Recurrence Pattern of Cervical Cancer Based on the Platinum Sensitivity Concept: A Multi-Institutional Study from the FRANCOGYN Group
Abstract
1. Introduction
2. Material and Methods
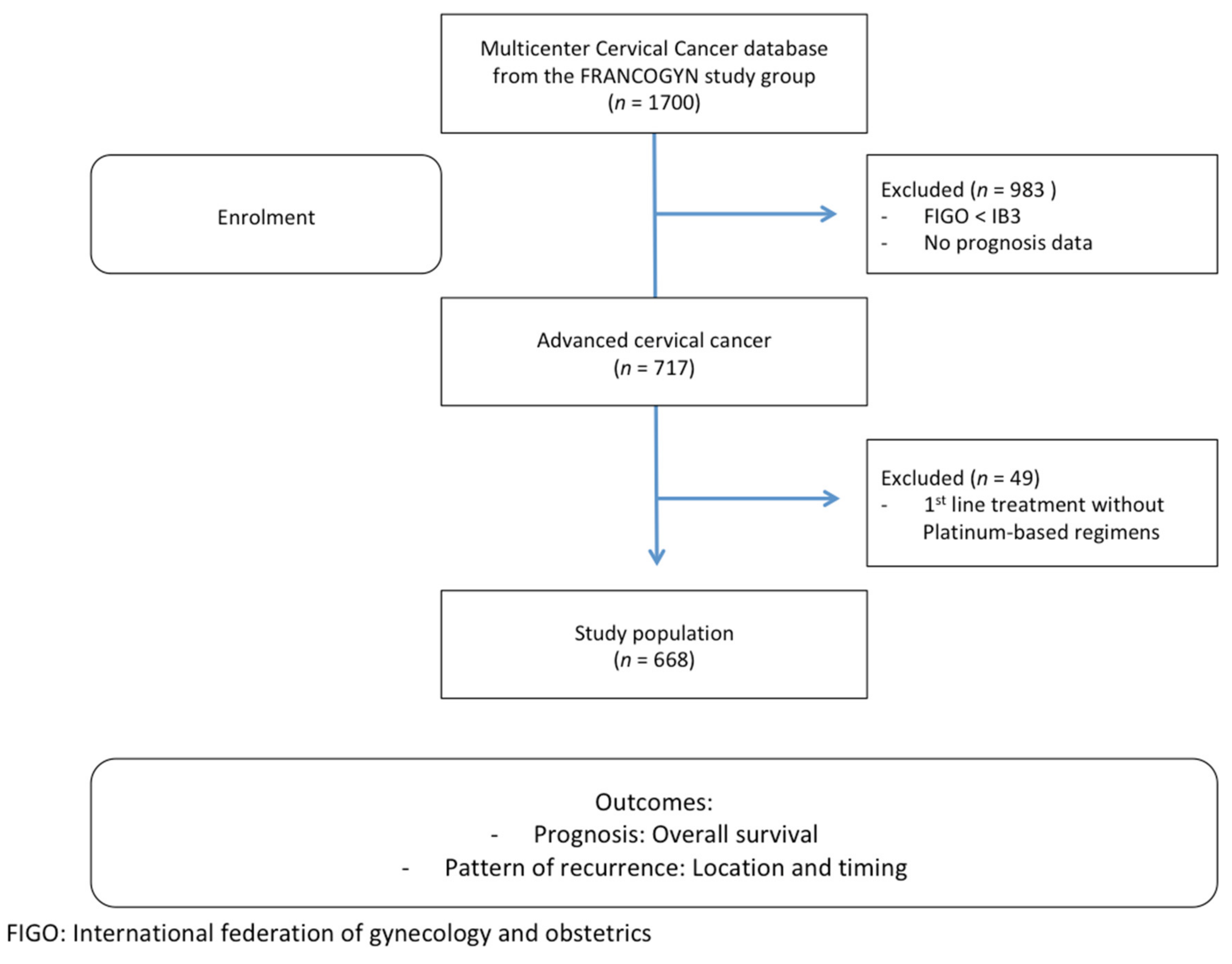
2.1. Study Population
2.2. Therapeutic Management
3. Definition and Classification of Recurrence
3.1. Definition of Platinum Sensitivity
3.2. Recurrence Classification
3.3. Statistical Analysis
4. Results
4.1. Population Characteristics
4.2. Overall Survival
4.2.1. Whole Population
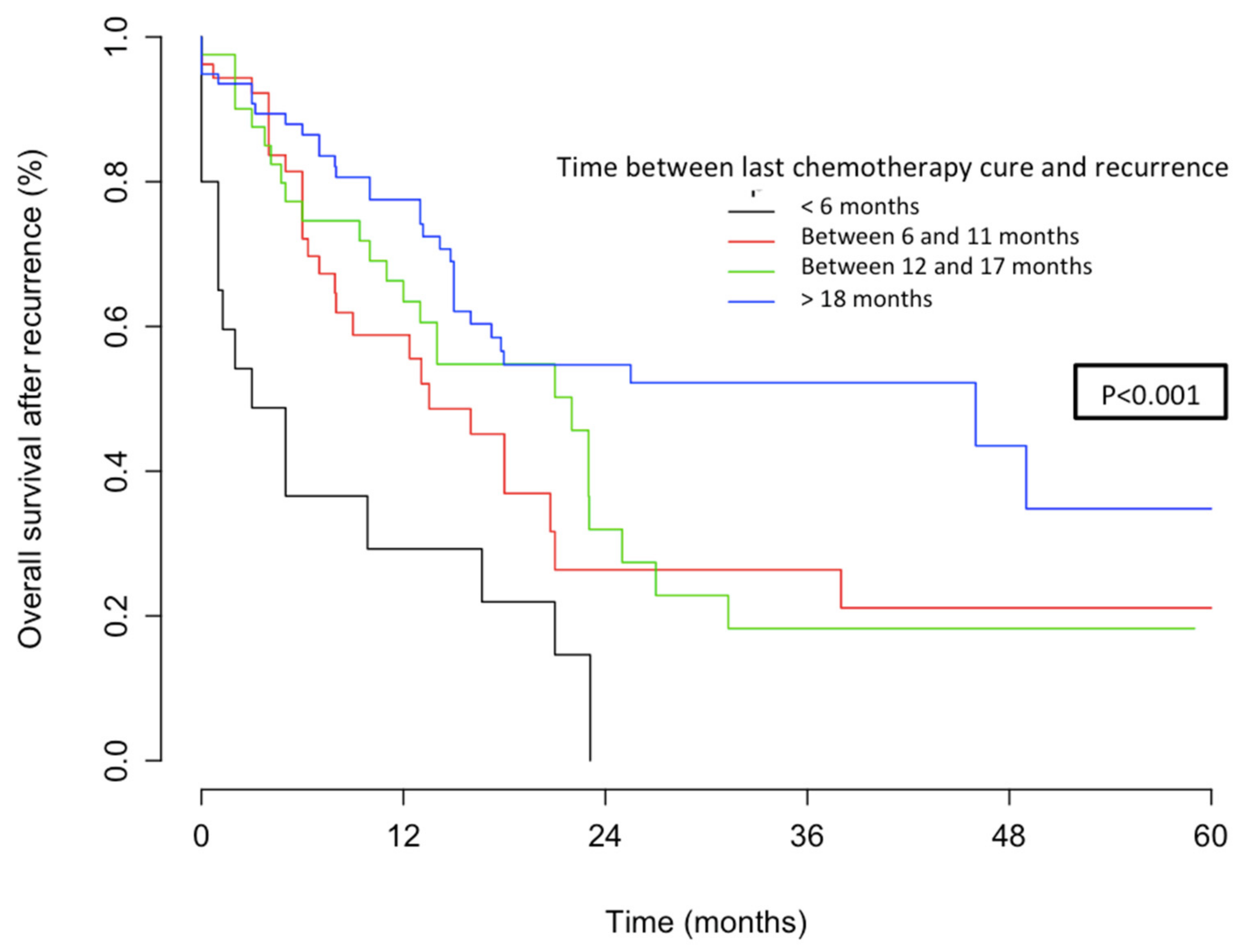
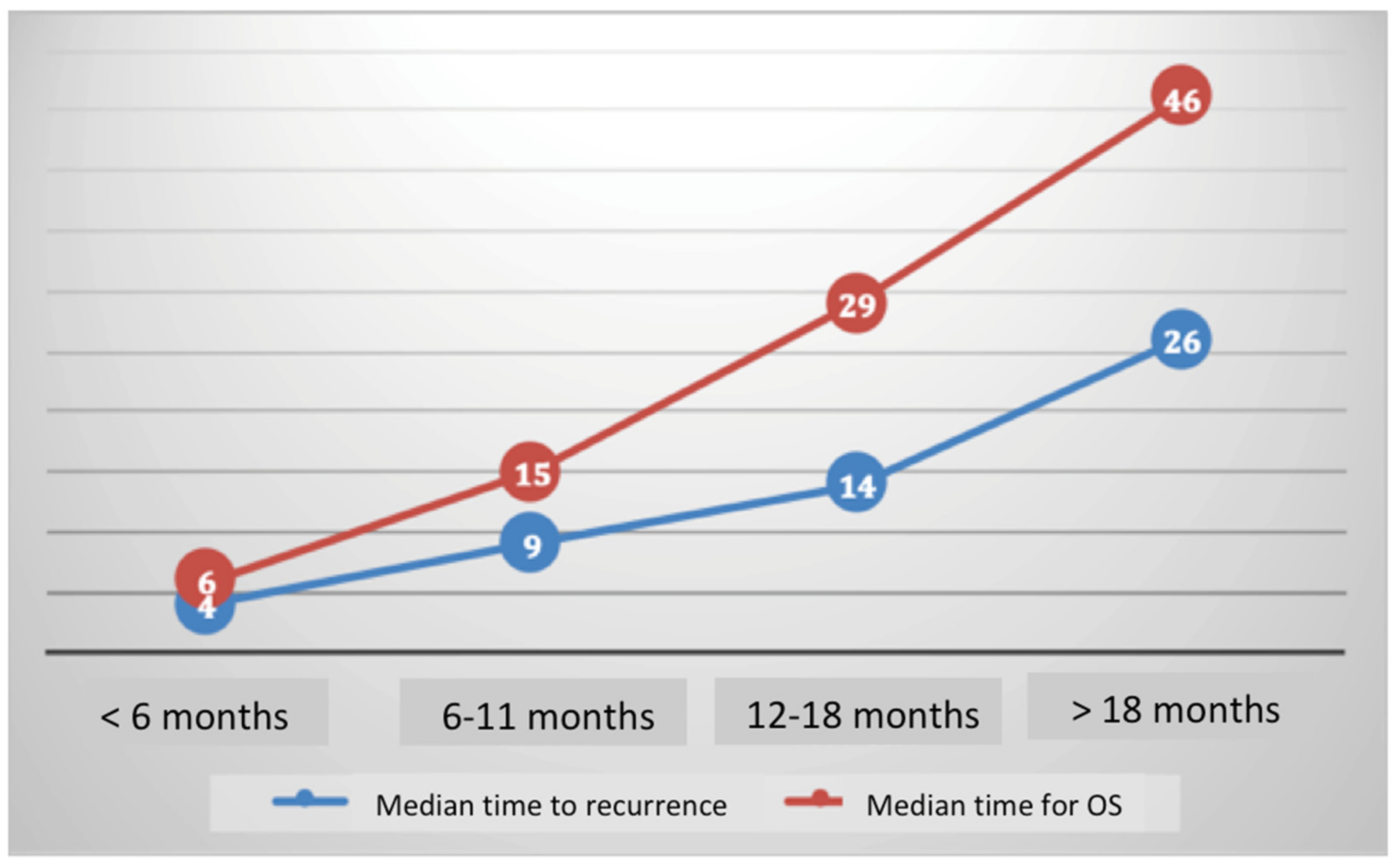
4.2.2. OS According to Platinum Sensitivity
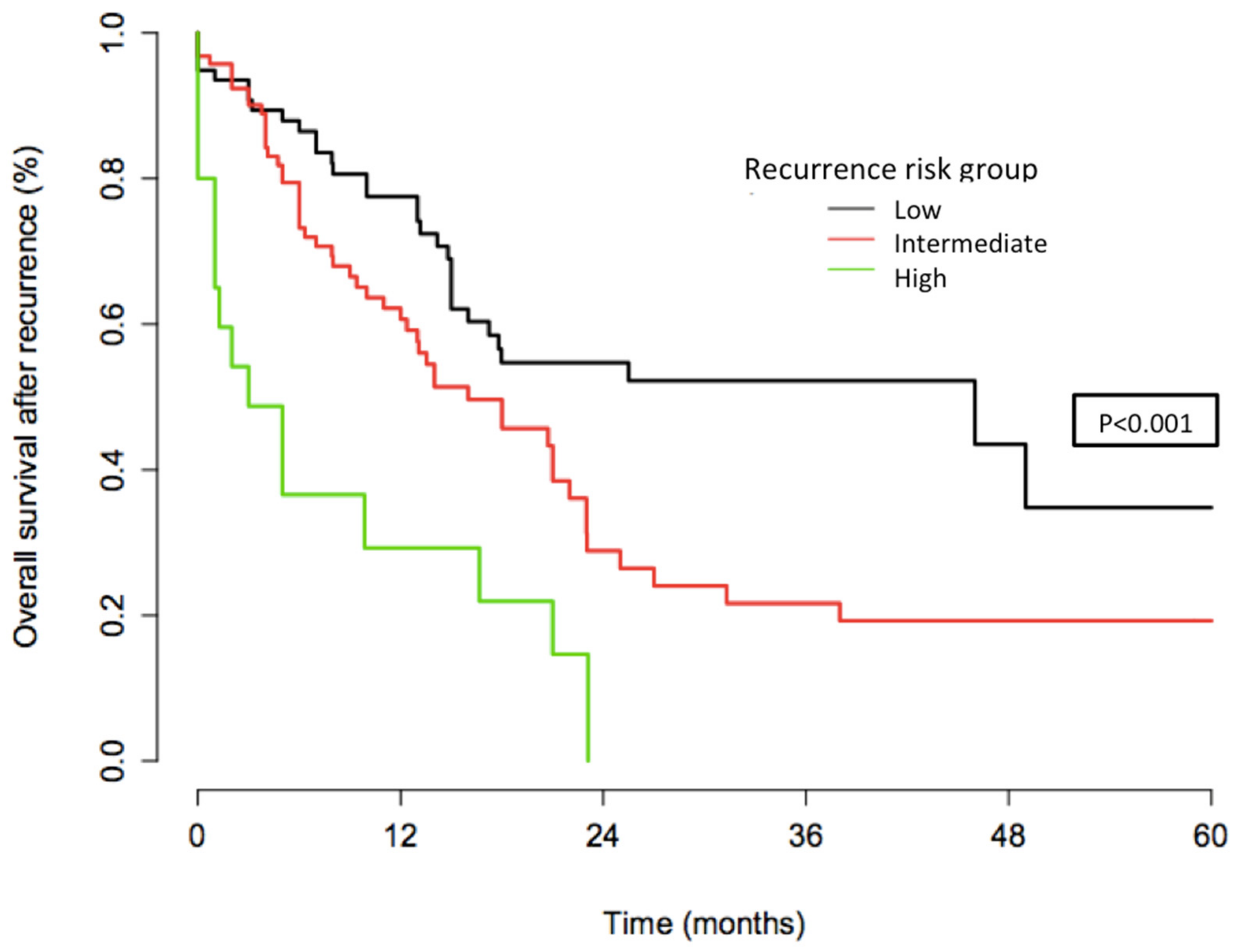
4.2.3. Stratification Analysis
4.3. Recurrence Pattern According to Platinum Sensitivity
4.3.1. Recurrence
4.3.2. Cumulative Recurrence Curves
4.4. Predictive Factors for OS in Multivariate Analysis
5. Discussion
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- International Agency for Research on Cancer. World Health Organization Cancer Today. Available online: http://gco.iarc.fr/today/home (accessed on 30 October 2020).
- Quinn, M.A.; Benedet, J.L.; Odicino, F.; Maisonneuve, P.; Beller, U.; Creasman, W.T.; Heintz, A.P.M.; Ngan, H.Y.S.; Pecorelli, S. Carcinoma of the cervix uteri. FIGO 26th Annual Report on the Results of Treatment in Gynecological Cancer. Int. J. Gynaecol. Obstet. Off. Organ. Int. Fed. Gynaecol. Obstet. 2006, 95 (Suppl. 1), S43-103. [Google Scholar] [CrossRef]
- Monk, B.J.; Tewari, K.S.; Koh, W.J. Multimodality therapy for locally advanced cervical carcinoma: State of the art and future directions. J. Clin Oncol. 2007, 25, 2952–2965. [Google Scholar] [CrossRef] [PubMed]
- Scatchard, K.; Forrest, J.L.; Flubacher, M.; Cornes, P.; Williams, C. Chemotherapy for metastatic and recurrent cervical cancer. Cochrane Database Syst. Rev. 2012, 10, CD006469. [Google Scholar] [CrossRef] [PubMed]
- Kitagawa, R.; Katsumata, N.; Ando, M.; Shimizu, C.; Fujiwara, Y.; Yoshikawa, H.; Satoh, T.; Nakanishi, T.; Ushijima, K.; Kamura, T. A multi-institutional phase II trial of paclitaxel and carboplatin in the treatment of advanced or recurrent cervical cancer. Gynecol. Oncol. 2012, 125, 307–311. [Google Scholar] [CrossRef] [PubMed]
- Moore, D.H. Chemotherapy for advanced, recurrent, and metastatic cervical cancer. J. Natl. Compr. Cancer Netw. 2008, 6, 53–57. [Google Scholar] [CrossRef]
- Gore, M.E.; Fryatt, I.; Wiltshaw, E.; Dawson, T. Treatment of relapsed carcinoma of the ovary with cisplatin or carboplatin following initial treatment with these compounds. Gynecol. Oncol. 1990, 36, 207–211. [Google Scholar] [CrossRef]
- Markman, M.; Rothman, R.; Hakes, T.; Reichman, B.; Hoskins, W.; Rubin, S.; Jones, W.; Almadrones, L.; Lewis, J.L. Second-line platinum therapy in patients with ovarian cancer previously treated with cisplatin. J. Clin. Oncol. 1991, 9, 389–393. [Google Scholar] [CrossRef]
- Fung, M.F.K.; Johnston, M.E.; Eisenhauer, E.A.; Elit, L.; Hirte, H.W.; Rosen, B. Chemotherapy for recurrent epithelial ovarian cancer previously treated with platinum—A systematic review of the evidence from randomized trials. Eur. J. Gynaecol. Oncol. 2002, 23, 104–110. [Google Scholar]
- Bhatla, N.; Berek, J.S.; Cuello Fredes, M.; Denny, L.A.; Grenman, S.; Karunaratne, K.; Kehoe, S.T.; Konishi, I.; Olawaiye, A.B.; Prat, J.; et al. Revised FIGO staging for carcinoma of the cervix uteri. Int. J. Gynaecol. Obstet. 2019, 145, 129–135. [Google Scholar] [CrossRef]
- de Foucher, T.; de Foucher, T.; Bendifallah, S.; Ouldamer, L.; Bricou, A.; Lavoué, V.; Varinot, J.; Canlorbe, G.; Carcopino, X.; Raimond, E.; et al. Patterns of recurrence and prognosis in locally advanced FIGO stage IB2 to IIB cervical cancer: Retrospective multicentre study from the FRANCOGYN group. Eur. J. Surg. Oncol. 2019, 45, 659–665. [Google Scholar] [CrossRef]
- Colombo, P.-E.; Fabbro, M.; Theillet, C.; Bibeau, F.; Rouanet, P.; Ray-Coquard, I. Sensitivity and resistance to treatment in the primary management of epithelial ovarian cancer. Crit. Rev. Oncol. Hematol. 2014, 89, 207–216. [Google Scholar] [CrossRef]
- Bendifallah, S.; de Foucher, T.; Bricou, A.; Ouldamer, L.; Lavoué, V.; Varinot, J.; Canlorbe, G.; Carcopino, X.; Raimond, E.; Huguet, F.; et al. Cervical cancer recurrence: Proposal for a classification based on anatomical dissemination pathways and prognosis. Surg. Oncol. 2019, 30, 40–46. [Google Scholar] [CrossRef] [PubMed]
- Takekuma, M.; Kuji, S.; Tanaka, A.; Takahashi, N.; Abe, M.; Hirashima, Y. Platinum sensitivity and non-cross-resistance of cisplatin analogue with cisplatin in recurrent cervical cancer. J. Gynecol. Oncol. 2015, 26, 185–192. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Takekuma, M.; Mori, K.; Iida, T.; Kurihara, K.; Saitou, M.; Tokunaga, H.; Kawana, K.; Ikeda, M.; Satoh, T.; Saito, T.; et al. The concept of platinum sensitivity could be applied to recurrent cervical cancer: A multi-institutional retrospective study from the Japanese Gynecologic Oncology Group. Cancer Chemother. Pharmacol. 2017, 80, 697–705. [Google Scholar] [CrossRef] [PubMed]
- Tanioka, M.; Katsumata, N.; Yonemori, K.; Kouno, T.; Shimizu, C.; Tamura, K.; Ando, M.; Fujiwara, Y. Second platinum therapy in patients with uterine cervical cancer previously treated with platinum chemotherapy. Cancer Chemother. Pharmacol. 2011, 68, 337–342. [Google Scholar] [CrossRef] [PubMed]
- Matoda, M.; Tanigawa, T.; Omatsu, K.; Ushioda, N.; Yamamoto, A.; Okamoto, S.; Kawamata, Y.; Kato, K.; Umayahara, K.; Takeshima, N. Platinum-free interval in second-line chemotherapy for recurrent cervical cancer. Int. J. Gynecol. Cancer 2013, 23, 1670–1674. [Google Scholar] [CrossRef]
- Nishio, S.; Kitagawa, R.; Shibata, T.; Yoshikawa, H.; Konishi, I.; Ushijima, K.; Kamura, T. Prognostic factors from a randomized phase III trial of paclitaxel and carboplatin versus paclitaxel and cisplatin in metastatic or recurrent cervical cancer: Japan Clinical Oncology Group (JCOG) trial: JCOG0505-S1. Cancer Chemother. Pharmacol. 2016, 78, 785–790. [Google Scholar] [CrossRef]
- Jiménez-Lima, R.; Arango-Bravo, E.; Galicia-Carmona, T.; Lino-Silva, L.S.; Trejo-Durán, G.E.; Alvarado-Silva, C.; Castañeda-Renderos, O.H.; Vanoye-Carlo, E.G.; La Torre, C.F.-D.; Dueñas-González, A.; et al. Immunotherapy Treatment against Cervical Cancer. Rev. Investig. Clin. Organo Hosp. Enferm. Nutr. 2020, 72, 231–238. [Google Scholar]
- Ballester, M.; Bendifallah, S.; Daraï, E. European guidelines (ESMO-ESGO-ESTRO consensus conference) for the management of endometrial cancer. Bull. Cancer 2017, 104, 1032–1038. [Google Scholar] [CrossRef]
- Moore, D.H. Combined chemotherapy and radiation therapy for cervical cancer. J. Natl. Compr. Cancer Netw. 2004, 2, 631–635. [Google Scholar] [CrossRef]
- Monk, B.J.; Sill, M.W.; McMeekin, D.S.; Cohn, D.E.; Ramondetta, L.M.; Boardman, C.H.; Benda, J.; Cella, D. Phase III trial of four cisplatin-containing doublet combinations in stage IVB, recurrent, or persistent cervical carcinoma: A Gynecologic Oncology Group study. J. Clin. Oncol. 2009, 27, 4649–4655. [Google Scholar] [CrossRef] [PubMed]
- Bloss, J.D.; Bloss, J.D.; Blessing, J.A.; Behrens, B.C.; Mannel, R.S.; Rader, J.S.; Sood, A.K.; Markman, M.; Benda, J. Randomized trial of cisplatin and ifosfamide with or without bleomycin in squamous carcinoma of the cervix: A gynecologic oncology group study. J. Clin. Oncol. 2002, 20, 1832–1837. [Google Scholar] [CrossRef] [PubMed]
- Tewari, K.S.; Sill, M.W.; Long, H.J., 3rd; Penson, R.T.; Huang, H.; Ramondetta, L.M.; Landrum, L.M.; Oaknin, A.; Reid, T.J.; Leitao, M.M.; et al. Improved survival with bevacizumab in advanced cervical cancer. N. Engl. J. Med. 2014, 370, 734–743. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Liu, M.; Wang, Z.; Huang, M.; Xu, N.; Wu, L. Serum MicroRNAs Related with Chemoradiotherapy Resistance in Advanced-Stage Cervical Squamous Cell Carcinoma. Transl. Oncol. 2017, 10, 378–384. [Google Scholar] [CrossRef]
- Dun, S.; Gao, L. Tanshinone I attenuates proliferation and chemoresistance of cervical cancer in a KRAS-dependent manner. J. Biochem. Mol. Toxicol. 2019, 33, e22267. [Google Scholar] [CrossRef]
- Cai, L.; Wang, Z.; Liu, D. Interference with endogenous EZH2 reverses the chemotherapy drug resistance in cervical cancer cells partly by up-regulating Dicer expression. Tumour Biol. 2016, 37, 6359–6369. [Google Scholar] [CrossRef]
- Guo, L.; Wu, H.; Zhu, J.; Zhang, C.; Ma, J.; Lan, J.; Xie, X. Genetic variations in the PI3K/AKT pathway predict platinum-based neoadjuvant chemotherapeutic sensitivity in squamous cervical cancer. Life Sci. 2015, 143, 217–224. [Google Scholar] [CrossRef]
- Omura, G.A. Current status of chemotherapy for cancer of the cervix. Oncology 1992, 6, 27–43. [Google Scholar]
- Thigpen, T.; Vance, R.; Khansur, T.; Malamud, F. The role of ifosfamide and systemic therapy in the management of carcinoma of the cervix. Semin. Oncol. 1996, 23 (Suppl. 6), 56–64. [Google Scholar]
| Features and Characteristics | Total Population (N = 668) |
|---|---|
| Age at diagnosis, median (IQ) | 52 (44−63) |
| BMI (kg/m2), median (IQ) | 24 (21–29) |
| Tumour size (mm), median (IQ) | 30 (24–35) |
| FIGO stage, N (%) | |
| 44 (7%) |
| 53 (8%) |
| 318 (47%) |
| 56 (8%) |
| 113 (17%) |
| 84 (13%) |
| Histology, N (%) | |
| 585 (88%) |
| 83 (12%) |
| Histological grade, N (%) | |
| 176 (27%) |
| 187 (28%) |
| 140 (21%) |
| 165 (24%) |
| Lympho-vascular space invasion, N (%) | |
| 63 (9%) |
| 225 (34%) |
| Treatments, N (%) | |
| 211 (35%) |
| 457 (65%) |
| Final lymph node status, N (%) | |
| 113 (17%) |
| 244 (36%) |
| 311 (47%) |
| Platinum sensitivity, N (%) | |
| 469 (70%) |
| 20 (3%) |
| 55 (8%) |
| 43 (6%) |
| 81 (12%) |
| Recurrence location, N (%) | |
| 57 (9%) |
| 20 (3%) |
| 122 (18%) |
| Population (N) | Recurrence Site (N) | 3 Years Cumulative Rates | p-Value | Figure |
|---|---|---|---|---|
| Overall Population (668) | - rT (57) - rN (20) - rM or multisite (122) | 41.9% (25.2–54.9) 28.4% (8.1–44.1) 49.8% (38.4–59) | p < 0.001 | 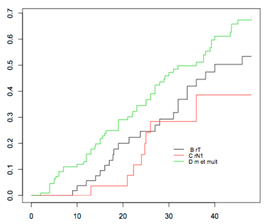 |
| Low-risk Group (81) | - rT (22) - rN (9) - rM or multisite (50) | 35.8% (10.2–54.1) 12.5% (0–32.7) 61.2% (40.1–74.9) | p = 0.057 | 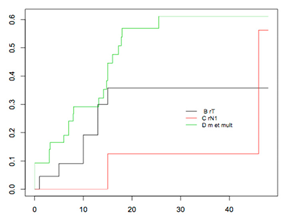 |
| Intermediate-risk group (98) | - rT (32) - rN (10) - rM or multisite (56) | 66.2% (29.8–83.7) 70.8% (13.2–90) 86.8% (64.2–95.1) | p = 0.1701 | 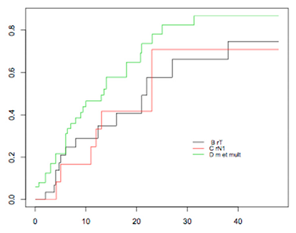 |
| High-risk Group (20) | - rT (3) - rN (1) - rM or multisite (16) | 75% (0–95.4) 77.8% (35–92.4) | p = 0.4522 | 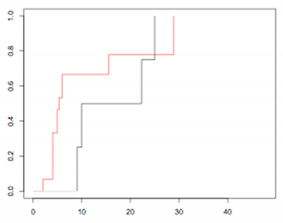 |
| HR | 95% CI | p | |
|---|---|---|---|
| Histology, N (%) | |||
| 1 | ||
| 1.92 | 0.64–5.69 | 0.23 |
| Histological grade, N (%) | |||
| 1 | ||
| 0.71 | 0.29–1.70 | |
| 1.99 | 0.92–4.33 | 0.079 |
| LVSI, N (%) | |||
| 1 | ||
| 0.39 | 0.20–0.76 | 0.005 |
| Final lymph node status, N (%) | |||
| 1 | ||
| 0.48 | 0.19–1.18 | |
| 0.21 | 0.08–0.53 | 0.001 |
| Treatments, N (%) | |||
| 1 | ||
| 0.74 | 0.34–1.60 | 0.459 |
| Platinum status | |||
| - Very sensitive | 3.08 | 1.36–6.96 | |
| - Sensitive | 7.87 | 3.15–19.65 | |
| - Partially sensitive | 19.0 | 5.85–61.67 | |
| - Resistant | 17.08 | 2.94–98.99 | <0.0001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Foucher, T.; Hennebert, C.; Dabi, Y.; Ouldamer, L.; Lavoué, V.; Dion, L.; Canlorbe, G.; Bolze, P.A.; Golfier, F.; Akladios, C.; et al. Recurrence Pattern of Cervical Cancer Based on the Platinum Sensitivity Concept: A Multi-Institutional Study from the FRANCOGYN Group. J. Clin. Med. 2020, 9, 3646. https://doi.org/10.3390/jcm9113646
de Foucher T, Hennebert C, Dabi Y, Ouldamer L, Lavoué V, Dion L, Canlorbe G, Bolze PA, Golfier F, Akladios C, et al. Recurrence Pattern of Cervical Cancer Based on the Platinum Sensitivity Concept: A Multi-Institutional Study from the FRANCOGYN Group. Journal of Clinical Medicine. 2020; 9(11):3646. https://doi.org/10.3390/jcm9113646
Chicago/Turabian Stylede Foucher, Tiphaine, Cecile Hennebert, Yohan Dabi, Lobna Ouldamer, Vincent Lavoué, Ludivine Dion, Geoffroy Canlorbe, Pierre Adrien Bolze, François Golfier, Cherif Akladios, and et al. 2020. "Recurrence Pattern of Cervical Cancer Based on the Platinum Sensitivity Concept: A Multi-Institutional Study from the FRANCOGYN Group" Journal of Clinical Medicine 9, no. 11: 3646. https://doi.org/10.3390/jcm9113646
APA Stylede Foucher, T., Hennebert, C., Dabi, Y., Ouldamer, L., Lavoué, V., Dion, L., Canlorbe, G., Bolze, P. A., Golfier, F., Akladios, C., Lecointre, L., Kerbage, Y., Collinet, P., Bricou, A., Carcopino, X., Huchon, C., Raimond, E., Graesslin, O., Owen, C., ... Bendifallah, S. (2020). Recurrence Pattern of Cervical Cancer Based on the Platinum Sensitivity Concept: A Multi-Institutional Study from the FRANCOGYN Group. Journal of Clinical Medicine, 9(11), 3646. https://doi.org/10.3390/jcm9113646







