostanes, NOx | PSG apnea or hypopnea 4% ↓SpO2 and arousal | AHI > 5 | HT | Metabolic disorders + snoring | ★▢▢★ | ★★ | ★★★ |
| Monneret et al. [57] | 2012 | TAC, GSH, GPx, Hcy, ur 15-F2t-isoprostane | PSG apnea or hypopnea 4% ↓SpO2 or EEG arousal | AHI > 10 | HT, IHD | Non-obese MetS | ★▢▢★ | ★★ | ★★★ |
| Piérola et al. [58] | 2011 | Plasma 8-isoprostanes | PSG apnea or hypopnea 4% ↓SpO2 or arousal | AHI > 10 | HT | Suspected of having OSA | ★★▢★ | ▢▢ | ★★★ |
| Wang et al. [59] | 2010 | Hcy, MDA, GSH | PSG apnea or hypopnea 3% ↓SpO2 | AHI > 5 | HT | Elderly and non-elderly | ★★▢★ | ★★ | ★★★ |
| Ortaç Ersoy et al. [60] | 2014 | NOx, Hcy, TAC | PSG with no oxygen desaturation cutoff value | AHI > 5 | CAD | Patients hospitalized after acute MI | ★▢▢★ | ★▢ | ★★★ |
| severe OSA vs non-severe OSA | |||||||||
| Chen et al. [61] | 2015 | TAC, urinary 8-OHdG | PSG apnea or hypopnea 3% ↓SpO2 or arousal | severe AHI > 30 | HT | Stable ischemic stroke patients | ★▢▢★ | ▢▢ | ★★★ |
| Gille et al. [62] | 2017 | 8-OHdG, MMP-7 | PSG with no oxygen desaturation cutoff value | AHI ⩾ 15 | HT, IHD, S/TIA | Patients with idiopathic pulmonary fibrosis | ★▢▢★ | ★★ | ★★★ |
| Yadav et al. [63] | 2014 | PON1 | PSG with no oxygen desaturation cutoff value | mean AHI = 21.3 | HT | Obese (BMI > 40) | ★▢▢★ | ★★ | ★★★ |
| Yamauchi et al. [64] | 2005 | Urinary 8-OHdG | PSG with no oxygen desaturation cutoff value | severe AHI > 30 | HT | Suspected of having OSA | ★★▢★ | ▢★ | ★★★ |
| Yardim-Akaydin et al. [65] | 2013 | MDA, 8-OHdG, | PSG apnea or hypopnea 4% ↓SpO2 | AHI > 5 | HT | Patients with and without MetS | ★▢▢★ | ★★ | ★★★ |
| OSA with CVC vs. OSA without CVC | |||||||||
| Feres et al. [66] | 2015 | oxLDL | PSG with no oxygen desaturation cutoff value | AHI ⩾ 15 | HT | Patients suspected of having OSA | ★★★★ | ▢★ | ★★★ |
| İn et al. [67] | 2015 | ADMA | PSG apnea or hypopnea 3% ↓SpO2 or arousal | AHI > 5 | HT | OSA patients with and without CV risk factors | ★★▢★ | ▢★ | ★★★ |
| Lavie et al. [68] | 2004 | PON1, TBARS, PD | PSG with no oxygen desaturation cutoff value. RDI | RDI > 10 + symptoms | HT, IHD, MI, CVA, | Untreated OSA patients | ★★▢★ | ▢★ | ★★★ |
| Murri et al. [69] | 2009 | CAT, GPx, SOD, TAC | PSG apnea or hypopnea 4% ↓SpO2 | AHI > 10 and ESS > 10 | HT | OSA patients that required CPAP | ★▢▢★ | ▢▢ | ★★★ |
| Author | Study and Control Characteristics | Biomarker Levels | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Sample Size (n) | Age ± SD | Male (%) | Mean BMI ± SD | Mean AHI ± SD | CVC (%) | Differences in Groups * | Results | Differences in Groups ^ | ||
| Anunciato et al. [53] | Study Group—Hypertensive Patients wWith OSA | No | NO Data presented graphically; therefore, parametric results cannot be displayed on this table. | No | ||||||
| 25 | 67.0 ± 6.5 | - | 30.3 ± 4.8 | 29.0 ± 13.7 | HT: 100 | |||||
| Control group—Hypertensive Patients Without OSA | ||||||||||
| 12 | 67.8 ±6.8 | - | 29.0 ± 5,0 | 3.1 ± 1.6 | HT: 100 | |||||
| Cherneva et al. [54] | Study Group—Patients with Minimally Symptomatic OSA | No | 8-isoprostane: 0.091 ± 0.007 pg/mkmol creatinine | 8-isoprostane | ||||||
| 86 | 54.66 ±11.59 | 88.4 | 31.1±4.63 | 38.94 ± 13.53 | HT: 41.21 | |||||
| Control Group—Patients Without OSA | 8-isoprostane: 0.078±0.004 pg/mkmol creatinine | |||||||||
| 45 | 52.17 ± 8.95 | 80 | 29.8 ± 3.92 | 4.10 ± 1.90 | HT: 44.53 | |||||
| De Lima et al. [55] | Study Group—Patients with OSA Not Treated With CPAP | No | NOx: 30.3 ± 7.9 µM | NOx | ||||||
| 10 | 57 ± 10.5 | 100 | 34.1 ± 1.3 | 29.5 ± 3.7 | HT: 17.2 | |||||
| Control Group—Patients Without OSA | NOx: 50.5 ± 2.9 µM | |||||||||
| 10 | 56.8 ± 4.7 | 100 | 33.1 ± 2.5 | 3.6 ± 0.1 | 13.8 | |||||
| Del Ben et al. [56] | Study Group—Severe OSA | BMI | 8-isoprostane: 337.6 ± 74.5 pg/mg creatinine NOx: 23.6 ± 16.0 uM/mL | 8-isoprostane (severe OSA vs control and mild/moderate OSA) | ||||||
| 30 | 56.6 ± 9.8 | 83.3 | 32.8 ± 5.1 | 42.8 ± 14.2 | HT: 66.7 | |||||
| Study Group—Mild/Moderate OSA | 8-isoprostane: 289.5 ± 77.0 pg/mg creatinine NOx: 27.0 ± 12.5 uM/mL | |||||||||
| 61 | 53.2 ± 11.7 | 70.5 | 30.5 ± 4.5 | 14.6 ± 8.0 | HT: 60.7 | |||||
| Control Group—Snorers | 8-isoprostane: 284.0 ± 77.3 pg/mg creatinine NOx: 27.1 ± 14.6 uM/mL | |||||||||
| 47 | 51.3 ± 11.7 | 66.0 | 29.3 ± 3.9 | 1.2 ± 1.4 | HT: 51.1 | |||||
| Monneret et al. [57] | Study Group—OSA + Metabolic Syndrome Patients | No | TAS: 1.59 ± 0.21 mmol/L GPx: 51.0 ± 9.7 U/g Hb GSH: 1022 ± 178 µmol/L Hcy: 12.8 ± 3.8 µmol/L F2-isoprostane: 21.2 ± 10.4 ng/mmol creatinine | Hcy | ||||||
| 26 | 61.5 ± 5.0 | 65.4 | 29.8 ± 3.7 | 31.7 ± 20.5 | HT: 88 IHD: 8 | |||||
| Control Group—Metabolic Syndrome Patients | TAS: 1.59 ± 0.10 mmol/L GPx: 51.4 ± 11.4 U/g Hb GSH: 1040 ± 137 µmol/L Hcy: 9.5 ± 2.5 µmol/L F2-isoprostane: 16.5 ± 4.5 ng/mmol creatinine | |||||||||
| 9 | 59.7 ± 3.4 | 33.3 | 29.1 ± 2.6 | 5.4 ± 3.6 | HT: 89 IHD: 0 | |||||
| Pierola et al. [58] | Study Group | Age, BMI, CVC | 8-isoprostane: 12.10 (IQR: 6.23–23.98) ng/dL | 8-isoprostane | ||||||
| 427 | 51 ± 12 | 82 | 31.4 ± 6 | 48 ± 24 | HT: 49 | |||||
| Control Group | 8-isoprostane: 5.07 (IQR: 1.41–11.56) ng/dL | |||||||||
| 139 | 45 ± 12 | 74 | 27.9 ± 5 | 3 ± 2 | HT: 24 | |||||
| Wang et al. [59] | Study Group—Elderly With OSA | No | Hcy: 18.70 ± 4.73 µmol/L MDA: 6.18 ± 1.23 nmol/mL GSH: 8.79 ± 2.68 mg/L | Hcy, MDA, GSH | ||||||
| 32 | 65.8 ± 7.2 | 90 | 23.34 ± 2.36 | 38.67 ± 21.28 | HT: 15.63 | |||||
| Control Group—The Elderly Without OSA | Hcy: 11.13 ± 3.05 µmol/L MDA: 5.04 ± 0.69 nmol/mL GSH: 6.68 ± 3.13 mg/L | |||||||||
| 29 | 69.4 ± 4.2 | 93.1 | 26.85 ± 2.9 | 2.93 ± 1.09 | HT: 13.79 | |||||
| Study Group—Non-Elderly with OSA | Hcy: 10.84 ± 2.56 µmol/L MDA: 5.18 ± 1.51 nmol/mL GSH: 6.42 ± 2.00 mg/L | Hcy, MDA, GSH | ||||||||
| 51 | 42.7 ± 8.3 | 90.2 | 28.36 ± 3.51 | 45.51 ± 25.20 | HT: 13.73 | |||||
| Control Group—Non-Elderly without OSA | Hcy: 8.90 ± 1.23 µmol/L MDA: 4.12 ± 1.09 nmol/mL GSH: 4.18 ± 1.19 mg/L | |||||||||
| 23 | 44.7 ± 12.3 | 87 | 25.13 ± 3.61 | 3.42 ± 2.10 | HT: 13.04 | |||||
| Ortaç Ersoy et al. [60] | OSA | AHI (OSA group vs OSA + CAD group) | NOx: 66.7 ± 20.3 μmol/L Hcy: 14.3 ± 2.7 μmol/L TAC: 2.2 ± 0.4 μmol/L | No | ||||||
| 15 | 53.7 ± 8.1 | - | 27.5 ± 3.2 | 41.6 ± 27.4 | CAD: 0 | |||||
| CAD | NOx: 77.6 ± 16.6 μmol/L Hcy: 16.4 ± 6.8 μmol/L TAC: 2.2 ± 0.2 μmol/L | |||||||||
| 15 | 53.2 ± 7.2 | - | 25.7 ± 3.6 | 1.9 ± 1.2 | CAD: 100 | |||||
| OSA + CAD | NOx: 83.7 ± 26.9 μmol/L Hcy: 16.9 ± 4.6 μmol/L TAC: 2.2 ± 0.3 μmol/L | |||||||||
| 12 | 54.7 ± 6.9 | - | 28.0 ± 3.4 | 16.2 ± 9.7 | CAD: 100 | |||||
| Normal | NOx: 73.8 ± 17.8 μmol/L Hcy: 12.3 ± 2.5 μmol/L TAC: 2.1 ± 0.3 μmol/L | |||||||||
| 10 | 48.8 ± 6.6 | - | 26.2 ± 1.6 | 2.7 ± 1.9 | CAD: 0 | |||||
| Author | Study and Control Characteristics | Biomarker Levels | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Sample Size (n) | Age ± SD | Male (%) | Mean BMI ± SD | Mean AHI ± SD | CVC (%) | Differences in Groups * | Results | Differences in Groups ^ | ||
| Chen et al. [61] | AHI < 30 | Age, HT | TAC: 596.3 ± 118.3 μmol/L 8-OHdG: 33.7 (IQR: 27.2–49.7) ng/mgcr | No | ||||||
| 34 | 58.1 ± 13.9 | 61.8 | 24.9 ± 4.2 | 18.1 (10.3–24.5) | Stroke: 100HT: 74 | |||||
| AHI > 30 | TAC: 606.1 ± 102.6 μmol/L 8-OHdG: 39.5 (IQR: 29.6–51.5) ng/mgcr | |||||||||
| 58 | 66.5 ± 11.0 | 72.4 | 24.9 ± 3.7 | 50.9 (38.3–60.9) | Stroke: 100 HT: 91 | |||||
| Gille et al. [62] | No OSA | Age (No OSA vs moderate OSA) | 8-OHdG MMP-7 Data presented graphically; parametric results cannot be displayed in this table. | 8-OHdG (moderate and severe OSA vs. mild and No OSA), MMP-7 (severe OSA vs. moderate, mild and No OSA) | ||||||
| 5 | 61 ± 10.9 | 60 | 27.7 ± 4.3 | 3 ± 1.7 | 41.2 (HT: 35.3, IHD: 11.8, Stroke/TIA: 0) $ | |||||
| Mild OSA | ||||||||||
| 12 | 67.6 ± 7.5 | 75 | 27.8 ± 2.5 | 10.8 ± 2.6 | 41.2 (HT: 35.3, IHD: 11.8, Stroke/TIA: 0) $ | |||||
| Moderate OSA | ||||||||||
| 10 | 72.1 ± 7.7 | 90 | 27.3 ± 4 | 22.6 ± 3.4 | 40 (HT: 30, IHD: 30, Stroke/TIA: 0) | |||||
| Severe OSA | ||||||||||
| 18 | 69.8 ± 8.6 | 94 | 28.6 ± 3.7 | 60.5 ± 25 | 100 (HT: 55.6, IHD: 61.1, Stroke/TIA: 16.7) | |||||
| Yadav et al. [63] | High AHI | No | PON1:101 ± 64 nmol/mL/min | PON1 | ||||||
| 20 | 49 ± 10 | 15 | 52 ± 6 | 21.3 (13.5–45.7) | HT: 65 | |||||
| Low AHI | PON1:186 ± 68 nmol/mL/min | |||||||||
| 21 | 45 ± 9 | 20 | 50 ± 8 | 4.3 (3.7–6.8) | HT: 50 | |||||
| Yamauchi et al. [64] | Non-Severe OSA | BMI | 8-OHdG: 8.5 ± 2.4 ng/mL/creatinine mg/mL | 8-OHdG | ||||||
| 70 | 49.3 ± 12.2 | 82.9 | 25.9 ± 3.8 | 12.1 ± 9.3 | HT: 21.4 | |||||
| Severe OSA | 8-OHdG: 9.5 ± 2.5 ng/mL/creatinine mg/mL | |||||||||
| 58 | 48.8 ± 11.0 | 98.0 | 29.5 ± 5.3 | 60.7 ± 19.2 | HT: 34.5 | |||||
| Yardim-Akaydin et al. [65] | Controls | Age (moderate and severe apnea vs. mild and control), BMI (severe apnea vs. moderate, severe apnea and control), HT (severe apnea vs. control) | MDA: 2.24 ± 0.91 µM 8-OHdG: 25.63 ± 8.14 nM/creatinine mM | MDA (controls vs. mild, moderate and severe apnea), 8-OHdG (mild apnea vs. severe apnea; controls vs. mild and moderate apnea) | ||||||
| 25 | 43.4 ± 8.2 | 56 | 27.2 ± 3.2 | 2.7 ± 1.2 | HT: 0 | |||||
| Mild Apnea | MDA: 3.01 ± 1.18 µM 8-OHdG: 38.20 ± 19.03 nM/creatinine mM | |||||||||
| 28 | 43.1 ± 9.7 | 64.29 | 30.03 ± 4.8 | 9.5 ± 3.3 | HT: 14.3 | |||||
| Moderate Apnea | MDA: 3.00 ± 0.89 µM 8-OHdG: 32.08 ± 12.69 nM/creatinine mM | |||||||||
| 30 | 52.9 ± 11.8 | 67 | 30.0 ± 4.0 | 22.5 ± 4.3 | HT: 13.3 | |||||
| Severe Apnea | MDA: 3.33 ± 1.11 µM 8-OHdG: 29.40 ± 16.35 nM/creatinine mM | |||||||||
| 59 | 51.2 ± 10.7 | 72.88 | 33.0 ± 5.8 | 56.3 ± 22.3 | HT: 32.2 | |||||
| Author | Study and Control Characteristics | Biomarker Levels | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Sample Size (n) | Age ± SD | Male (%) | Mean BMI ± SD | Mean AHI ± SD | CVC (%) | Differences in Groups * | Results | Differences in Groups ^ | |||
| Feres et al. [66] | OSA + comorbidities | Age (OSA + comorbidities and OSA vs control), BMI (OSA + comorbidities vs control) | oxLDL: 89.42 ± 29.53 U/L | No | |||||||
| 48 | 53.77 ± 8.03 | 36 | 28.97 ± 3.11 | 25.02 ± 15.97 | HT: 41.6 | ||||||
| OSA | oxLDL: 83.63 ± 25.2 U/L | ||||||||||
| 24 | 50.75 ± 7.43 | 50 | 28.13 ± 3.74 | 23.07 ± 18.59 | HT: 0 | ||||||
| Control | oxLDL: 69.46 ± 24.79 U/L | ||||||||||
| 27 | 44.38 ± 7.03 | 38 | 25.79 ± 4.49 | 1.98 ± 1.69 | HT: 0 | ||||||
| İn et al. [67] | OSA with CV Risk Factors (obesity, hypercholesterolemia, DM, HT and smoking) | Age (OSA with CV risk factors vs OSA without CV risk factors and control subjects), BMI (OSA with CV risk factors vs OSA without CV risk factors and control subjects) | ADMA: 0.64 ± 0.12 µmol/L | ADMA (control subjects vs OSA with and without CV risk factors) | |||||||
| 26 | 57.5 ± 10.8 | 65.4 | 38.5 ± 8.6 | 34.8 ± 23.3 | 80.7# | ||||||
| OSA without CV Risk Factors | ADMA: 0.67 ± 0.13 µmol/L | ||||||||||
| 14 | 45.9 ± 8.8 | 85.7 | 28.5 ± 1.6 | 28.3 ± 24.6 | 0# | ||||||
| Control Subjects | ADMA: 0.47 ± 0.19 µmol/L | ||||||||||
| 20 | 49.3 ± 10.9 | 70 | 26.7 ± 2.3 | 1.9 ± 1.2 | 0 | ||||||
| Lavie et al. [68] | OSA + CVD | Age (OSA + CVD vs OSA − CVD and controls), BMI (OSA + CVD vs OSA − CVD and controls; OSA − CVD vs controls) | TBARS: 18.6 ± 7.3 nmol MDA/mL PON1:79.5 ± 13.6 U/min/mL | TBARS (controls vs OSA + CVD and OSA − CVD), PON1 (controls vs OSA + CVD) | |||||||
| 59 | 58.5 ± 11.3 | 83.0 | 30.6 ± 5.5 | 31.3 ± 18.5 | IHD: 37.9 MI: 8.5 HT: 75.9 | ||||||
| OSA - CVD | TBARS: 17.2 ± 6.3 nmol MDA/mL PON1:86.7 ± 17.6 U/min/mL | ||||||||||
| 55 | 46.8 ± 10.2 | 85.5 | 28.4 ± 3.5 | 26.9 ± 13.8 | IHD: 0 MI: 0 HT: 0 | ||||||
| Controls | TBARS: 12.9 ± 3.5 nmol MDA/mL PON1:92.1 ± 14.4 U/min/mL | ||||||||||
| 30 | 42.9 ± 13.8 | 90.0 | 26.0 ± 3.2 | 8.2 ± 2.6 | IHD: 3.3 MI: 0 HT: 13.3 | ||||||
| Murri et al. [69] | Hypertensive OSA | Data provided for the whole studied population only $ | CAT: 2.52 ± 1.15 nmol/min/m GPx: 20.49 ± 5.52 mmol/min/mL SOD: 0.066 ± 0.025 U/mL TAC: 3.605 ± 1.338 mmol/L | CAT, TAC | |||||||
| 60 | 55.32 ± 11.28 $ | - | 32.21 ± 5.19 $ | 54.77 ± 19.34 $ | HT: 100 | ||||||
| Normotensive OSA | CAT: 2.66 ± 1.19 nmol/min/m GPx: 23.34 ± 7.64 mmol/min/mL SOD: 0.072 ± 0.021 U/mL TAC: 4.127 ± 1.256 mmol/L | ||||||||||
| 18 | 55.32 ± 11.28 $ | - | 32.21 ± 5.19 $ | 54.77 ± 19.34 $ | HT: 0 | ||||||
| Ortaç Ersoy et al. [60] | OSA | AHI (OSA group vs OSA + CAD group) | NOx: 66.7 ± 20.3 μmol/L Hcy: 14.3 ± 2.7 μmol/L TAC: 2.2 ± 0.4 μmol/L | No | |||||||
| 15 | 53.7 ± 8.1 | - | 27.5 ± 3.2 | 41.6 ± 27.4 | CAD: 0 | ||||||
| CAD | NOx: 77.6 ± 16.6 μmol/L Hcy: 16.4 ± 6.8 μmol/L TAC: 2.2 ± 0.2 μmol/L | ||||||||||
| 15 | 53.2 ± 7.2 | - | 25.7 ± 3.6 | 1.9 ± 1.2 | CAD: 100 | ||||||
| OSA + CAD | NOx: 83.7 ± 26.9 μmol/L Hcy: 16.9 ± 4.6 μmol/L TAC: 2.2 ± 0.3 μmol/L | ||||||||||
| 12 | 54.7 ± 6.9 | - | 28.0 ± 3.4 | 16.2 ± 9.7 | CAD: 100 | ||||||
| Normal | NOx: 73.8 ± 17.8 μmol/L Hcy: 12.3 ± 2.5 μmol/L TAC: 2.1 ± 0.3 μmol/L | ||||||||||
| 10 | 48.8 ± 6.6 | - | 26.2 ± 1.6 | 2.7 ± 1.9 | CAD: 0 | ||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fiedorczuk, P.; Stróżyński, A.; Olszewska, E. Is the Oxidative Stress in Obstructive Sleep Apnea Associated with Cardiovascular Complications?—Systematic Review. J. Clin. Med. 2020, 9, 3734. https://doi.org/10.3390/jcm9113734
Fiedorczuk P, Stróżyński A, Olszewska E. Is the Oxidative Stress in Obstructive Sleep Apnea Associated with Cardiovascular Complications?—Systematic Review. Journal of Clinical Medicine. 2020; 9(11):3734. https://doi.org/10.3390/jcm9113734
Chicago/Turabian StyleFiedorczuk, Piotr, Adam Stróżyński, and Ewa Olszewska. 2020. "Is the Oxidative Stress in Obstructive Sleep Apnea Associated with Cardiovascular Complications?—Systematic Review" Journal of Clinical Medicine 9, no. 11: 3734. https://doi.org/10.3390/jcm9113734
APA StyleFiedorczuk, P., Stróżyński, A., & Olszewska, E. (2020). Is the Oxidative Stress in Obstructive Sleep Apnea Associated with Cardiovascular Complications?—Systematic Review. Journal of Clinical Medicine, 9(11), 3734. https://doi.org/10.3390/jcm9113734





