CD40/CD40L Signaling as a Promising Therapeutic Target for the Treatment of Renal Disease
Abstract
1. Introduction
2. CD40 and CD40L as Markers of Renal Function in Kidney Disease
3. Implications of CD40 Expression in Immune Cells in Kidney Disease
4. CD40 Signaling in Kidney Cells is Associated with Pathological Changes of Kidney Disease
4.1. Renal Expression of CD40 Contributes to Inflammatory Responses in Kidney Disease
4.2. Renal Expression of CD40 is Tightly Regulated and Associated with Homeostatic Conditions
5. Targeting CD40 Contributes to Therapeutic Treatments of Kidney Disease
6. CD40 and CD40L Blockade in Kidney Transplantation
7. Summary
Author Contributions
Funding
Conflicts of Interest
References
- Eckardt, K.-U.; Coresh, J.; Devuyst, O.; Johnson, R.J.; Köttgen, A.; Levey, A.S.; Levin, A. Evolving importance of kidney disease: From subspecialty to global health burden. Lancet 2013, 382, 158–169. [Google Scholar] [CrossRef]
- Hill, N.R.; Fatoba, S.T.; Oke, J.L.; Hirst, J.A.; O’Callaghan, C.A.; Lasserson, D.S.; Hobbs, F.D.R. Global Prevalence of Chronic Kidney Disease—A Systematic Review and Meta-Analysis. PLoS ONE 2016, 11, e0158765. [Google Scholar] [CrossRef]
- Desideri, G.; Panichi, V.; Paoletti, S.; Grassi, D.; Bigazzi, R.; Beati, S.; Bernabini, G.; Rosati, A.; Ferri, C.; Taddei, S.; et al. Soluble CD40 ligand is predictive of combined cardiovascular morbidity and mortality in patients on haemodialysis at a relatively short-term follow-up. Nephrol. Dial. Transplant. 2011, 26, 2983–2988. [Google Scholar] [CrossRef]
- Haller, S.; Adlakha, S.; Reed, G.; Brewster, P.; Kennedy, D.; Burket, M.W.; Colyer, W.; Yu, H.; Zhang, D.; Shapiro, J.I.; et al. Platelet Activation in Patients with Atherosclerotic Renal Artery Stenosis Undergoing Stent Revascularization. Clin. J. Am. Soc. Nephrol. 2011, 6, 2185–2191. [Google Scholar] [CrossRef] [PubMed]
- Tecklenborg, J.; Clayton, D.; Siebert, S.; Coley, S.M. The role of the immune system in kidney disease. Clin. Exp. Immunol. 2018, 192, 142–150. [Google Scholar] [CrossRef] [PubMed]
- Grewal, I.S.; Flavell, R.A. CD40 and CD154 in cell-mediated immunity. Annu. Rev. Immunol. 1998, 16, 111–135. [Google Scholar] [CrossRef] [PubMed]
- Van Kooten, C.; Banchereau, J. CD40-CD40 ligand. J. Leukoc. Biol. 2000, 67, 2–17. [Google Scholar] [CrossRef]
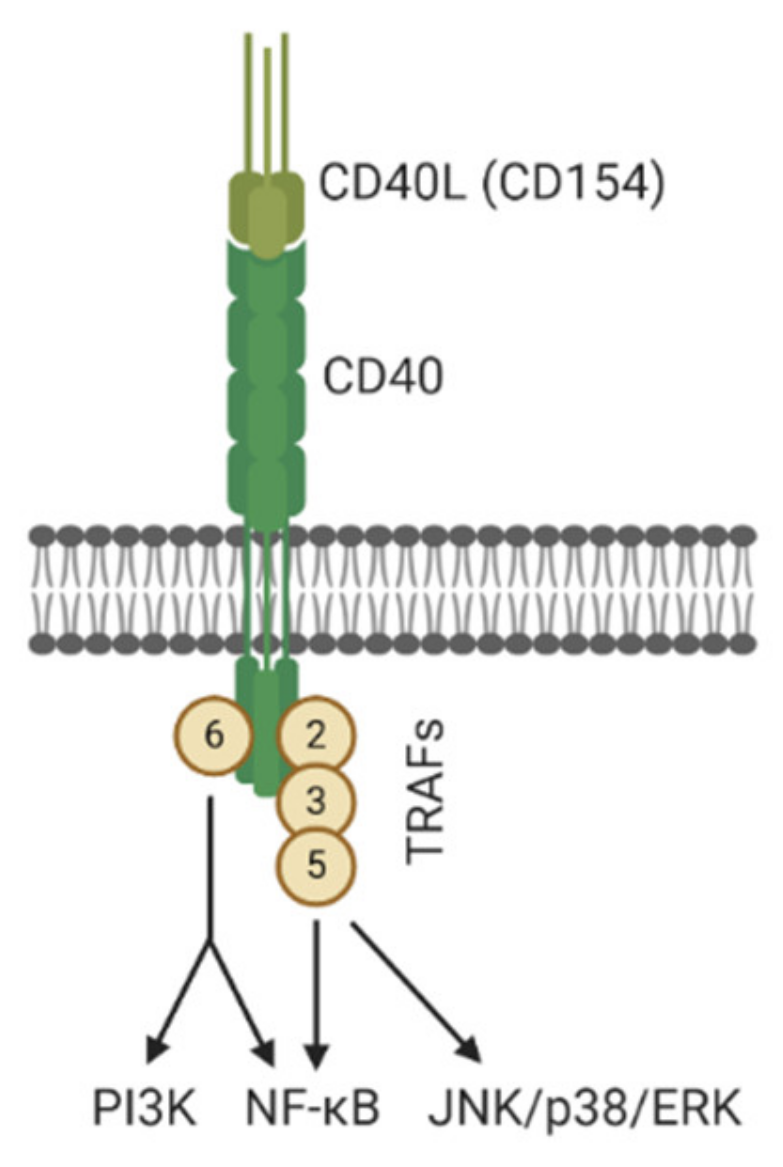
- Jabara, H.H.; Laouini, D.; Tsitsikov, E.; Mizoguchi, E.; Bhan, A.K.; Castigli, E.; Dedeoglu, F.; Pivniouk, V.; Brodeur, S.R.; Geha, R.S. The Binding Site for TRAF2 and TRAF3 but Not for TRAF6 Is Essential for CD40-Mediated Immunoglobulin Class Switching. Immunity 2002, 17, 265–276. [Google Scholar] [CrossRef]
- Razani, B.; Reichardt, A.; Cheng, G. Non-canonical NF-κB signaling activation and regulation: Principles and perspectives. Immunol. Rev. 2011, 244, 44–54. [Google Scholar] [CrossRef]
- Elgueta, R.; Benson, M.J.; De Vries, V.C.; Wasiuk, A.; Guo, Y.; Noelle, R.J. Molecular mechanism and function of CD40/CD40L engagement in the immune system. Immunol. Rev. 2009, 229, 152–172. [Google Scholar] [CrossRef]
- Portillo, J.-A.C.; Greene, J.A.; Schwartz, I.; Subauste, M.C.; Subauste, C.S. Blockade of CD40–TRAF2,3 or CD40–TRAF6 is sufficient to inhibit pro-inflammatory responses in non-haematopoietic cells. Immunology 2015, 144, 21–33. [Google Scholar] [CrossRef] [PubMed]
- Van Kooten, C.; Banchereau, J. CD40-CD40 Ligand: A Multifunctional Receptor-Ligand Pair. In Advances in Immunology; Dixon, F.J., Ed.; Academic Press: Cambridge, MA, USA, 1996; Volume 61, pp. 1–77. [Google Scholar]
- Dugger, K.; Lowder, T.W.; Tucker, T.A.; Schwiebert, L.M. Epithelial cells as immune effector cells: The role of CD40. Semin. Immunol. 2009, 21, 289–292. [Google Scholar] [CrossRef] [PubMed]
- Malvezzi, P.; Jouve, T.; Rostaing, L. Costimulation Blockade in Kidney Transplantation: An Update. Transplantation 2016, 100, 2315–2323. [Google Scholar] [CrossRef] [PubMed]
- Laxmanan, S.; Datta, D.; Geehan, C.; Briscoe, D.M.; Pal, S. CD40: A Mediator of Pro- and Anti-Inflammatory Signals in Renal Tubular Epithelial Cells. J. Am. Soc. Nephrol. 2005, 16, 2714–2723. [Google Scholar] [CrossRef] [PubMed]
- Bertelli, R.; Bonanni, A.; Di Donato, A.; Cioni, M.; Ravani, P.; Ghiggeri, G.M. Regulatory T cells and minimal change nephropathy: In the midst of a complex network. Clin. Exp. Immunol. 2016, 183, 166–174. [Google Scholar] [CrossRef] [PubMed]
- Pontrelli, P.; Ursi, M.; Ranieri, E.; Capobianco, C.; Schena, F.P.; Gesualdo, L.; Grandaliano, G. CD40L Proinflammatory and Profibrotic Effects on Proximal Tubular Epithelial Cells: Role of NF-κB and Lyn. J. Am. Soc. Nephrol. 2006, 17, 627–636. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Breidenbach, J.D.; Khalaf, F.K.; Dube, P.R.; Mohammed, C.J.; Lad, A.; Stepkowski, S.; Hinds Terry, D.; Kumarasamy, S.; Kleinhenz, A.; et al. Renal Fibrosis Is Significantly Attenuated Following Targeted Disruption of Cd40 in Experimental Renal Ischemia. J. Am. Heart Assoc. 2020, 9, e014072. [Google Scholar] [CrossRef]
- Rigothier, C.; Daculsi, R.; Lepreux, S.; Auguste, P.; Villeneuve, J.; Dewitte, A.; Doudnikoff, E.; Saleem, M.; Bourget, C.; Combe, C.; et al. CD154 Induces Matrix Metalloproteinase-9 Secretion in Human Podocytes. J. Cell. Biochem. 2016, 117, 2737–2747. [Google Scholar] [CrossRef]
- André, P.; Nannizzi-Alaimo, L.; Prasad, S.K.; Phillips, D.R. Platelet-Derived CD40L. Circulation 2002, 106, 896–899. [Google Scholar] [CrossRef]
- Mazzei, G.J.; Edgerton, M.D.; Losberger, C.; Lecoanet-Henchoz, S.; Graber, P.; Durandy, A.; Gauchat, J.-F.; Bernard, A.; Allet, B.; Bonnefoy, J.-Y. Recombinant Soluble Trimeric CD40 Ligand Is Biologically Active. J. Biol. Chem. 1995, 270, 7025–7028. [Google Scholar] [CrossRef]
- Bontekoe, J.; Bansal, V.; Lee, J.; Syed, M.; Hoppensteadt, D.; Maia, P.; Walborn, A.; Liles, J.; Vasaiwala, S.; Fareed, J. Procalcitonin as a Marker of Comorbid Atrial Fibrillation in Chronic Kidney Disease and History of Sepsis. Clin. Appl. Thromb. Hemost. 2020, 26, 1076029620932228. [Google Scholar] [CrossRef] [PubMed]
- Rusu, C.; Racasan, S.; Moldovan, D.; Kacso, I.M.; Potra, A.; Bondor, C.I.; Patiu, I.M.; Vladutiu, D.; Caprioara, M.G. Soluble CD40 ligand in haemodialysis patients: Survival impact and cardiovascular prognostic role. Biomarkers 2017, 22, 232–238. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.X.; Alderson, H.; Ritchie, J.; Kalra, P.A.; Xie, Y.; Ren, K.; Nguyen, H.; Chen, T.; Brewster, P.; Gupta, R.; et al. Circulating CD40 and sCD40L Predict Changes in Renal Function in Subjects with Chronic Kidney Disease. Sci. Rep. 2017, 7, 7942. [Google Scholar] [CrossRef]
- Gremmel, T.; Müller, M.; Steiner, S.; Seidinger, D.; Koppensteiner, R.; Kopp, C.W.; Panzer, S. Chronic kidney disease is associated with increased platelet activation and poor response to antiplatelet therapy. Nephrol. Dial. Transplant. 2013, 28, 2116–2122. [Google Scholar] [CrossRef] [PubMed]
- Yagmur, E.; Frank, R.D.; Neulen, J.; Floege, J.; Mühlfeld, A.S. Platelet Hyperaggregability is Highly Prevalent in Patients with Chronic Kidney Disease: An Underestimated Risk Indicator of Thromboembolic Events. Clin. Appl. Thromb. Hemost. 2013, 21, 132–138. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Yamamoto, H. Interaction of receptor for advanced glycation end products with advanced oxidation protein products induces podocyte injury. Kidney Int. 2012, 82, 733–735. [Google Scholar] [CrossRef] [PubMed]
- Witko-Sarsat, V.; Friedlander, M.; Capeillère-Blandin, C.; Nguyen-Khoa, T.; Nguyen, A.T.; Zingraff, J.; Jungers, P.; Descamps-Latscha, B. Advanced oxidation protein products as a novel marker of oxidative stress in uremia. Kidney Int. 1996, 49, 1304–1313. [Google Scholar] [CrossRef]
- Pasterk, L.; Lemesch, S.; Leber, B.; Trieb, M.; Curcic, S.; Stadlbauer, V.; Schuligoi, R.; Schicho, R.; Heinemann, A.; Marsche, G. Oxidized plasma albumin promotes platelet-endothelial crosstalk and endothelial tissue factor expression. Sci. Rep. 2016, 6, 22104. [Google Scholar] [CrossRef]
- Garibaldi, S.; Barisione, C.; Marengo, B.; Ameri, P.; Brunelli, C.; Balbi, M.; Ghigliotti, G. Advanced Oxidation Protein Products-Modified Albumin Induces Differentiation of RAW264.7 Macrophages into Dendritic-Like Cells Which Is Modulated by Cell Surface Thiols. Toxins 2017, 9, 27. [Google Scholar] [CrossRef]
- Trappenburg, M.C.; van Schilfgaarde, M.; Frerichs, F.C.P.; Spronk, H.M.H.; ten Cate, H.; de Fijter, C.W.H.; Terpstra, W.E.; Leyte, A. Chronic renal failure is accompanied by endothelial activation and a large increase in microparticle numbers with reduced procoagulant capacity. Nephrol. Dial. Transplant. 2011, 27, 1446–1453. [Google Scholar] [CrossRef]
- Nomura, S. Microparticle and Atherothrombotic Diseases. J. Atheroscler. Thromb. 2016, 23, 1–9. [Google Scholar] [CrossRef]
- Boulanger, C.M.; Loyer, X.; Rautou, P.-E.; Amabile, N. Extracellular vesicles in coronary artery disease. Nat. Rev. Cardiol. 2017, 14, 259–272. [Google Scholar] [CrossRef] [PubMed]
- Almquist, T.; Mobarrez, F.; Jacobson, S.H.; Wallén, H.; Hjemdahl, P. Effects of lipid-lowering treatment on circulating microparticles in patients with diabetes mellitus and chronic kidney disease. Nephrol. Dial. Transplant. 2015, 31, 944–952. [Google Scholar] [CrossRef] [PubMed]
- Mörtberg, J.; Lundwall, K.; Mobarrez, F.; Wallén, H.; Jacobson, S.H.; Spaak, J. Increased concentrations of platelet- and endothelial-derived microparticles in patients with myocardial infarction and reduced renal function- a descriptive study. BMC Nephrol. 2019, 20, 71. [Google Scholar] [CrossRef]
- Lundwall, K.; Mörtberg, J.; Mobarrez, F.; Jacobson, S.H.; Jörneskog, G.; Spaak, J. Changes in microparticle profiles by vitamin D receptor activation in chronic kidney disease —A randomized trial. BMC Nephrol. 2019, 20, 290. [Google Scholar] [CrossRef]
- Piccoli, G.B.; Grassi, G.; Cabiddu, G.; Nazha, M.; Roggero, S.; Capizzi, I.; De Pascale, A.; Priola, A.M.; Di Vico, C.; Maxia, S.; et al. Diabetic Kidney Disease: A Syndrome Rather Than a Single Disease. Rev. Diabet. Stud. 2015, 12, 87–109. [Google Scholar] [CrossRef] [PubMed]
- Schena, F.P.; Gesualdo, L. Pathogenetic Mechanisms of Diabetic Nephropathy. J. Am. Soc. Nephrol. 2005, 16, S30–S33. [Google Scholar] [CrossRef] [PubMed]
- Lim, A.K. Diabetic nephropathy—Complications and treatment. Int. J. Nephrol. Renovasc. Dis. 2014, 7, 361–381. [Google Scholar] [CrossRef]
- Cherney, D.Z.I.; Scholey, J.W.; Sochett, E.; Bradley, T.J.; Reich, H.N. The Acute Effect of Clamped Hyperglycemia on the Urinary Excretion of Inflammatory Cytokines/Chemokines in Uncomplicated Type 1 Diabetes. Diabetes Care 2011, 34, 177–180. [Google Scholar] [CrossRef]
- Lajer, M.; Tarnow, I.; Michelson, A.D.; Jorsal, A.; Frelinger, A.L.; Parving, H.-H.; Rossing, P.; Tarnow, L. Soluble CD40 ligand is elevated in Type 1 diabetic nephropathy but not predictive of mortality, cardiovascular events or kidney function. Platelets 2010, 21, 525–532. [Google Scholar] [CrossRef]
- Kuo, H.-L.; Huang, C.-C.; Lin, T.-Y.; Lin, C.-Y. IL-17 and CD40 ligand synergistically stimulate the chronicity of diabetic nephropathy. Nephrol. Dial. Transplant. 2017, 33, 248–256. [Google Scholar] [CrossRef]
- Frieri, M. Accelerated Atherosclerosis in Systemic Lupus Erythematosus: Role of Proinflammatory Cytokines and Therapeutic Approaches. Curr. Allergy Asthma Rep. 2012, 12, 25–32. [Google Scholar] [CrossRef]
- Seghal, R.; Jabaar, A.; Anand, P.; Capetandes, A.; Frieri, M. Monitoring CD40Ligand in Patients with Systemic Lupus Erythematosus (SLE). J. Allergy Clin. Immunol. 2009, 123. [Google Scholar] [CrossRef]
- Tapia-Llanos, R.; Muñoz-Valle, J.F.; Román-Fernández, I.V.; Marín-Rosales, M.; Salazar-Camarena, D.C.; Cruz, A.; Orozco-Barocio, G.; Guareña-Casillas, J.A.; Oregon-Romero, E.; Palafox-Sánchez, C.A. Association of soluble CD40 levels with −1 C > T CD40 polymorphism and chronic kidney disease in systemic lupus erythematosus. Mol. Genet. Genom. Med. 2019, 7, e1014. [Google Scholar] [CrossRef] [PubMed]
- Abrey Recalde, M.J.; Alvarez, R.S.; Alberto, F.; Mejias, M.P.; Ramos, M.V.; Fernandez Brando, R.J.; Bruballa, A.C.; Exeni, R.A.; Alconcher, L.; Ibarra, C.A.; et al. Soluble CD40 Ligand and Oxidative Response Are Reciprocally Stimulated during Shiga Toxin-Associated Hemolytic Uremic Syndrome. Toxins 2017, 9, 331. [Google Scholar] [CrossRef] [PubMed]
- Doublier, S.; Zennaro, C.; Musante, L.; Spatola, T.; Candiano, G.; Bruschi, M.; Besso, L.; Cedrino, M.; Carraro, M.; Ghiggeri, G.M.; et al. Soluble CD40 ligand directly alters glomerular permeability and may act as a circulating permeability factor in FSGS. PLoS ONE 2017, 12, e0188045. [Google Scholar] [CrossRef] [PubMed]
- Königshausen, E.; Sellin, L. Circulating Permeability Factors in Primary Focal Segmental Glomerulosclerosis: A Review of Proposed Candidates. Biomed. Res. Int. 2016, 2016, 3765608. [Google Scholar] [CrossRef] [PubMed]
- Peev, V.; Hahm, E.; Reiser, J. Unwinding focal segmental glomerulosclerosis. F1000Res 2017, 6, 466. [Google Scholar] [CrossRef]
- Delville, M.; Sigdel, T.K.; Wei, C.; Li, J.; Hsieh, S.-C.; Fornoni, A.; Burke, G.W.; Bruneval, P.; Naesens, M.; Jackson, A.; et al. A circulating antibody panel for pretransplant prediction of FSGS recurrence after kidney transplantation. Sci. Transl. Med. 2014, 6, 256ra136. [Google Scholar] [CrossRef]
- Levin, A.; Linas, S.; Luft, F.C.; Chapman, A.B.; Textor, S. Controversies in Renal Artery Stenosis: A Review by the American Society of Nephrology Advisory Group on Hypertension. Am. J. Nephrol. 2007, 27, 212–220. [Google Scholar] [CrossRef]
- Balk, E.; Raman, G.; Chung, M.; Ip, S.; Tatsioni, A.; Alonso, A.; Chew, P.; Gilbert, S.J.; Lau, J. Effectiveness of Management Strategies for Renal Artery Stenosis: A Systematic Review. Ann. Intern. Med. 2006, 145, 901–912. [Google Scholar] [CrossRef]
- Safian, R.D.; Textor, S.C. Renal-Artery Stenosis. N. Engl. J. Med. 2001, 344, 431–442. [Google Scholar] [CrossRef] [PubMed]
- Haller, S.T.; Kalra, P.A.; Ritchie, J.P.; Chrysochou, T.; Brewster, P.; He, W.; Yu, H.; Shapiro, J.I.; Cooper, C.J. Effect of CD40 and sCD40L on renal function and survival in patients with renal artery stenosis. Hypertension 2013, 61, 894–900. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Contin, C.; Pitard, V.; Delmas, Y.; Pelletier, N.; Defrance, T.; Moreau, J.-F.; Merville, P.; Déchanet-Merville, J. Potential role of soluble CD40 in the humoral immune response impairment of uraemic patients. Immunology 2003, 110, 131–140. [Google Scholar] [CrossRef] [PubMed]
- Contin, C.; Pitard, V.; Itai, T.; Nagata, S.; Moreau, J.-F.; Déchanet-Merville, J. Membrane-anchored CD40 Is Processed by the Tumor Necrosis Factor-α-converting Enzyme: Implications for CD40 Signaling. J. Biol. Chem. 2003, 278, 32801–32809. [Google Scholar] [CrossRef]
- Contin-Bordes, C.; Lacraz, A.; de Précigout, V. Potential role of the soluble form of CD40 in deficient immunological function of dialysis patients: New findings of its amelioration using polymethylmethacrylate (PMMA) membrane. NDT Plus 2010, 3, i20–i27. [Google Scholar] [CrossRef] [PubMed]
- Nelson, K.; Thethi, I.; Cunanan, J.; Hoppensteadt, D.; Bajwa, R.; Fareed, J.; Bansal, V. Upregulation of Surrogate Markers of Inflammation and Thrombogenesis in Patients With ESRD: Pathophysiologic and Therapeutic Implications. Clin. Appl. Thromb. Hemost. 2010, 17, 302–304. [Google Scholar] [CrossRef]
- Opalinska, M.; Stompor, T.; Pach, D.; Mikolajczak, R.; Fedak, D.; Krzanowski, M.; Rakowski, T.; Sowa-Staszczak, A.; Glowa, B.; Garnuszek, P.; et al. Imaging of inflamed carotid artery atherosclerotic plaques with the use of 99mTc-HYNIC-IL-2 scintigraphy in end-stage renal disease patients. Eur. J. Nucl. Med. Mol. Imaging 2012, 39, 673–682. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Liew, A. Perspectives in renal replacement therapy: Haemodialysis. Nephrology 2018, 23, 95–99. [Google Scholar] [CrossRef]
- Rios, D.R.A.; das Graças Carvalho, M.; Lwaleed, B.A.; e Silva, A.C.S.; Borges, K.B.G.; Dusse, L.M.S. Hemostatic changes in patients with end stage renal disease undergoing hemodialysis. Clin. Chim. Acta 2010, 411, 135–139. [Google Scholar] [CrossRef]
- Wang, X.-F.; Zhang, B.-H.; Lu, X.-Q.; Wang, P. Efficacy of different hemodialysis methods on dendritic cell marker CD40 and CD80 and platelet activation marker CD62P and P10 in patients with chronic renal failure. J. Clin. Lab. Anal. 2019, 33, e22713. [Google Scholar] [CrossRef] [PubMed]
- Gamboa, J.L.; Pretorius, M.; Todd-Tzanetos, D.R.; Luther, J.M.; Yu, C.; Ikizler, T.A.; Brown, N.J. Comparative Effects of Angiotensin-Converting Enzyme Inhibition and Angiotensin-Receptor Blockade on Inflammation during Hemodialysis. J. Am. Soc. Nephrol. 2012, 23, 334–342. [Google Scholar] [CrossRef] [PubMed]
- Pawlak, K.; Mysliwiec, M.; Pawlak, D. Impact of residual renal function and HCV seropositivity on plasma CD40/CD40L system and oxidative status in haemodialysis patients. Clin. Biochem. 2010, 43, 1393–1398. [Google Scholar] [CrossRef] [PubMed]
- Ulusoy, S.; Ozkan, G.; Menteşe, A.; Yavuz, A.; Karahan, S.C.; Sümer, A.U. Signal peptide-CUB-EGF domain-containing protein 1 (SCUBE1) level in hemodialysis patients and parameters affecting that level. Clin. Biochem. 2012, 45, 1444–1449. [Google Scholar] [CrossRef] [PubMed]
- Stępniewska, J.; Dołęgowska, B.; Chruściana, M.; Gołembiewska, E.; Malinowska-Jędraszczyk, A.; Marchelek-Myśliwiec, M.; Ciechanowski, K. Platelet—Derived CD154 antigen in patients with chronic kidney disease. Clin. Biochem. 2016, 49, 243–247. [Google Scholar] [CrossRef]
- Esposito, P.; Rampino, T.; Gregorini, M.; Gabanti, E.; Bianzina, S.; Dal Canton, A. Mechanisms underlying sCD40 production in hemodialysis patients. Cell. Immunol. 2012, 278, 10–15. [Google Scholar] [CrossRef] [PubMed]
- Woo, J.S.; Kim, W.; Lee, S.R.; Jung, K.H.; Kim, W.S.; Lew, J.H.; Lee, T.W.; Lim, C.K. Platelet reactivity in patients with chronic kidney disease receiving adjunctive cilostazol compared with a high-maintenance dose of clopidogrel: Results of the Effect of Platelet Inhibition According to Clopidogrel Dose in Patients with Chronic Kidney Disease (PIANO-2 CKD) randomized study. Am. Heart J. 2011, 162, 1018–1025. [Google Scholar] [CrossRef] [PubMed]
- Peters, A.L.; Stunz, L.L.; Meyerholz, D.K.; Mohan, C.; Bishop, G.A. Latent Membrane Protein 1, the EBV-Encoded Oncogenic Mimic of CD40, Accelerates Autoimmunity in B6.Sle1 Mice. J. Immunol. 2010, 185, 4053–4062. [Google Scholar] [CrossRef]
- Zhang, Y.U.; Saha, S.; Rosenfeld, G.; Gonzalez, J.; Pepeljugoski, K.P.; Peeva, E. Raloxifene Modulates Estrogen-mediated B Cell Autoreactivity in NZB/W F1 Mice. J. Rheumatol. 2010, 37, 1646–1657. [Google Scholar] [CrossRef]
- Zhao, L.-D.; Liang, D.; Wu, X.-N.; Li, Y.; Niu, J.-W.; Zhou, C.; Wang, L.; Chen, H.; Zheng, W.-J.; Fei, Y.-Y.; et al. Contribution and underlying mechanisms of CXCR4 overexpression in patients with systemic lupus erythematosus. Cell. Mol. Immunol. 2017, 14, 842–849. [Google Scholar] [CrossRef]
- Yin, S.; Mao, Y.; Li, X.; Yue, C.; Zhou, C.; Huang, L.; Mo, W.; Liang, D.; Zhang, J.; He, W.; et al. Hyperactivation and in situ recruitment of inflammatory Vδ2 T cells contributes to disease pathogenesis in systemic lupus erythematosus. Sci. Rep. 2015, 5, 14432. [Google Scholar] [CrossRef]
- Desai-Mehta, A.; Lu, L.; Ramsey-Goldman, R.; Datta, S.K. Hyperexpression of CD40 ligand by B and T cells in human lupus and its role in pathogenic autoantibody production. J. Clin. Investig. 1996, 97, 2063–2073. [Google Scholar] [CrossRef]
- Zhou, Y.; Yuan, J.; Pan, Y.; Fei, Y.; Qiu, X.; Hu, N.; Luo, Y.; Lei, W.; Li, Y.; Long, H.; et al. T cell CD40LG gene expression and the production of IgG by autologous B cells in systemic lupus erythematosus. Clin. Immunol. 2009, 132, 362–370. [Google Scholar] [CrossRef] [PubMed]
- Pau, E.; Chang, N.-H.; Loh, C.; Lajoie, G.; Wither, J.E. Abrogation of pathogenic IgG autoantibody production in CD40L gene-deleted lupus-prone New Zealand Black mice. Clin. Immunol. 2011, 139, 215–227. [Google Scholar] [CrossRef]
- Strickland, F.M.; Hewagama, A.; Lu, Q.; Wu, A.; Hinderer, R.; Webb, R.; Johnson, K.; Sawalha, A.H.; Delaney, C.; Yung, R.; et al. Environmental exposure, estrogen and two X chromosomes are required for disease development in an epigenetic model of lupus. J. Autoimmun. 2012, 38, J135–J143. [Google Scholar] [CrossRef] [PubMed]
- Lu, Q.; Wu, A.; Tesmer, L.; Ray, D.; Yousif, N.; Richardson, B. Demethylation of CD40LG on the Inactive X in T Cells from Women with Lupus. J. Immunol. 2007, 179, 6352–6358. [Google Scholar] [CrossRef] [PubMed]
- Sasidhar, M.V.; Itoh, N.; Gold, S.M.; Lawson, G.W.; Voskuhl, R.R. The XX sex chromosome complement in mice is associated with increased spontaneous lupus compared with XY. Ann. Rheum. Dis. 2012, 71, 1418–1422. [Google Scholar] [CrossRef] [PubMed]
- Strickland, F.M.; Hewagama, A.; Wu, A.; Sawalha, A.H.; Delaney, C.; Hoeltzel, M.F.; Yung, R.; Johnson, K.; Mickelson, B.; Richardson, B.C. Diet Influences Expression of Autoimmune-Associated Genes and Disease Severity by Epigenetic Mechanisms in a Transgenic Mouse Model of Lupus. Arthritis Rheum. 2013, 65, 1872–1881. [Google Scholar] [CrossRef]
- Yang, J.; Fang, P.; Yu, D.; Zhang, L.; Zhang, D.; Jiang, X.; Yang William, Y.; Bottiglieri, T.; Kunapuli Satya, P.; Yu, J.; et al. Chronic Kidney Disease Induces Inflammatory CD40+ Monocyte Differentiation via Homocysteine Elevation and DNA Hypomethylation. Circ. Res. 2016, 119, 1226–1241. [Google Scholar] [CrossRef]
- Dai, J.; Fang, P.; Saredy, J.; Xi, H.; Ramon, C.; Yang, W.; Choi, E.T.; Ji, Y.; Mao, W.; Yang, X.; et al. Metabolism-associated danger signal-induced immune response and reverse immune checkpoint-activated CD40+ monocyte differentiation. J. Hematol. Oncol. 2017, 10, 141. [Google Scholar] [CrossRef]
- Fell, L.H.; Seiler-Mußler, S.; Sellier, A.B.; Rotter, B.; Winter, P.; Sester, M.; Fliser, D.; Heine, G.H.; Zawada, A.M. Impact of individual intravenous iron preparations on the differentiation of monocytes towards macrophages and dendritic cells. Nephrol. Dial. Transplant. 2016, 31, 1835–1845. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Md, S.J.; Xiaofeng, Y.; Hong, W. Hyperhomocysteinemia, DNA methylation and vascular disease. Clin. Chem. Lab. Med. 2007, 45, 1660–1666. [Google Scholar] [CrossRef]
- Prontera, C.; Martelli, N.; Evangelista, V.; D’Urbano, E.; Manarini, S.; Recchiuti, A.; Dragani, A.; Passeri, C.; Davì, G.; Romano, M. Homocysteine Modulates the CD40/CD40L System. J. Am. Coll. Cardiol. 2007, 49, 2182–2190. [Google Scholar] [CrossRef]
- Brightbill, H.D.; Suto, E.; Blaquiere, N.; Ramamoorthi, N.; Sujatha-Bhaskar, S.; Gogol, E.B.; Castanedo, G.M.; Jackson, B.T.; Kwon, Y.C.; Haller, S.; et al. NF-κB inducing kinase is a therapeutic target for systemic lupus erythematosus. Nat. Commun. 2018, 9, 179. [Google Scholar] [CrossRef]
- Katakam, A.K.; Brightbill, H.; Franci, C.; Kung, C.; Nunez, V.; Jones, C.; Peng, I.; Jeet, S.; Wu, L.C.; Mellman, I.; et al. Dendritic cells require NIK for CD40-dependent cross-priming of CD8+ T cells. Proc. Natl. Acad. Sci. USA 2015, 112, 14664–14669. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, M.; Taubitz, A.; Eltrich, N.; Mulay, S.R.; Allam, R.; Vielhauer, V. Analysis of TNF-mediated recruitment and activation of glomerular dendritic cells in mouse kidneys by compartment-specific flow cytometry. Kidney Int. 2013, 84, 116–129. [Google Scholar] [CrossRef][Green Version]
- Haller, S.T.; Kumarasamy, S.; Folt, D.A.; Wuescher, L.M.; Stepkowski, S.; Karamchandani, M.; Waghulde, H.; Mell, B.; Chaudhry, M.; Maxwell, K.; et al. Targeted disruption of Cd40 in a genetically hypertensive rat model attenuates renal fibrosis and proteinuria, independent of blood pressure. Kidney Int. 2017, 91, 365–374. [Google Scholar] [CrossRef] [PubMed]
- De Miguel, C.; Das, S.; Lund, H.; Mattson, D.L. T lymphocytes mediate hypertension and kidney damage in Dahl salt-sensitive rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2010, 298, R1136–R1142. [Google Scholar] [CrossRef]
- Yellin, M.J.; D’Agati, V.; Parkinson, G.; Han, A.S.-Y.; Szema, A.; Baum, D.; Estes, D.; Szabolcs, M.; Chess, L. Immunohistologic analysis of renal CD40 and CD40L expression in lupus nephritis and other glomerulonephritides. Arthritis Rheum. 1997, 40, 124–134. [Google Scholar] [CrossRef]
- Donner, A.J.; Yeh, S.T.; Hung, G.; Graham, M.J.; Crooke, R.M.; Mullick, A.E. CD40 Generation 2.5 Antisense Oligonucleotide Treatment Attenuates Doxorubicin-induced Nephropathy and Kidney Inflammation. Mol. Ther. Nucleic Acids 2015, 4, e265. [Google Scholar] [CrossRef]
- Wei, C.; Sigdel, T.K.; Sarwal, M.M.; Reiser, J. Circulating CD40 autoantibody and suPAR synergy drives glomerular injury. Ann. Transl. Med. 2015, 3, 300. [Google Scholar] [PubMed]
- Gewin, L.S. Renal fibrosis: Primacy of the proximal tubule. Matrix Biol. 2018, 68–69, 248–262. [Google Scholar] [CrossRef] [PubMed]
- Dewitte, A.; Villeneuve, J.; Lepreux, S.; Bouchecareilh, M.; Gauthereau, X.; Rigothier, C.; Combe, C.; Ouattara, A.; Ripoche, J. CD154 Induces Interleukin-6 Secretion by Kidney Tubular Epithelial Cells under Hypoxic Conditions: Inhibition by Chloroquine. Mediat. Inflamm. 2020, 2020, 6357046. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Lai, C.-F.; Chang-Panesso, M.; Humphreys, B.D. Proximal Tubule Translational Profiling during Kidney Fibrosis Reveals Proinflammatory and Long Noncoding RNA Expression Patterns with Sexual Dimorphism. J. Am. Soc. Nephrol. 2020, 31, 23–38. [Google Scholar] [CrossRef]
- Perper, S.J.; Westmoreland, S.V.; Karman, J.; Twomey, R.; Seagal, J.; Wang, R.; McRae, B.L.; Clarke, S.H. Treatment with a CD40 Antagonist Antibody Reverses Severe Proteinuria and Loss of Saliva Production and Restores Glomerular Morphology in Murine Systemic Lupus Erythematosus. J. Immunol. 2019. [Google Scholar] [CrossRef]
- Kelley, V.; Singer, G. The antigen presentation function of renal tubular epithelial cells. Exp. Nephrol. 1993, 1, 102–111. [Google Scholar]
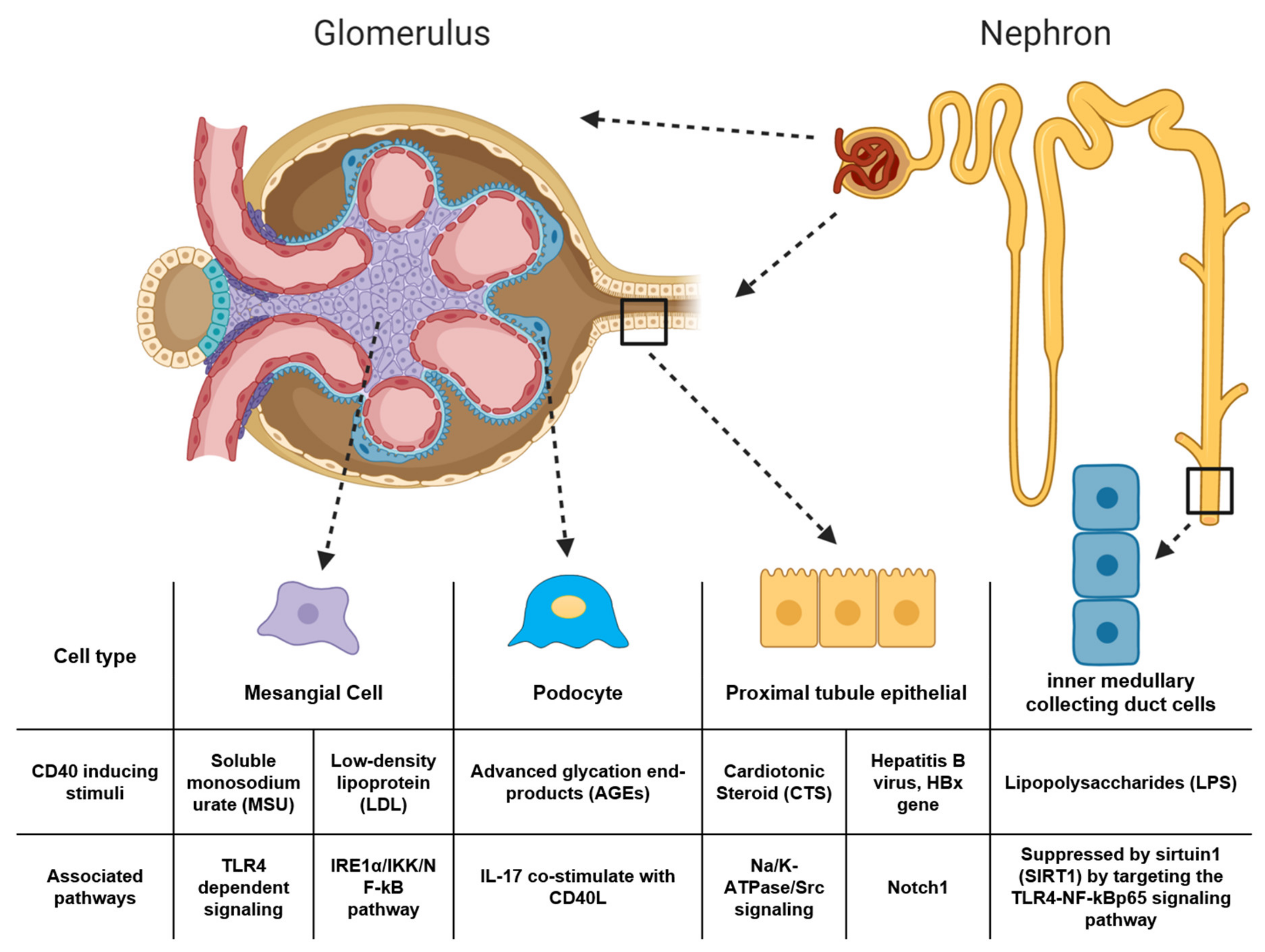
- Wang, X.; Wang, L.; Zhu, N.; Zhou, Y.; Gu, L.-J.; Yuan, W.-J. Hepatitis B virus X protein modulates renal tubular epithelial cell-induced T-cell and macrophage responses. Immunol. Cell Biol. 2016, 94, 266–273. [Google Scholar] [CrossRef]
- Wang, X.; Zhou, Y.; Zhu, N.; Yuan, W.-J. Effects of hepatitis B virus X gene on apoptosis and expression of immune molecules of human proximal tubular epithelial cells. Arch. Virol. 2013, 158, 2479–2485. [Google Scholar] [CrossRef]
- Wang, X.; Zhou, Y.; Zhu, N.; Wang, L.; Gu, L.J.; Yuan, W.J. The deposition of Notch1 in hepatitis B virus-associated nephropathy and its role in hepatitis B virus X protein-induced epithelial–mesenchymal transdifferentiation and immunity disorder in renal tubular epithelial cells. J. Viral Hepat. 2014, 21, 734–743. [Google Scholar] [CrossRef]
- Liu, J.; Tian, J.; Haas, M.; Shapiro, J.I.; Askari, A.; Xie, Z. Ouabain Interaction with Cardiac Na+/K+-ATPase Initiates Signal Cascades Independent of Changes in Intracellular Na+ and Ca2+ Concentrations. J. Biol. Chem. 2000. [Google Scholar] [CrossRef]
- Fedorova, L.V.; Raju, V.; El-Okdi, N.; Shidyak, A.; Kennedy, D.J.; Vetteth, S.; Giovannucci, D.R.; Bagrov, A.Y.; Fedorova, O.V.; Shapiro, J.I.; et al. The cardiotonic steroid hormone marinobufagenin induces renal fibrosis: Implication of epithelial-to-mesenchymal transition. Am. J. Physiol. Ren. Physiol. 2009, 296, F922–F934. [Google Scholar] [CrossRef] [PubMed]
- Komiyama, Y.; Dong, X.H.; Nishimura, N.; Masaki, H.; Yoshika, M.; Masuda, M.; Takahashi, H. A novel endogenous digitalis, telocinobufagin, exhibits elevated plasma levels in patients with terminal renal failure. Clin. Biochem. 2005, 38, 36–45. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.X.; Zhang, S.; Cui, X.; Zhang, J.; Yu, H.; Khalaf, F.K.; Malhotra, D.; Kennedy, D.J.; Shapiro, J.I.; Tian, J.; et al. Na/K-ATPase/src complex mediates regulation of CD40 in renal parenchyma. Nephrol. Dial. Transplant. 2017, 33, 1138–1149. [Google Scholar] [CrossRef]
- Yu, H.; Cui, S.; Mei, Y.; Li, Q.; Wu, L.; Duan, S.; Cai, G.; Zhu, H.; Fu, B.; Zhang, L.; et al. Mesangial Cells Exhibit Features of Antigen-Presenting Cells and Activate CD4+ T Cell Responses. J. Immunol. Res. 2019, 2019, 2121849. [Google Scholar] [CrossRef]
- Xiao, J.; Fu, C.; Zhang, X.; Zhu, D.; Chen, W.; Lu, Y.; Ye, Z. Soluble monosodium urate, but not its crystal, induces toll like receptor 4-dependent immune activation in renal mesangial cells. Mol. Immunol. 2015, 66, 310–318. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Zhang, L.; Liu, Q.; Tang, L.; Sun, H.; Guo, H. Endoplasmic reticulum stress preconditioning antagonizes low-density lipoprotein-induced inflammation in human mesangial cells through upregulation of XBP1 and suppression of the IRE1α/IKK/NF-B pathway. Mol. Med. Rep. 2014, 11. [Google Scholar] [CrossRef]
- Martillo, M.A.; Nazzal, L.; Crittenden, D.B. The crystallization of monosodium urate. Curr. Rheumatol. Rep. 2014, 16, 400. [Google Scholar] [CrossRef] [PubMed]
- Tsai, C.-W.; Lin, S.-Y.; Kuo, C.-C.; Huang, C.-C. Serum Uric Acid and Progression of Kidney Disease: A Longitudinal Analysis and Mini-Review. PLoS ONE 2017, 12, e0170393. [Google Scholar] [CrossRef] [PubMed]
- Nitta, K. Clinical assessment and management of dyslipidemia in patients with chronic kidney disease. Clin. Exp. Nephrol. 2012, 16, 522–529. [Google Scholar] [CrossRef]
- Vaziri, N.D.; Vaziri, N.D.; Norris, K. Lipid Disorders and Their Relevance to Outcomes in Chronic Kidney Disease. Blood Purif. 2011, 31, 189–196. [Google Scholar] [CrossRef]
- Lin, Q.-Q.; Geng, Y.-W.; Jiang, Z.-W.; Tian, Z.-J. SIRT1 regulates lipopolysaccharide-induced CD40 expression in renal medullary collecting duct cells by suppressing the TLR4-NF-κB signaling pathway. Life Sci. 2017, 170, 100–107. [Google Scholar] [CrossRef] [PubMed]
- Lin, Q.; Geng, Y.; Zhao, M.; Lin, S.; Zhu, Q.; Tian, Z. MiR-21 Regulates TNF-α-Induced CD40 Expression via the SIRT1-NF-κB Pathway in Renal Inner Medullary Collecting Duct Cells. Cell. Physiol. Biochem. 2017, 41, 124–136. [Google Scholar] [CrossRef]
- Mardomi, A.; Mohammadi, N.; Khosroshahi, H.T.; Abediankenari, S. An update on potentials and promises of T cell co-signaling molecules in transplantation. J. Cell. Physiol. 2020, 235, 4183–4197. [Google Scholar] [CrossRef] [PubMed]
- Harland, R.C.; Klintmalm, G.; Jensik, S.; Yang, H.; Bromberg, J.; Holman, J.; Kumar, M.S.A.; Santos, V.; Larson, T.J.; Wang, X. Efficacy and safety of bleselumab in kidney transplant recipients: A phase 2, randomized, open-label, noninferiority study. Am. J. Transplant. 2019. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.-M.; Liu, J.-H.; Xue, C.-B.; Li, M.-Q.; Xing, S.; Zhang, X.; He, W.-T.; Jiang, F.-C.; Lu, X.; Zhou, P. Pharmacological inhibition of MyD88 homodimerization counteracts renal ischemia reperfusion-induced progressive renal injury in vivo and in vitro. Sci. Rep. 2016, 6, 26954. [Google Scholar] [CrossRef] [PubMed]
- Thomson, A.W.; Ezzelarab, M.B. A Tale of Two Pathways: Renewing the Promise of Anti-CD40L Blockade. Am. J. Transplant. 2017, 17, 1156–1157. [Google Scholar] [CrossRef][Green Version]
- Xiao, Z.; Juan, L.; Song, Y.; Zhijian, Z.; Jing, J.; Kun, Y.; Yuna, H.; Dongfa, D.; Lili, D.; Liuxin, T.; et al. Evaluation of humoral and cellular immune responses to a DNA vaccine encoding chicken type II collagen for rheumatoid arthritis in normal rats. Hum. Vaccines Immunother. 2015, 11, 938–945. [Google Scholar] [CrossRef]
- Wang, Y.M.; Zhou, J.J.; Wang, Y.; Watson, D.; Zhang, G.Y.; Hu, M.; Wu, H.; Zheng, G.; Wang, Y.; Durkan, A.M.; et al. Daedalic DNA vaccination against self antigens as a treatment for chronic kidney disease. Int. J. Clin. Exp. Pathol. 2013, 6, 326–333. [Google Scholar]
- Wang, Y.; Wang, Y.M.; Wang, Y.; Zheng, G.; Zhang, G.Y.; Zhou, J.J.; Tan, T.K.; Cao, Q.; Hu, M.; Watson, D.; et al. DNA vaccine encoding CD40 targeted to dendritic cells in situ prevents the development of Heymann nephritis in rats. Kidney Int. 2013, 83, 223–232. [Google Scholar] [CrossRef]
- Li, Q.; Cao, Q.; Wang, C.; Nguyen, H.; Wang, X.M.; Zheng, G.; Wang, Y.M.; Hu, S.; Alexander, S.I.; Harris, D.C.H.; et al. Dendritic cell-targeted CD40 DNA vaccine suppresses Th17 and ameliorates progression of experimental autoimmune glomerulonephritis. J. Leukoc. Biol. 2019, 105, 809–819. [Google Scholar] [CrossRef]
- McManus, M.T.; Sharp, P.A. Gene silencing in mammals by small interfering RNAs. Nat. Rev. Genet. 2002, 3, 737–747. [Google Scholar] [CrossRef]
- Narváez, A.; Guiteras, R.; Sola, A.; Manonelles, A.; Morote, J.; Torras, J.; Grinyó, J.M.; Cruzado, J.M. siRNA-silencing of CD40 attenuates unilateral ureteral obstruction-induced kidney injury in mice. PLoS ONE 2019, 14, e0215232. [Google Scholar] [CrossRef] [PubMed]
- De Ramon, L.; Ripoll, E.; Merino, A.; Lúcia, M.; Aran, J.M.; Pérez-Rentero, S.; Lloberas, N.; Cruzado, J.M.; Grinyó, J.M.; Torras, J. CD154-CD40 T-cell co-stimulation pathway is a key mechanism in kidney ischemia-reperfusion injury. Kidney Int. 2015, 88, 538–549. [Google Scholar] [CrossRef] [PubMed]
- Ripoll, È.; Merino, A.; Goma, M.; Aran, J.M.; Bolaños, N.; de Ramon, L.; Herrero-Fresneda, I.; Bestard, O.; Cruzado, J.M.; Grinyó, J.M.; et al. CD40 Gene Silencing Reduces the Progression of Experimental Lupus Nephritis Modulating Local Milieu and Systemic Mechanisms. PLoS ONE 2013, 8, e65068. [Google Scholar] [CrossRef]
- Hueso, M.; Casas, A.; Mallén, A.; de Ramón, L.; Bolaños, N.; Varela, C.; Cruzado, J.M.; Torras, J.; Navarro, E. The double edge of anti-CD40 siRNA therapy: It increases renal microcapillar density but favours the generation of an inflammatory milieu in the kidneys of ApoE−/− mice. J. Inflamm. 2019, 16, 25. [Google Scholar] [CrossRef] [PubMed]
- Frazier, K.S. Antisense Oligonucleotide Therapies: The Promise and the Challenges from a Toxicologic Pathologist’s Perspective. Toxicol. Pathol. 2014, 43, 78–89. [Google Scholar] [CrossRef]
- Zhang, T.; Pierson, R.N.; Azimzadeh, A.M. Update on CD40 and CD154 blockade in transplant models. Immunotherapy 2015, 7, 899–911. [Google Scholar] [CrossRef]
- Van der Zwan, M.; Hesselink, D.A.; van den Hoogen, M.W.F.; Baan, C.C. Costimulation Blockade in Kidney Transplant Recipients. Drugs 2020, 80, 33–46. [Google Scholar] [CrossRef]
- Burghuber, C.K.; Manook, M.; Ezekian, B.; Gibby, A.C.; Leopardi, F.V.; Song, M.; Jenks, J.; Saccoccio, F.; Permar, S.; Farris, A.B.; et al. Dual targeting: Combining costimulation blockade and bortezomib to permit kidney transplantation in sensitized recipients. Am. J. Transplant. 2019, 19, 724–736. [Google Scholar] [CrossRef]
- Vincenti, F.; Klintmalm, G.; Yang, H.; Ram Peddi, V.; Blahunka, P.; Conkle, A.; Santos, V.; Holman, J. A randomized, phase 1b study of the pharmacokinetics, pharmacodynamics, safety, and tolerability of bleselumab, a fully human, anti-CD40 monoclonal antibody, in kidney transplantation. Am. J. Transplant. 2020, 20, 172–180. [Google Scholar] [CrossRef]
- Ristov, J.; Espie, P.; Ulrich, P.; Sickert, D.; Flandre, T.; Dimitrova, M.; Müller-Ristig, D.; Weider, D.; Robert, G.; Schmutz, P.; et al. Characterization of the in vitro and in vivo properties of CFZ533, a blocking and non-depleting anti-CD40 monoclonal antibody. Am. J. Transplant. 2018, 18, 2895–2904. [Google Scholar] [CrossRef] [PubMed]
- Cordoba, F.; Wieczorek, G.; Audet, M.; Roth, L.; Schneider, M.A.; Kunkler, A.; Stuber, N.; Erard, M.; Ceci, M.; Baumgartner, R.; et al. A novel, blocking, Fc-silent anti-CD40 monoclonal antibody prolongs nonhuman primate renal allograft survival in the absence of B cell depletion. Am. J. Transplant. 2015, 15, 2825–2836. [Google Scholar] [CrossRef]
- Nashan, B.; Tedesco, H.; van den Hoogen, M.W.; Berger, S.P.; Cibrik, D.; Mulgaonkar, S.; Leeser, D.; Alloway, R.; Patel, A.; Pratschke, J.; et al. CD40 Inhibition with CFZ533—A New, Fully Human, Non-Depleting, Fc Silent Mab—Improves Renal Allograft Function While Demonstrating Comparable Efficacy vs. Tacrolimus in De-Novo CNI-Free Kidney Transplant Recipients. Transplantation 2018, 102, S366. [Google Scholar] [CrossRef]
- Goldwater, R.; Keirns, J.; Blahunka, P.; First, R.; Sawamoto, T.; Zhang, W.; Kowalski, D.; Kaibara, A.; Holman, J. A phase 1, randomized ascending single-dose study of antagonist anti-human CD40 ASKP1240 in healthy subjects. Am. J. Transplant. 2013, 13, 1040–1046. [Google Scholar] [CrossRef]
- Yang, H.; Vincenti, F.; Klintmalm, G.; Steinberg, S.; Wang, L.; Zhang, W.; Conkle, A.; Shrivastava, A.; Blahunka, P.; First, R.; et al. A Phase 1b, Randomized, Double-Blind, Parallel Group, Placebo-Controlled, Single-Dose, Pharmacokinetic, Pharmacodynamic, Safety and Tolerability Study of ASKP1240 in de novo Kidney Transplantation: 2546. Transplantation 2012, 94, 80. [Google Scholar] [CrossRef]
- Kim, S.C.; Wakwe, W.; Higginbotham, L.B.; Mathews, D.V.; Breeden, C.P.; Stephenson, A.C.; Jenkins, J.; Strobert, E.; Price, K.; Price, L.; et al. Fc-Silent Anti-CD154 Domain Antibody Effectively Prevents Nonhuman Primate Renal Allograft Rejection. Am. J. Transplant. 2017, 17, 1182–1192. [Google Scholar] [CrossRef] [PubMed]
| Disease Condition | Presence of CD40/CD40L | Associations with Kidney Function |
|---|---|---|
| Chronic kidney disease | CD40 ligand expression in platelet-derived microparticles (PMP) | Concentration of CD40 ligand + PMPs is positively associated with severity of CKD and inversely correlated with eGFR [35] |
| Circulating sCD40L and sCD40 | Increased plasma sCD40L levels are associated with declined eGFR, and sCD40 levels are negatively associated with the reduction in eGFR [24] | |
| Diabetic nephropathy | Urinary sCD40L | Elevated in patients with type 1 diabetes [40] |
| Circulating sCD40L | Circulating sCD40L is increased compared with normoalbuminuric patients but not associated with a decline in kidney function [41] | |
| Tubular expression of CD40 | Upregulated in kidney biopsy [42] | |
| Infiltrating CD40L expressing cells | ||
| Systemic lupus erythematosus (SLE) | Circulating sCD40L and sCD40 | sCD40L levels are elevated [43,44,45] and circulating levels of the CD40 receptor are negatively associated with eGFR [45] |
| Shiga toxin-associated hemolytic uremic syndrome | Circulating sCD40L | Negatively correlated with levels of urea and creatinine [46] |
| Nephrotic syndrome and focal segmental glomerulosclerosis (FSGS) | Serum sCD40L | Increased in patients but not correlated with proteinuria and eGFR [47] |
| Renal artery stenosis | Circulating sCD40L and sCD40 | Lower circulating levels of CD40 receptor (sCD40) are associated with a decline in renal function [4,54] |
| Approach | Distribution | Kidney Disease Model | Prevention Effects | Drawbacks |
|---|---|---|---|---|
| CD40L antagonist (MR1) | Non-specific | Renal ischemia reperfusion (IRI) in mouse | MR1 in combination with MyD88 inhibitor restored survival rate, decreased serum creatinine (Cr), blood urea nitrogen (BUN), attenuated tubular damage and apoptosis, and reduced inflammatory cytokines in the kidney [116] | Risk of thromboembolism events by CD40L antibody |
| DNA vaccination | Dendritic cells | Heymann nephritis (HN) in rats | Block B-cell activation and CD8+ T-cells proliferation, reduced proteinuria, glomerulosclerosis, tubular atrophy, immune cell infiltration and IgG deposition [120] | Targeting dendritic cells, limited to autoimmune-mediated kidney disease |
| Autoimmune glomerulonephritis in rats | Attenuated glomerulosclerosis and tubular atrophy; reduced immune cell infiltration [121] | |||
| siRNA | Non-specific | Unilateral ureteral obstruction (UUO) in mouse | Attenuated tubular dilation and interstitial fibrosis, reduced macrophage and CD3+ T-cells infiltration, and reduced gene expression of pro-fibrotic cytokines [123] | Lack of ability to target distribution, triggers immune responses and has off-target side effects |
| Renal ischemia reperfusion (IRI) in rats | Attenuated renal injury, CD68+ macrophages and CD3+ T-cells infiltration, pro-inflammatory genes expression, and suppressed overexpression of genes related to cell cycle [124] | |||
| Lupus nephritis in mouse | Cholesterol-conjugated anti-CD40-siRNA attenuated proteinuria, extra-capillary proliferation, interstitial infiltrates, tubular atrophy, and interstitial fibrosis; reduced serum anti-dsDNA antibodies and circulating pro-inflammatory cytokines [125] | |||
| Generation 2.5 antisense oligonucleotide (ASO) | Favorable distribution into organs including kidney | Doxorubicin (DOX)-induced nephropathy and UUO model in mouse | Improved glomerular nephropathy, interstitial and mesangial expansion, granular tubular casts, and reduced renal injury markers [91] | Potential for nephrotoxicity and hepatotoxicity |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, S.; Breidenbach, J.D.; Russell, B.H.; George, J.; Haller, S.T. CD40/CD40L Signaling as a Promising Therapeutic Target for the Treatment of Renal Disease. J. Clin. Med. 2020, 9, 3653. https://doi.org/10.3390/jcm9113653
Zhang S, Breidenbach JD, Russell BH, George J, Haller ST. CD40/CD40L Signaling as a Promising Therapeutic Target for the Treatment of Renal Disease. Journal of Clinical Medicine. 2020; 9(11):3653. https://doi.org/10.3390/jcm9113653
Chicago/Turabian StyleZhang, Shungang, Joshua D. Breidenbach, Benjamin H. Russell, Jerrin George, and Steven T. Haller. 2020. "CD40/CD40L Signaling as a Promising Therapeutic Target for the Treatment of Renal Disease" Journal of Clinical Medicine 9, no. 11: 3653. https://doi.org/10.3390/jcm9113653
APA StyleZhang, S., Breidenbach, J. D., Russell, B. H., George, J., & Haller, S. T. (2020). CD40/CD40L Signaling as a Promising Therapeutic Target for the Treatment of Renal Disease. Journal of Clinical Medicine, 9(11), 3653. https://doi.org/10.3390/jcm9113653





