Levosimendan Plus Dobutamine in Acute Decompensated Heart Failure Refractory to Dobutamine
Abstract
1. Introduction
2. Methods
2.1. Population
2.2. Cardiac Assessment and Hemodynamic Monitoring
2.3. Management of ADHF
2.4. Outcome and Follow-Up
2.5. Statistical Analysis
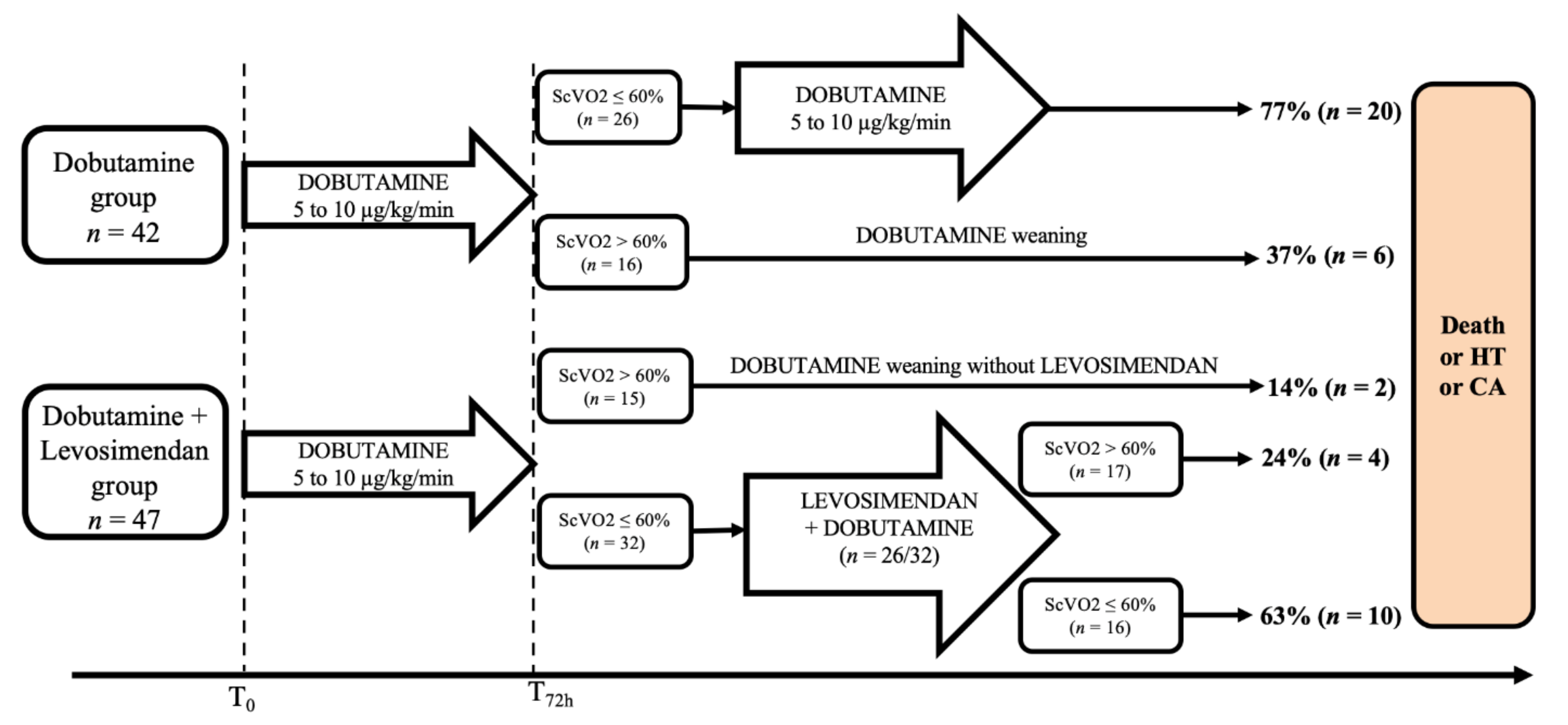
3. Results
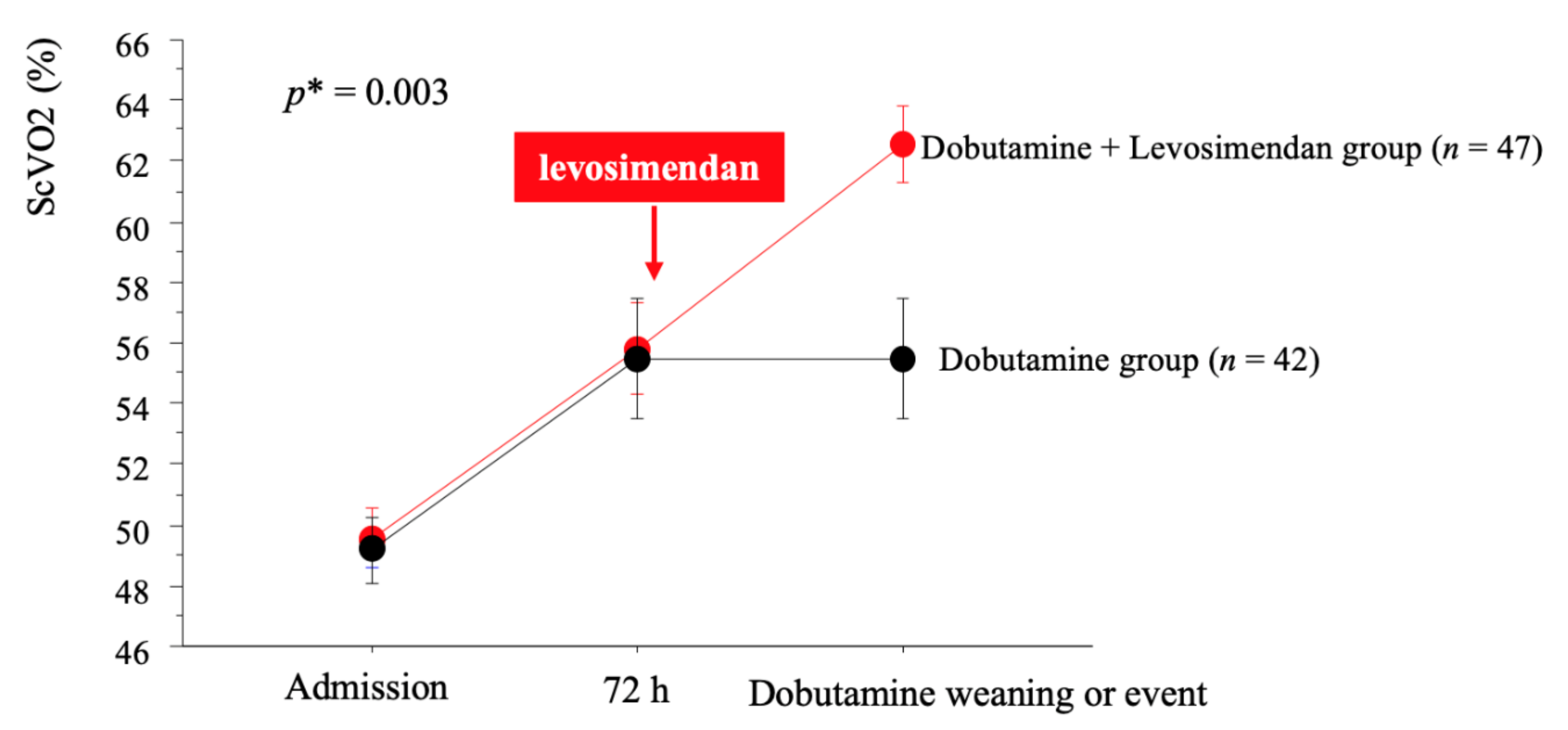
3.1. Effect of Levosimendan
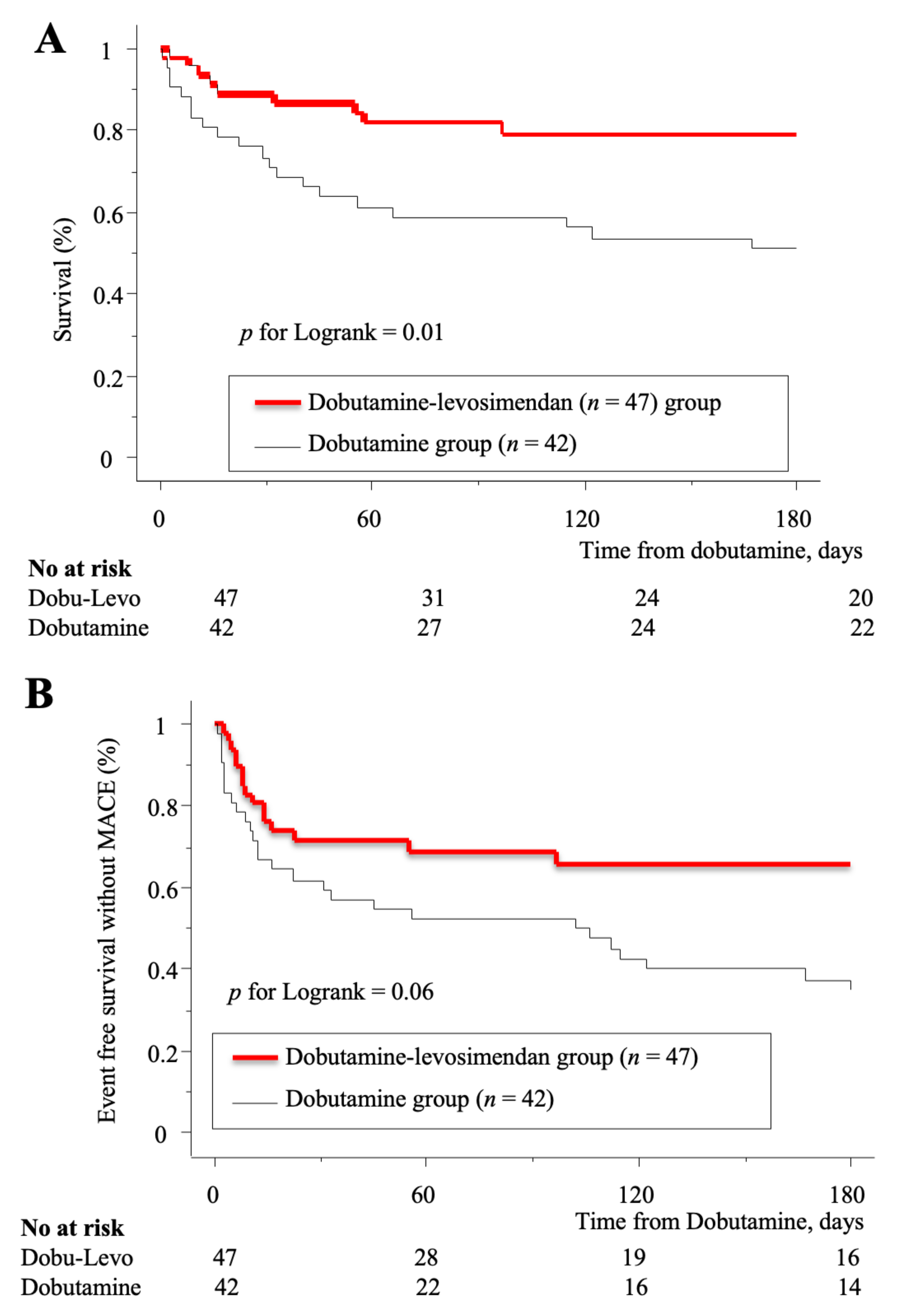
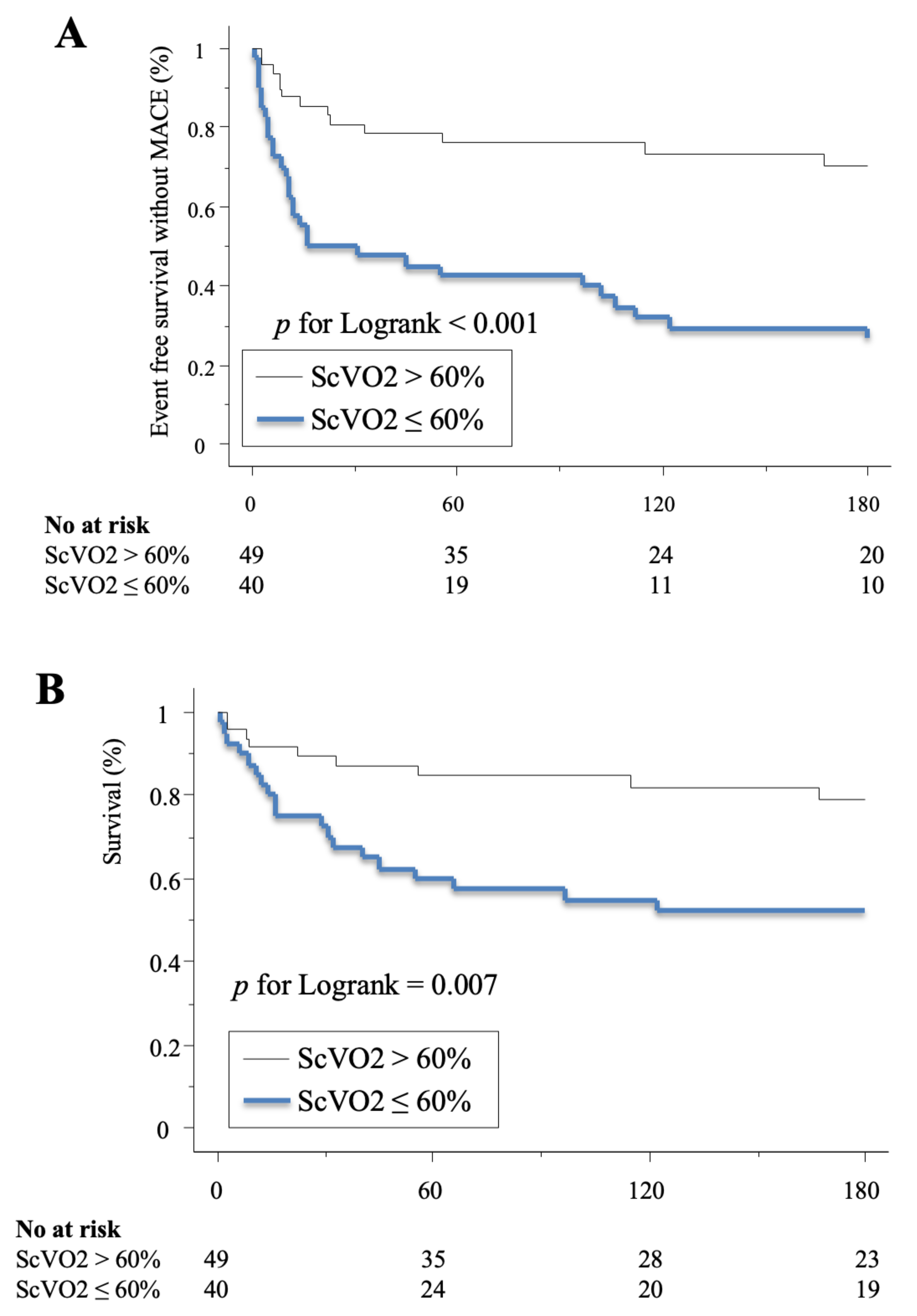
3.2. Outcome
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Thackray, S.; Easthaugh, J.; Freemantle, N.; Cleland, J.G.F. The effectiveness and relative effectiveness of intravenous inotropic drugs acting through the adrenergic pathway in patients with heart failure-a meta-regression analysis. Eur. J. Heart Fail. 2002, 4, 515–529. [Google Scholar] [CrossRef]
- Bayram, M.; De Luca, L.; Massie, M.B.; Gheorghiade, M. Reassessment of Dobutamine, Dopamine and Milrinone in the Management of Acute Heart Failure Syndromes. Am. J. Cardiol. 2005, 96, 47–58. [Google Scholar] [CrossRef] [PubMed]
- Tacon, C.L.; McCaffrey, J.; Delaney, A. Dobutamine for patients with severe heart failure: A systematic review and meta-analysis of randomised controlled trials. Intensive Care Med. 2012, 38, 359–367. [Google Scholar] [CrossRef] [PubMed]
- Nieminen, M.S.; Akkila, J.; Hasenfuss, G.; Kleber, F.X.; Lehtonen, L.A.; Mitrovic, V.; Nyquist, O.; Remme, W.J. Hemodynamic and neurohumoral effects of continuous infusion of levosimendan in patients with congestive heart failure. J. Am. Coll. Cardiol. 2000, 36, 1903–1912. [Google Scholar] [CrossRef]
- Follath, F.; Cleland, J.; Just, H.; Papp, J.; Scholz, H.; Peuhkurinen, K.; Harjola, V.; Mitrovic, V.; Abdalla, M.; Sandell, E.-P.; et al. Efficacy and safety of intravenous levosimendan compared with dobutamine in severe low-output heart failure (the LIDO study): A randomised double-blind trial. Lancet 2002, 360, 196–202. [Google Scholar] [CrossRef]
- Mebazaa, A.; Nieminen, M.S.; Packer, M.; Cohen-Solal, A.; Kleber, F.X.; Pocock, S.J.; Thakkar, R.; Padley, R.J.; Põder, P.; Kivikko, M.; et al. Levosimendan vs dobutamine for patients with acute decompensated heart failure: The SURVIVE Randomized Trial. JAMA 2007, 297, 1883–1891. [Google Scholar] [CrossRef] [PubMed]
- Nanas, J.N.; Papazoglou, P.P.; Terrovitis, J.V.; Kanakakis, J.; Dalianis, A.; Tsolakis, E.; Tsagalou, E.P.; Agrios, N.; Christodoulou, K.; Anastasiou-Nana, M.I. Hemodynamic effects of levosimendan added to dobutamine in patients with decompensated advanced heart failure refractory to dobutamine alone. Am. J. Cardiol. 2004, 94, 1329–1332. [Google Scholar] [CrossRef]
- Ponikowski, P.; Voors, A.A.; Anker, S.D.; Bueno, H.; Cleland, J.G.F.; Coats, A.J.S.; Falk, V.; González-Juanatey, J.R.; Harjola, V.-P.; Jankowska, E.A.; et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC)Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur. Heart J. 2016, 37, 2129–2200. [Google Scholar] [CrossRef] [PubMed]
- Adamopoulos, S.; Anker, S.D.; Auricchio, A.; Bohm, M.; Dickstein, K.; Falk, V.; Filippatos, G.; Fonseca, C.; Gomez-Sanchez, M.A.; Jaarsma, T.; et al. ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: The Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2012 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association (HFA) of the ESC. Eur. Heart J. 2012, 33, 1787–1847. [Google Scholar] [CrossRef]
- Rivers, E.; Muzzin, A. Early Goal-Directed Therapy in the Treatment of Severe Sepsis and Septic Shock. N. Engl. J. Med. 2001, 10, 1368–1377. [Google Scholar] [CrossRef]
- The PRISM Investigators Early, Goal-Directed Therapy for Septic Shock—A Patient-Level Meta-Analysis. N. Engl. J. Med. 2017, 376, 2223–2234. [CrossRef]
- Bracht, H.; Hänggi, M.; Jeker, B.; Wegmüller, N.; Porta, F.; Tüller, D.; Takala, J.; Jakob, S.M. Incidence of low central venous oxygen saturation during unplanned admissions in a multidisciplinary intensive care unit: An observational study. Crit. Care 2007, 11, 8. [Google Scholar] [CrossRef]
- Pearse, R.; Dawson, D.; Fawcett, J.; Rhodes, A.; Grounds, R.M.; Bennett, D. Changes in central venous saturation after major surgery and association with outcome. Crit. Care 2005, 9, 6. [Google Scholar]
- Gallet, R.; Lellouche, N.; Mitchell-Heggs, L.; Bouhemad, B.; Bensaid, A.; Dubois-Randé, J.-L.; Gueret, P.; Lim, P. Prognosis value of central venous oxygen saturation in acute decompensated heart failure. Arch. Cardiovasc. Dis. 2012, 105, 5–12. [Google Scholar] [CrossRef]
- Felker, G.M.; Stevenson, L.W.; Rouleau, J.L.; McNulty, S.E.; Givertz, M.M.; Braunwald, E. Diuretic Strategies in Patients with Acute Decompensated Heart Failure. N. Engl. J. Med. 2011, 9, 797–805. [Google Scholar] [CrossRef]
- Valente, M.A.E.; Voors, A.A.; Damman, K.; Van Veldhuisen, D.J.; Massie, B.M.; O’Connor, C.M.; Metra, M.; Ponikowski, P.; Teerlink, J.R.; Cotter, G.; et al. Diuretic response in acute heart failure: Clinical characteristics and prognostic significance. Eur. Heart J. 2014, 35, 1284–1293. [Google Scholar] [CrossRef] [PubMed]
- Kiernan, M.S.; Stevens, S.R.; Tang, W.H.W.; Butler, J.; Anstrom, K.J.; Birati, E.Y.; Grodin, J.L.; Gupta, D.; Margulies, K.B.; LaRue, S.; et al. Determinants of Diuretic Responsiveness and Associated Outcomes During Acute Heart Failure Hospitalization: An Analysis From the NHLBI Heart Failure Network Clinical Trials. J. Card. Fail. 2018, 24, 428–438. [Google Scholar] [CrossRef]
- Haikala, H. Cardiac troponin C as a target protein for a novel calcium sensitizing drug, levosimendan. J. Mol. Cell. Cardiol. 1995, 27, 1859–1866. [Google Scholar] [CrossRef]
- Moiseyev, V. Safety and efficacy of a novel calcium sensitizer, levosimendan, in patients with left ventricular failure due to an acute myocardial infarction. A randomized, placebo-controlled, double-blind study (RUSSLAN). Eur. Heart J. 2002, 23, 1422–1432. [Google Scholar] [CrossRef]
- Slawsky, M.T.; Colucci, W.S.; Gottlieb, S.S.; Greenberg, B.H.; Haeusslein, E.; Hare, J.; Hutchins, S.; Leier, C.V.; LeJemtel, T.H.; Loh, E.; et al. Acute Hemodynamic and Clinical Effects of Levosimendan in Patients With Severe Heart Failure. Circulation 2000, 102, 2222–2227. [Google Scholar] [CrossRef] [PubMed]
- Fang, M.; Cao, H.; Wang, Z. Levosimendan in patients with cardiogenic shock complicating myocardial infarction: A meta-analysis. Med. Intensiva 2017. [CrossRef]
- Landoni, G.; Lomivorotov, V.V.; Alvaro, G.; Lobreglio, R.; Pisano, A.; Guarracino, F.; Calabrò, M.G.; Grigoryev, E.V.; Likhvantsev, V.V.; Salgado-Filho, M.F.; et al. Levosimendan for Hemodynamic Support after Cardiac Surgery. N. Engl. J. Med. 2017, 376, 2021–2031. [Google Scholar] [CrossRef]
- Mehta, R.H.; Leimberger, J.D.; van Diepen, S.; Meza, J.; Wang, A.; Jankowich, R.; Harrison, R.W.; Hay, D.; Fremes, S.; Duncan, A.; et al. Levosimendan in Patients with Left Ventricular Dysfunction Undergoing Cardiac Surgery. N. Engl. J. Med. 2017, 376, 2032–2042. [Google Scholar] [CrossRef]
- Cholley, B.; Caruba, T.; Grosjean, S.; Amour, J.; Ouattara, A.; Villacorta, J.; Miguet, B.; Guinet, P.; Lévy, F.; Squara, P.; et al. Effect of Levosimendan on Low Cardiac Output Syndrome in Patients with Low Ejection Fraction Undergoing Coronary Artery Bypass Grafting With Cardiopulmonary Bypass: The LICORN Randomized Clinical Trial. JAMA 2017, 318, 548. [Google Scholar] [CrossRef] [PubMed]
- Cavusoglu, Y.; Beyaztas, A.; Entok, E. Additive Improvement of Systolic and Diastolic Functions by the Concomitant Administration of Levosimendan and Dobutamine in Left Ventricular Dysfunction: A Radionuclide Ventriculographic Study. Circulation 2006, 114, 619. [Google Scholar]
| Item | All (n = 89) | Dobutamine Alone (n = 42) | Dobutamine-Levosimendan (n = 47) | p |
|---|---|---|---|---|
| Age, years | 61 ± 15 | 60 ± 16 | 62 ± 15 | 0.43 |
| Sex, male, n (%) | 64 (72) | 29 (69) | 35 (74) | 0.64 |
| LV dysfunction etiology | ||||
| CAD, n (%) | 29 (33) | 14 (33) | 15 (32) | 0.89 |
| Dilated CM, n (%) | 58 (65) | 28 (67) | 32 (64) | 0.54 |
| Others, n (%) | 2 (2) | 0 (0) | 2 (4) | 0.18 |
| Diabetes, n (%) | 24 (27) | 14 (33) | 10 (21) | 0.24 |
| Atrial fibrillation, n (%) | 27 (30) | 9 (21) | 18 (38) | 0.11 |
| Newly diagnosed HF, n (%) | 14 (16) | 6 (14) | 8 (17) | 0,72 |
| Care limitation, n (%) | 39 (44) | 17 (40) | 22 (47) | 0.67 |
| Betablocker, n (%) | 53 (60) | 28 (66) | 25 (53) | 0.28 |
| ACE inhibitor, n (%) | 50 (56) | 28 (60) | 22 (53) | 0.09 |
| Aldosterone inhibitor, n (%) | 46 (52) | 23 (55) | 23 (49) | 0.67 |
| CRT, n (%) | 20 (22) | 10 (24) | 10 (21) | 0.80 |
| LVEF, % | 20 ± 6 | 20 ± 6 | 19 ± 6 | 0.72 |
| Cardiac Index, L/min/m2 | 1.7 ± 0.6 | 1.7 ± 0.5 | 1.8 ± 0.6 | 0.33 |
| TAPSE, mm | 12 ± 4 | 12 ± 4 | 12 ± 3 | 0.50 |
| Admission | ||||
| SBP, mmHg | 104 ± 20 | 103 ± 20 | 105 ± 19 | 0.71 |
| DBP, mmHg | 63 ± 14 | 61 ± 13 | 66 ± 13 | 0.11 |
| HR, bpm | 91 ± 20 | 90 ± 20 | 93 ± 20 | 0.50 |
| Lactate, mM/L | 3.1 ± 2.6 | 3.3 ± 3.0 | 3.0 ± 2.1 | 0.66 |
| Creatinine, μM/L | 154 ± 84 | 165 ± 84 | 143 ± 83 | 0.22 |
| eGFR, mL/min | 53 ± 28 | 48 ± 28 | 58 ± 29 | 0.11 |
| Nt-proBNP, pg/mL | 10,651 (5746–19,757) | 12,930 (5641–26,691) | 10,291 (5798–15,847) | 0.22 |
| ScVO2, % | 50 ± 9 | 49 ± 7 | 50 ± 9 | 0.79 |
| Dobutamine, γ/kg/min | 6.8 ± 3.5 | 7.8 ± 4.3 | 5.8 ± 2.0 | 0.005 |
| Furosemide, mg/h | 30 ± 15 | 27 ± 15 | 32 ± 15 | 0.15 |
| Mean norepinephrine, mg/h | 0.9 ± 0.5 | 0.9 ± 0.5 | 0.9 ± 0.6 | 0.87 |
| At 72 h | ||||
| SBP, mmHg | 105 ± 16 | 104 ± 16 | 104 ± 16 | 0.80 |
| DBP, mmHg | 62 ± 12 | 61 ± 12 | 63 ± 11 | 0.42 |
| HR, bpm | 93 ± 21 | 92 ± 15 | 93 ± 24 | 0.88 |
| Diuresis, L per day | 3.9 ± 1.9 | 3.7 ± 2.0 | 4.0 ± 1.9 | 0.46 |
| Dobutamine, γ/kg/min | 6.6 ± 3.4 | 8.4 ± 3.9 | 5.0 ± 1.6 | <0.001 |
| Furosemide, mg/h | 26 ± 16 | 21 ± 15 | 30 ± 16 | 0.007 |
| Mean norepinephrine, mg/h | 1.1 ± 0.7 | 1.2 ± 0.7 | 0.8 ± 0.5 | 0.34 |
| Lactate, mmol/L | 1.9 ± 0.6 | 2.0 ± 0.8 | 1.8 ± 0.5 | 0.13 |
| ScVO2, % | 56 ± 12 | 55 ± 13 | 56 ± 10 | 0.88 |
| ScVO2 > 60%, n (%) | 34 (36) | 15 (36) | 15 (32) | 0.82 |
| At dobutamine weaning or at MACE | ||||
| ScVO2, % | 59 ± 11 | 54 ± 13 | 63 ± 9 | 0.003 |
| ScVO2 > 60%, n (%) | 49 (55) | 15 (36) | 34 (72) | 0.03 |
| Item | MACE (n = 42) | Event Free (n = 47) | p |
|---|---|---|---|
| Age, years | 61 ± 16 | 61 ± 15 | 0.96 |
| Sex, male, n (%) | 31 (74) | 33 (70) | 0.70 |
| CAD, n (%) | 14 (33) | 15 (32) | 0.89 |
| Diabetes, n (%) | 11 (26) | 13 (28) | 0.87 |
| Atrial fibrillation, n (%) | 11 (26) | 16 (34) | 0.42 |
| Care limitation, n (%) | 18 (43) | 21 (45) | 0.86 |
| Beta-blocker, n (%) | 27 (64) | 26 (55) | 0.74 |
| ACE inhibitor, n (%) | 24 (57) | 26 (55) | 0.86 |
| Aldosterone inhibitor, n (%) | 23 (55) | 23 (49) | 0.58 |
| CRT, n (%) | 13 (31) | 7 (15) | 0.08 |
| LVEF, % | 19 ± 6 | 20 ± 20 | 0.34 |
| Cardiac Index, L/min/m2 | 1.6 ± 0.5 | 1.8 ± 0.6 | 0.06 |
| TAPSE, mm | 12 ± 4 | 12 ± 4 | 0.94 |
| Admission | |||
| SBP, mmHg | 99 ± 14 | 109 ± 22 | 0.01 |
| DBP, mmHg | 60 ± 13 | 66 ± 13 | 0.04 |
| HR, bpm | 89 ± 18 | 93 ± 20 | 0.36 |
| Lactate, mM/L | 3.0 ± 2.6 | 3.2 ± 2.6 | 0.71 |
| Creatinine, μM/L | 169 ± 100 | 137 ± 62 | 0.10 |
| Nt-proBNP, pg/mL | 10,651 (5852–27,156) | 10,651 (5534–19,394) | 0.33 |
| ScVO2, % | 50 ± 7 | 49 ± 6 | 0.28 |
| Dobutamine, γ/kg/min | 6.7 ± 3.9 | 6.7 ± 3.9 | 0.98 |
| Furosemide, mg/h | 615 ± 387 | 800 ± 325 | 0.01 |
| At 72 h | |||
| SBP, mmHg | 101 ± 10 | 108 ± 19 | 0.04 |
| DBP, mmHg | 60 ± 10 | 63 ± 13 | 0.23 |
| HR, bpm | 93 ± 15 | 92 ± 25 | 0.89 |
| Diuresis, L per day | 3.4 ± 1.8 | 4.4 ± 1.9 | 0.02 |
| Dobutamine, γ/kg/min | 7.1 ± 3.9 | 6.2 ± 2.8 | 0.21 |
| Furosemide, mg/h | 582 ± 373 | 652 ± 382 | 0.38 |
| Norepinephrine, n (%) | 10 (24) | 3 (6) | 0.03 |
| Lactate, mmol/L | 2.0 ± 0.6 | 1.8 ± 0.6 | 0.38 |
| ScVO2, % | 50 ± 11 | 60 ± 10 | <0.001 |
| ScVO2 > 60% | 8 (19) | 22 (47) | 0.006 |
| At dobutamine weaning or at MACE | |||
| ScVO2, % | 53 ± 12 | 65 ± 6 | <0.001 |
| ScVO2 >60% | 13 (31) | 36 (77) | <0.001 |
| ScVO2 not Included in the Model | OR | p |
|---|---|---|
| Dobutamine-Levosimendan | 0.44 (0.23–0.84) | 0.01 |
| Admission SBP | 0.98 (0.96–0.99) | 0.02 |
| ScVO2 included in the model | OR | p |
| ScVO2 <60% | 4.30 (2.20–8.50) | <0.0001 |
| Admission SBP | 0.98 (0.96–0.99) | 0.01 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Juguet, W.; Fard, D.; Faivre, L.; Koutsoukis, A.; Deguillard, C.; Mongardon, N.; Mekontso-Dessap, A.; Huguet, R.; Lim, P. Levosimendan Plus Dobutamine in Acute Decompensated Heart Failure Refractory to Dobutamine. J. Clin. Med. 2020, 9, 3605. https://doi.org/10.3390/jcm9113605
Juguet W, Fard D, Faivre L, Koutsoukis A, Deguillard C, Mongardon N, Mekontso-Dessap A, Huguet R, Lim P. Levosimendan Plus Dobutamine in Acute Decompensated Heart Failure Refractory to Dobutamine. Journal of Clinical Medicine. 2020; 9(11):3605. https://doi.org/10.3390/jcm9113605
Chicago/Turabian StyleJuguet, William, Damien Fard, Laureline Faivre, Athanasios Koutsoukis, Camille Deguillard, Nicolas Mongardon, Armand Mekontso-Dessap, Raphaelle Huguet, and Pascal Lim. 2020. "Levosimendan Plus Dobutamine in Acute Decompensated Heart Failure Refractory to Dobutamine" Journal of Clinical Medicine 9, no. 11: 3605. https://doi.org/10.3390/jcm9113605
APA StyleJuguet, W., Fard, D., Faivre, L., Koutsoukis, A., Deguillard, C., Mongardon, N., Mekontso-Dessap, A., Huguet, R., & Lim, P. (2020). Levosimendan Plus Dobutamine in Acute Decompensated Heart Failure Refractory to Dobutamine. Journal of Clinical Medicine, 9(11), 3605. https://doi.org/10.3390/jcm9113605






