Development of Monitoring System for Assessing Rheumatoid Arthritis within 5 Minutes Using a Drop of Bio-Fluids
Abstract
1. Introduction
2. Materials and Methods
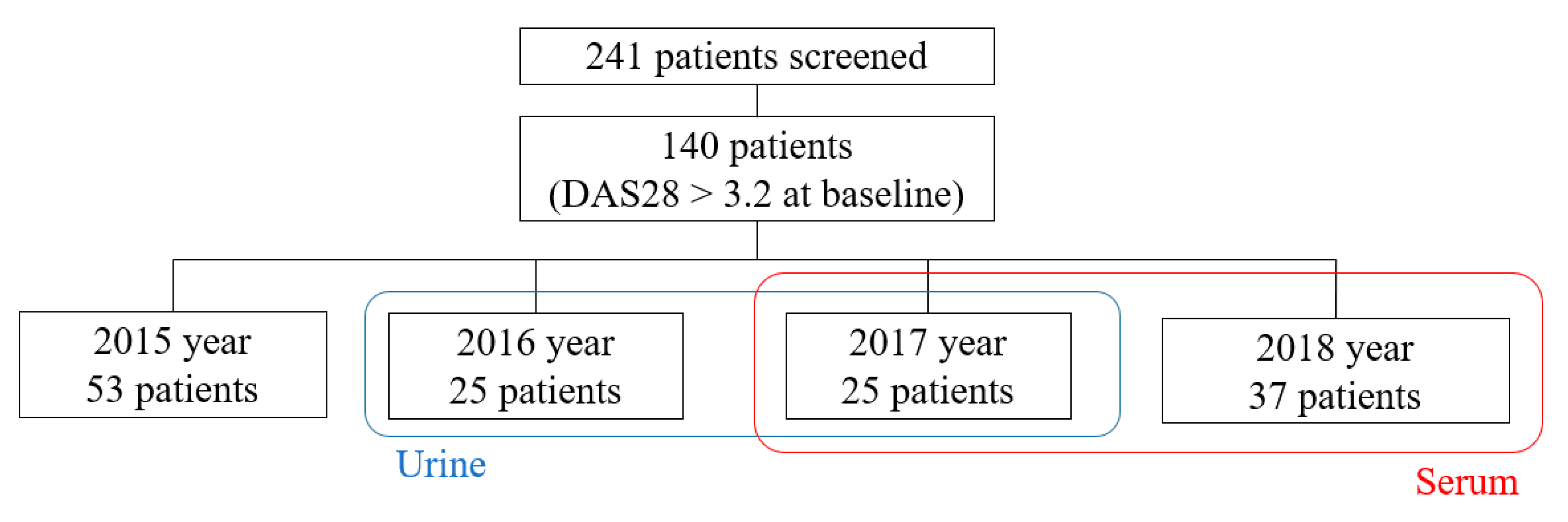
2.1. Study Population
2.2. Assessment of Clinical Parameters
2.3. Collection of Serum and Urine
2.4. Validation of the sCD14 Test Cartridge
2.5. Statistical Analysis
3. Results
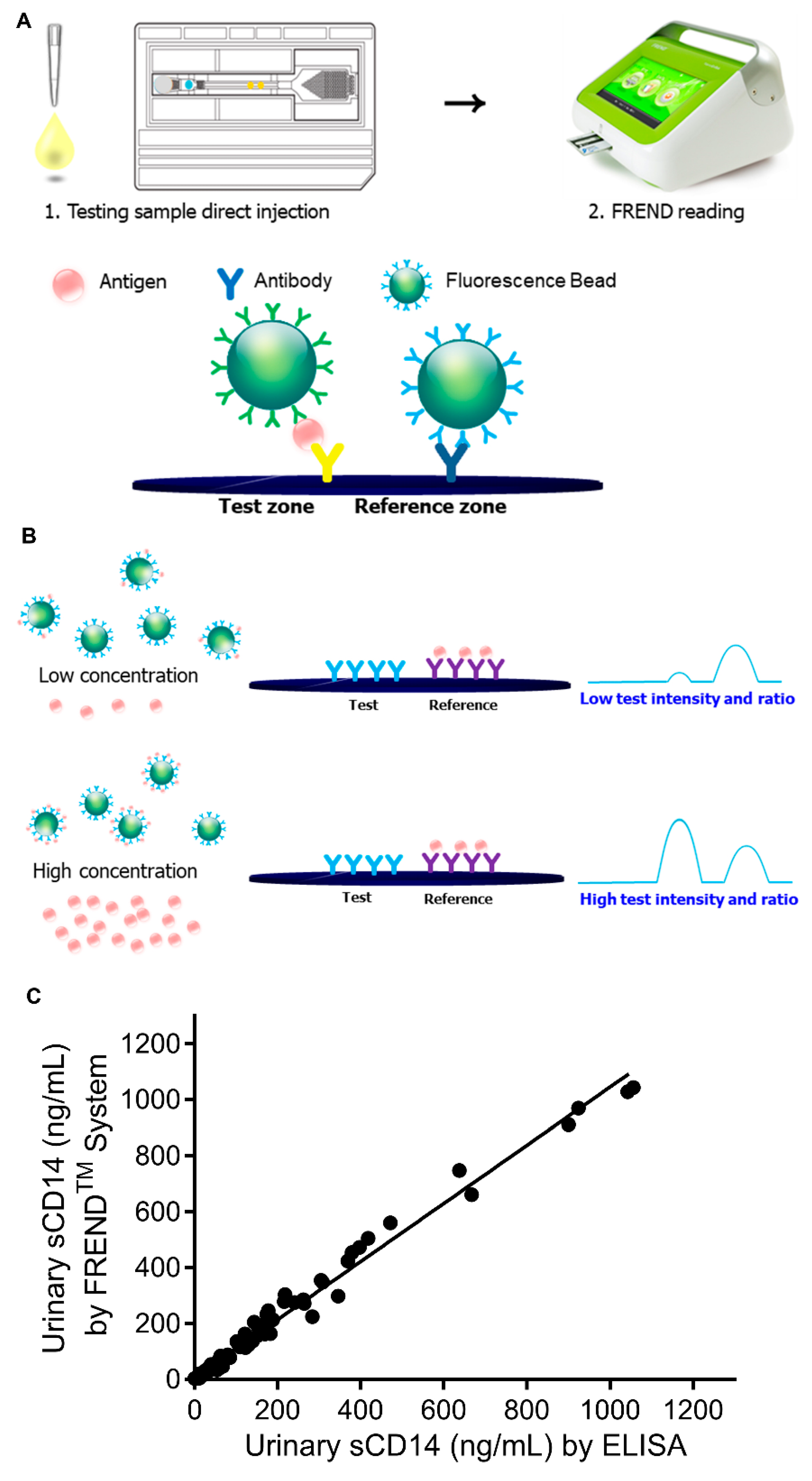
3.1. Development of the FREND System for Rapid Detection of sCD14 in Bio-Fluids
3.2. Validity and Reliability of FRENDTM-CD14 System
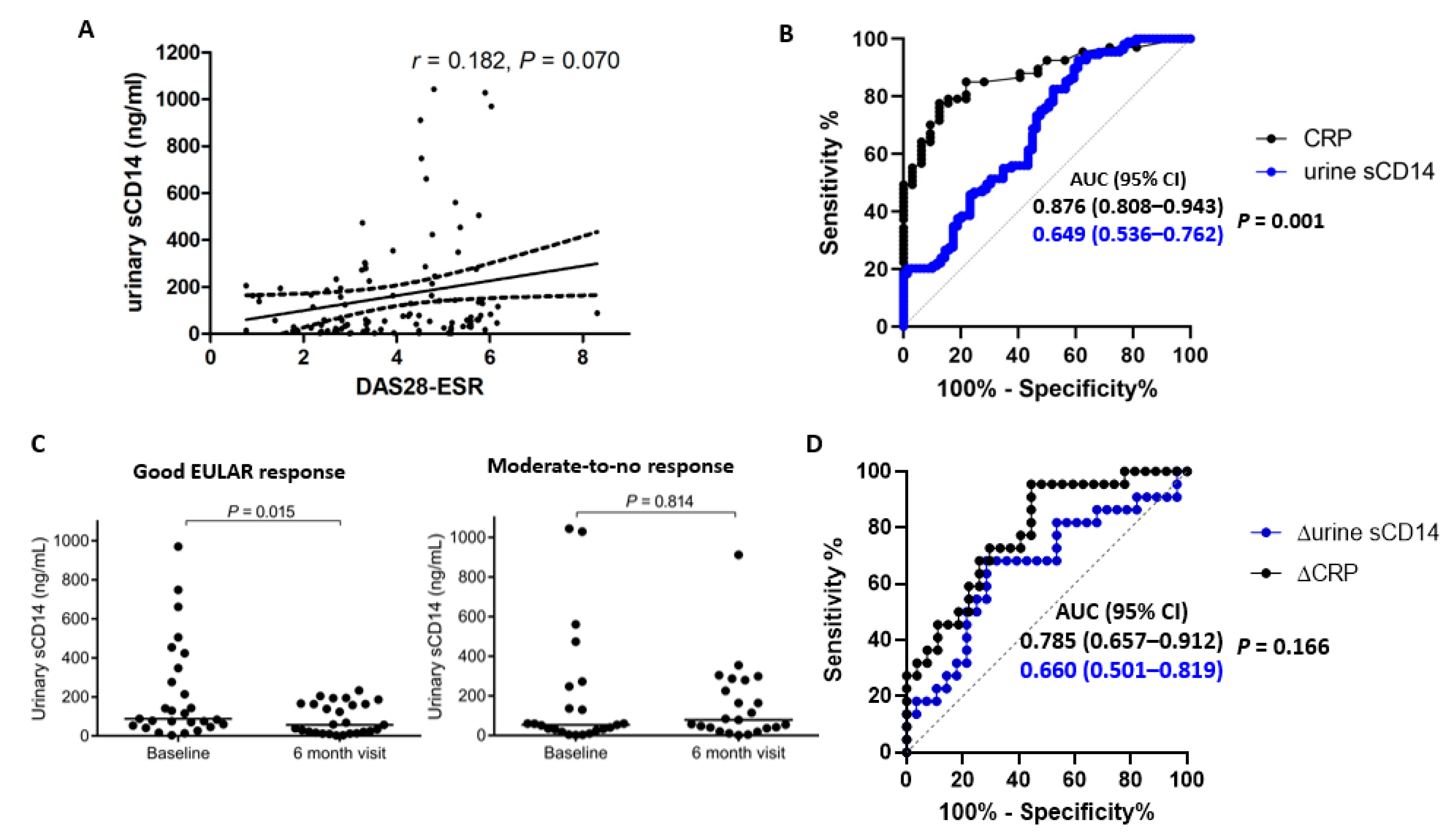
3.3. The FREND™-CD14 System Shows Weak Diagnostic Performance When Testing Urinary sCD14
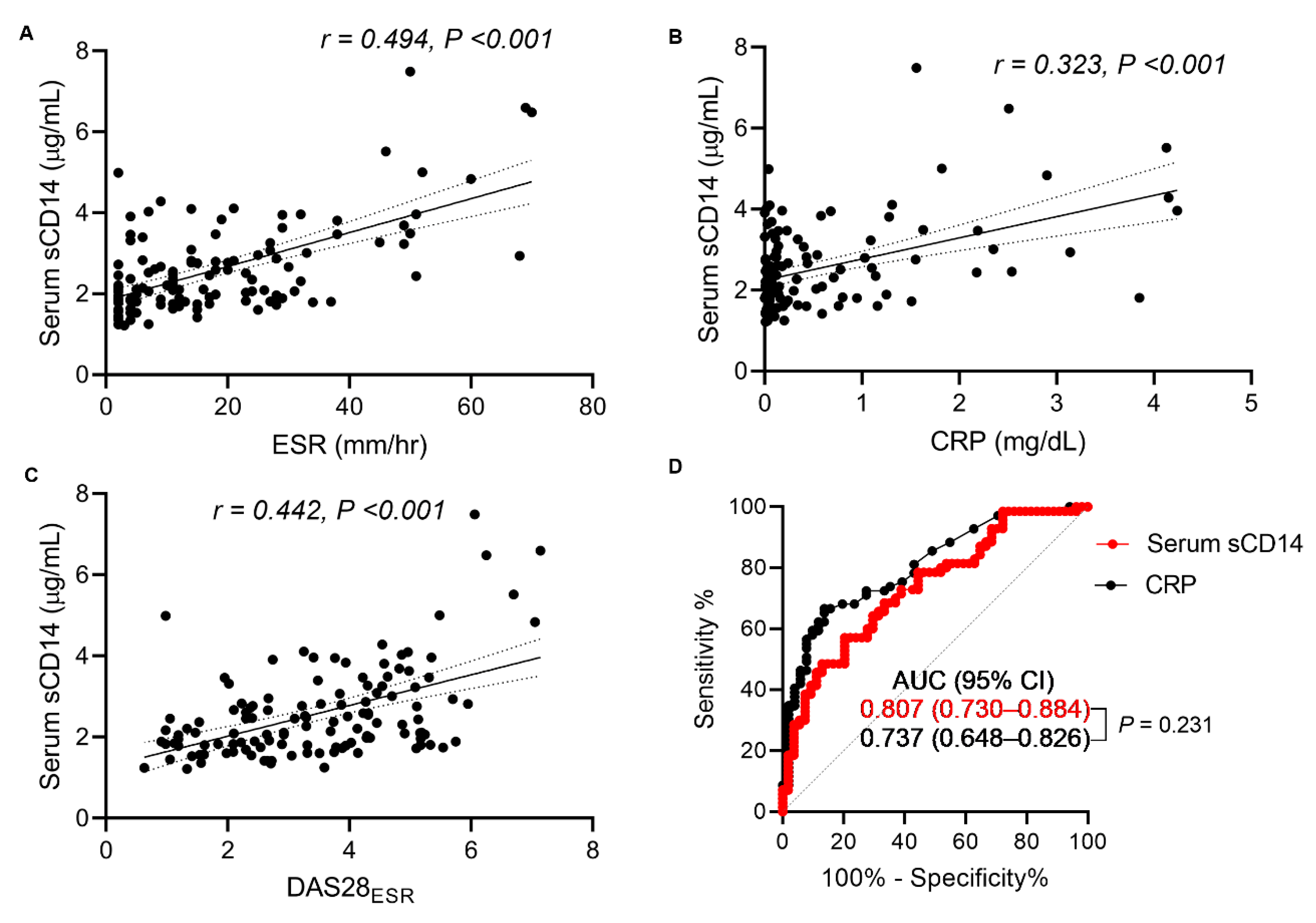
3.4. Strong Diagnostic Performance of Serum sCD14 as Measured by the FREND™-CD14 System
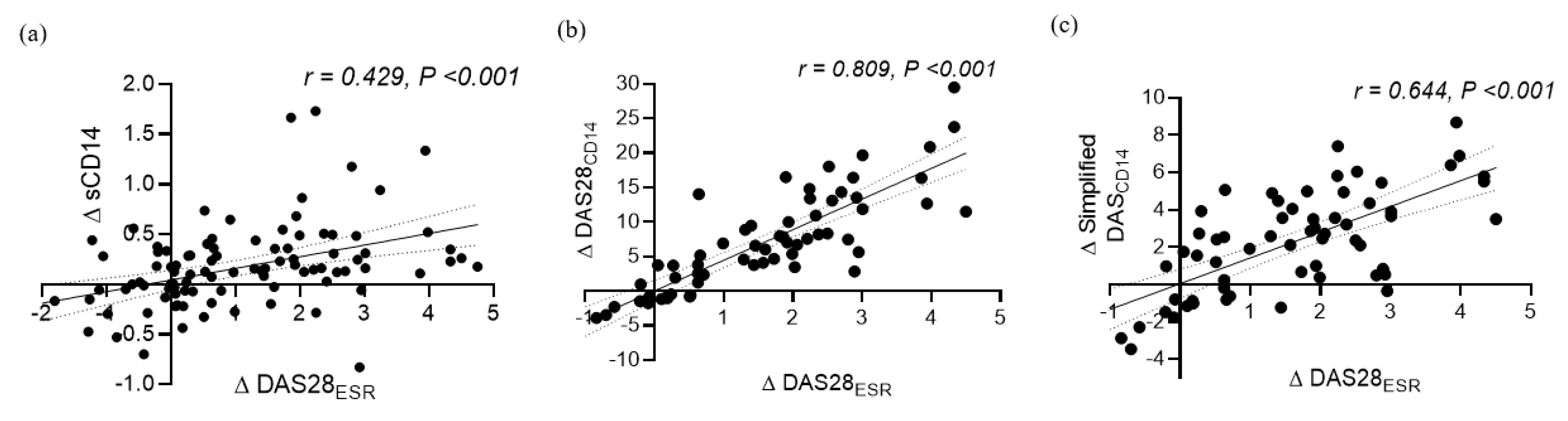
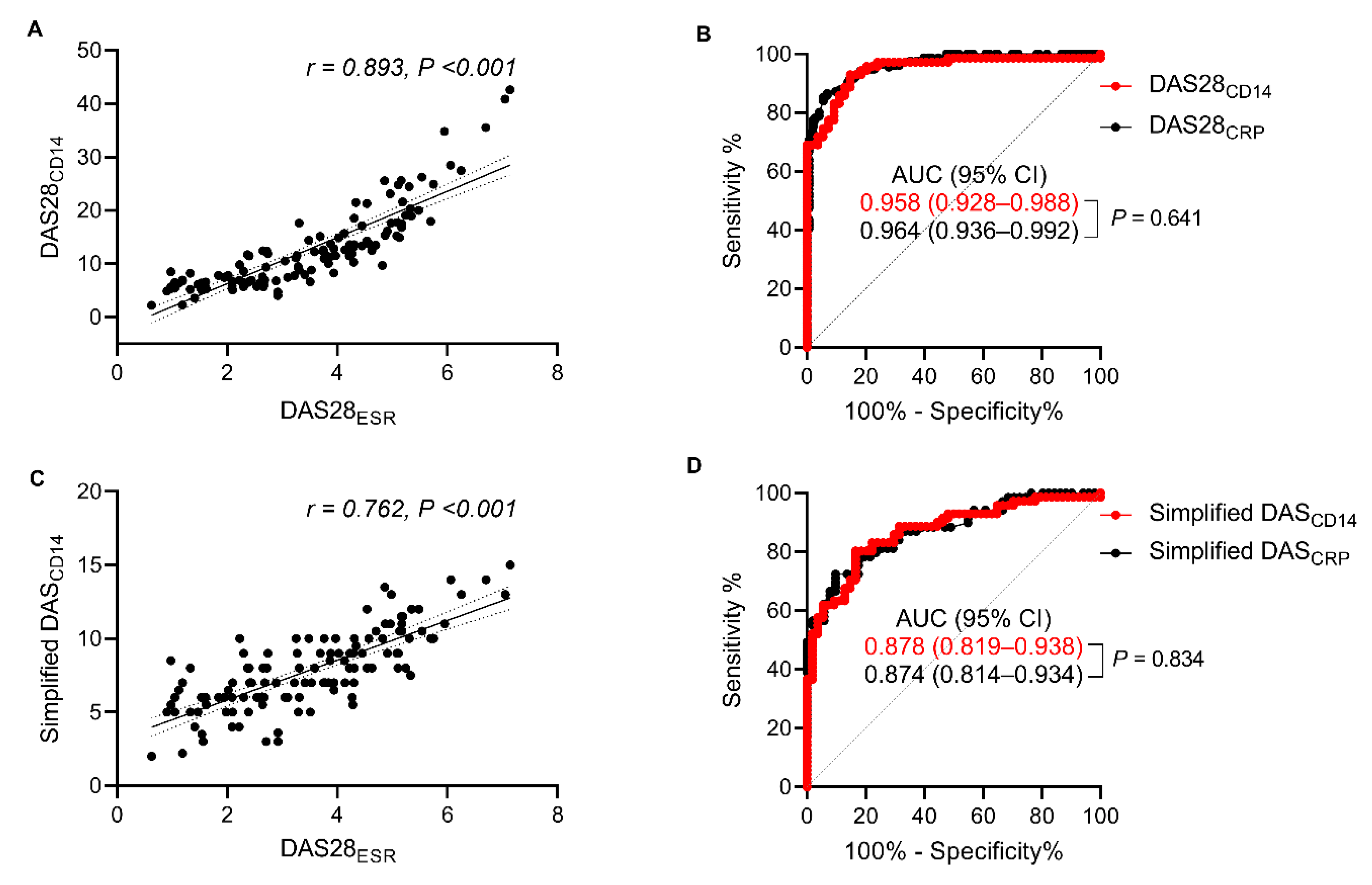
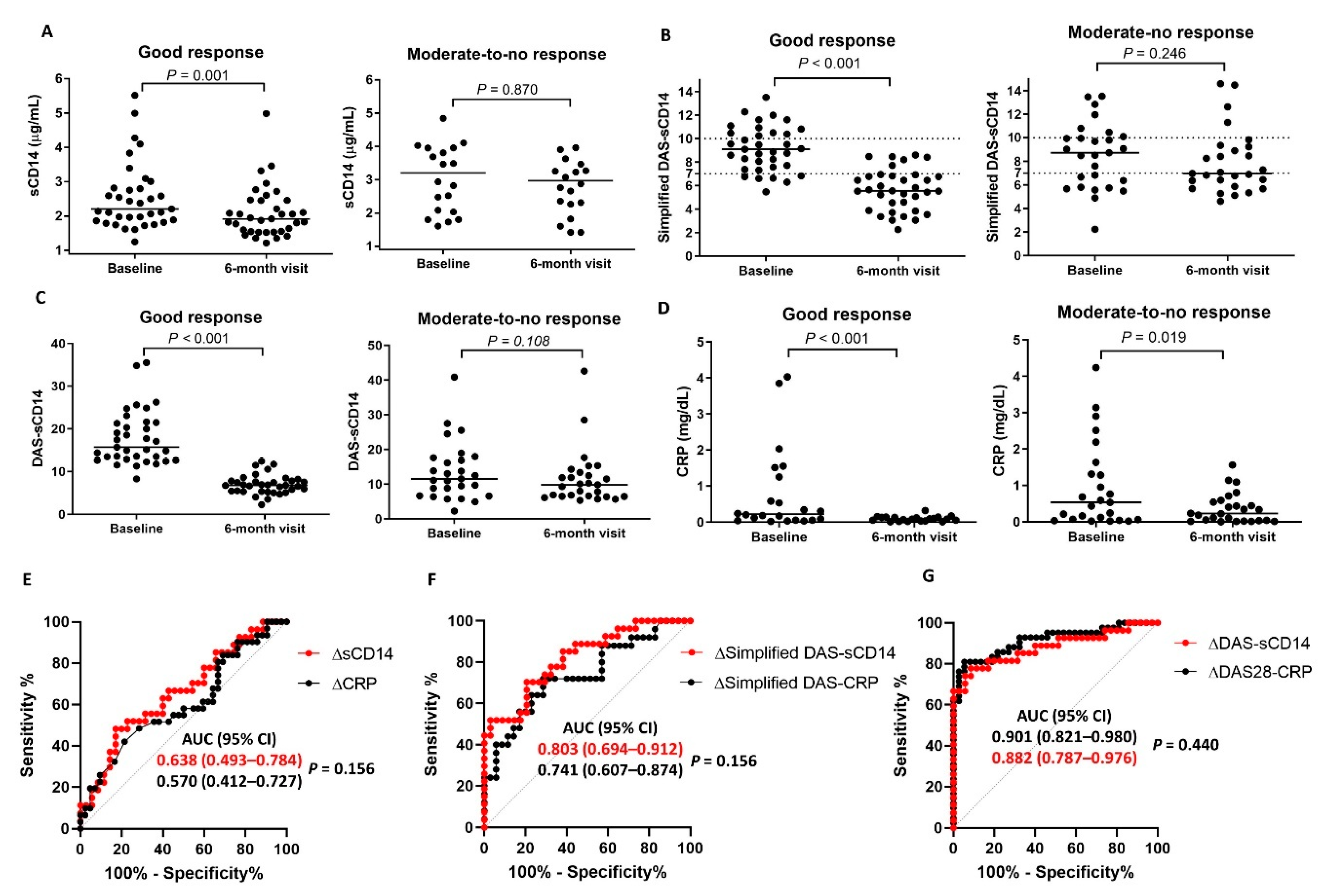
3.5. Diagnostic Performance of DAS28CD14 and the Simplified DASCD14 for Tracking Treatment Responses
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Appendix A
| Study I | Study II | |
|---|---|---|
| Number of patients/samples | 50/100 | 62/124 |
| Age, yr (IQR) | 55 (46–62) | 58 (52–64) |
| Female, n (%) | 44 (88) | 52 (84) |
| ACPA positive, n (%) | 39/44 (88.6) | 46/50 (92) |
| RF positive, n (%) | 44 (88) | 49/61 (80) |
| Smoker, n (%) | 2 (4) | 3 (4.8) |
| Methotrexate, n (%) | 71 (71) | 80 (64.5) |
| Leflunomide, n (%) | 43 (43) | 40 (32.2) |
| HCQ, n (%) | 47 (47) | 59 (47.6) |
| bDMARDs, n (%) | 32 (32) | 58 (46.8) |
| TJC | 2 (0–5) | 2 (0–4) |
| SJC | 1 (0–3) | 1 (0–2) |
| PGA | 55 (40–74) | 50 (36–70) |
| ESR, mm/hr | 29 (13–45) | 14 (5–30) |
| CRP, mg/dL | 0.41 (0.1–1.7) | 0.16 (0.05–0.80) |
| DAS28ESR | 4.0 (2.8–5.2) | 3.4 (2.2–4.6) |
| Baseline DAS28ESR | 5.2 (4.5–5.7) | 4.4 (3.8–5.2) |
| Follow-up DAS28ESR | 2.8 (2.4–3.4) | 2.6 (2.0–3.3) |
| Model 1 * | Model 2 † | ||||||
|---|---|---|---|---|---|---|---|
| Beta | SE | p | Beta | SE | p | ||
| Age | Per 1 Year | 0.016 | 0.011 | 0.167 | 0.014 | 0.009 | 0.118 |
| Sex | Female vs. Male (ref) | 0.034 | 0.326 | 0.917 | 0.218 | 0.244 | 0.374 |
| Body Mass Index | Per 1 kg/m2 | 0.038 | 0.038 | 0.318 | 0.051 | 0.028 | 0.077 |
| Hypertension | Yes vs. No (ref) | −0.389 | 0.304 | 0.203 | −0.085 | 0.227 | 0.710 |
| Diabetes Mellitus | Yes vs. No (ref) | −0.061 | 0.498 | 0.903 | 0.480 | 0.373 | 0.202 |
| Rheumatoid Factor Positive | Yes vs. No (ref) | 0.411 | 0.293 | 0.165 | 0.351 | 0.220 | 0.114 |
| Acpa Positive | Yes vs. No (ref) | 0.302 | 0.412 | 0.465 | 0.226 | 0.309 | 0.467 |
| Methotrexate | Yes vs. No (ref) | −0.475 | 0.232 | 0.044 | 0.020 | 0.178 | 0.912 |
| Leflunomide | Yes vs. No (ref) | 0.244 | 0.230 | 0.293 | 0.160 | 0.173 | 0.357 |
| Biologic Dmards | Yes vs. No (ref) | −0.353 | 0.236 | 0.137 | -0.199 | 0.173 | 0.357 |
| Simplified Dascd14 | Per 1 Score | 0.453 | 0.038 | <0.001 | |||
| Das28cd14 | Per 1 Score | 0.169 | 0.010 | <0.001 | |||
References
- Grigor, C.; Capell, H.; Stirling, A.; McMahon, A.D.; Lock, P.; Vallance, R.; Kincaid, W.; Porter, D. Effect of a treatment strategy of tight control for rheumatoid arthritis (the TICORA study): A single-blind randomised controlled trial. Lancet 2004, 364, 263–269. [Google Scholar] [CrossRef]
- Smolen, J.S.; Breedveld, F.C.; Burmester, G.R.; Bykerk, V.; Dougados, M.; Emery, P.; Kvien, T.K.; Navarro-Compan, M.V.; Oliver, S.; Schoels, M.; et al. Treating rheumatoid arthritis to target: 2014 update of the recommendations of an international task force. Ann. Rheum. Dis. 2016, 75, 3–15. [Google Scholar] [CrossRef] [PubMed]
- Pincus, T.; Braun, J.; Kavanaugh, A.; Smolen, J.S. Optimisation of assessment for rheumatic diseases in clinical trials, observational studies and routine clinical care. Clin. Exp. Rheumatol. 2014, 32, 1. [Google Scholar]
- Wolfe, F.; Michaud, K. The clinical and research significance of the erythrocyte sedimentation rate. J. Rheumatol. 1994, 21, 1227–1237. [Google Scholar]
- Wang, J.; Devenport, J.; Low, J.M.; Yu, D.; Hitraya, E. Relationship Between Baseline and Early Changes in C-Reactive Protein and Interleukin-6 Levels and Clinical Response to Tocilizumab in Rheumatoid Arthritis. Arthritis Care Res. 2016, 68, 882–885. [Google Scholar] [CrossRef]
- Centola, M.; Cavet, G.; Shen, Y.; Ramanujan, S.; Knowlton, N.; Swan, K.A.; Turner, M.; Sutton, C.; Smith, D.R.; Haney, D.J.; et al. Development of a multi-biomarker disease activity test for rheumatoid arthritis. PLoS ONE 2013, 8, e60635. [Google Scholar] [CrossRef]
- Bechman, K.; Tweehuysen, L.; Garrood, T.; Scott, D.L.; Cope, A.P.; Galloway, J.B.; Ma, M.H.Y. Flares in Rheumatoid Arthritis Patients with Low Disease Activity: Predictability and Association with Worse Clinical Outcomes. J. Rheumatol. 2018, 45, 1515–1521. [Google Scholar] [CrossRef] [PubMed]
- Fleischmann, R.; Connolly, S.E.; Maldonado, M.A.; Schiff, M. Brief Report: Estimating Disease Activity Using Multi-Biomarker Disease Activity Scores in Rheumatoid Arthritis Patients Treated With Abatacept or Adalimumab. Arthritis Rheumatol. 2016, 68, 2083–2089. [Google Scholar] [CrossRef] [PubMed]
- Bouman, C.A.M.; van der Maas, A.; van Herwaarden, N.; Sasso, E.H.; van den Hoogen, F.H.J.; den Broeder, A.A. A multi-biomarker score measuring disease activity in rheumatoid arthritis patients tapering adalimumab or etanercept: Predictive value for clinical and radiographic outcomes. Rheumatology 2017, 56, 973–980. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Park, Y.J.; Yoo, S.A.; Hwang, D.; Cho, C.S.; Kim, W.U. Identification of novel urinary biomarkers for assessing disease activity and prognosis of rheumatoid arthritis. Exp. Mol. Med. 2016, 48, e211. [Google Scholar] [CrossRef]
- Kang, M.J.; Park, Y.J.; You, S.; Yoo, S.A.; Choi, S.; Kim, D.H.; Cho, C.S.; Yi, E.C.; Hwang, D.; Kim, W.U. Urinary proteome profile predictive of disease activity in rheumatoid arthritis. J. Proteome Res. 2014, 13, 5206–5217. [Google Scholar] [CrossRef] [PubMed]
- Bas, S.; Gauthier, B.R.; Spenato, U.; Stingelin, S.; Gabay, C. CD14 Is an Acute-Phase Protein. J. Immunol. 2004, 172, 4470–4479. [Google Scholar] [CrossRef] [PubMed]
- Navarro-Compán, V.; Gherghe, A.M.; Smolen, J.S.; Aletaha, D.; Landewé, R.; van der Heijde, D. Relationship between disease activity indices and their individual components and radiographic progression in RA: A systematic literature review. Rheumatology 2015, 54, 994–1007. [Google Scholar]
- Aletaha, D.; Neogi, T.; Silman, A.J.; Funovits, J.; Felson, D.T.; Bingham, C.O., 3rd; Birnbaum, N.S.; Burmester, G.R.; Bykerk, V.P.; Cohen, M.D.; et al. Rheumatoid arthritis classification criteria: An American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis Rheum. 2010, 62, 2569–2581. [Google Scholar] [CrossRef]
- Anderson, J.; Caplan, L.; Yazdany, J.; Robbins, M.L.; Neogi, T.; Michaud, K.; Saag, K.G.; O’Dell, J.R.; Kazi, S. Rheumatoid arthritis disease activity measures: American College of Rheumatology recommendations for use in clinical practice. Arthritis Care Res. 2012, 64, 640–647. [Google Scholar] [CrossRef]
- van Gestel, A.M.; Prevoo, M.L.; van’t Hof, M.A.; van Rijswijk, M.H.; van de Putte, L.B.; van Riel, P.L. Development and validation of the European League Against Rheumatism response criteria for rheumatoid arthritis. Comparison with the preliminary American College of Rheumatology and the World Health Organization/International League Against Rheumatism Criteria. Arthritis Rheum. 1996, 39, 34–40. [Google Scholar]
- van Gestel, A.M.; Haagsma, C.J.; van Riel, P.L. Validation of rheumatoid arthritis improvement criteria that include simplified joint counts. Arthritis Rheum. 1998, 41, 1845–1850. [Google Scholar] [CrossRef]
- DeLong, E.R.; DeLong, D.M.; Clarke-Pearson, D.L. Comparing the areas under two or more correlated receiver operating characteristic curves: A nonparametric approach. Biometrics 1988, 44, 837–845. [Google Scholar] [CrossRef]
- Park, H.-I.; Lee, S.; Kim, Y.; Shin, D.-Y.; Lee, C.; Han, S.; Chung, C.; Chang, J.K.; Seo, I.B. Analytical performance of a new one-step quantitative prostate-specific antigen assay, the FREND™ PSA Plus. Clin. Chem. Lab. Med. 2014, 52, 715–723. [Google Scholar] [CrossRef]
- Decramer, S.; Gonzalez de Peredo, A.; Breuil, B.; Mischak, H.; Monsarrat, B.; Bascands, J.L.; Schanstra, J.P. Urine in clinical proteomics. Mol. Cell. Proteom. MCP 2008, 7, 1850–1862. [Google Scholar] [CrossRef]
- Harpole, M.; Davis, J.; Espina, V. Current state of the art for enhancing urine biomarker discovery. Expert Rev. Proteom. 2016, 13, 609–626. [Google Scholar] [CrossRef] [PubMed]
- Anderson, J.K.; Zimmerman, L.; Caplan, L.; Michaud, K. Measures of rheumatoid arthritis disease activity: Patient (PtGA) and Provider (PrGA) Global Assessment of Disease Activity, Disease Activity Score (DAS) and Disease Activity Score with 28-Joint Counts (DAS28), Simplified Disease Activity Index (SDAI), Clinical Disease Activity Index (CDAI), Patient Activity Score (PAS) and Patient Activity Score-II (PASII), Routine Assessment of Patient Index Data (RAPID), Rheumatoid Arthritis Disease Activity Index (RADAI) and Rheumatoid Arthritis Disease Activity Index-5 (RADAI-5), Chronic Arthritis Systemic Index (CASI), Patient-Based Disease Activity Score With ESR (PDAS1) and Patient-Based Disease Activity Score without ESR (PDAS2), and Mean Overall Index for Rheumatoid Arthritis (MOI-RA). Arthritis Care Res. 2011, 63 (Suppl. 11), S14–S36. [Google Scholar]
- Smolen, J.S.; Landewé, R.; Bijlsma, J.; Burmester, G.; Chatzidionysiou, K.; Dougados, M.; Nam, J.; Ramiro, S.; Voshaar, M.; van Vollenhoven, R.; et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2016 update. Ann. Rheum. Dis. 2017, 76, 960–977. [Google Scholar] [CrossRef] [PubMed]
- Singh, J.A.; Saag, K.G.; Bridges, S.L., Jr.; Akl, E.A.; Bannuru, R.R.; Sullivan, M.C.; Vaysbrot, E.; McNaughton, C.; Osani, M.; Shmerling, R.H.; et al. 2015 American College of Rheumatology Guideline for the Treatment of Rheumatoid Arthritis. Arthritis Rheumatol. 2016, 68, 1–26. [Google Scholar] [CrossRef]
- Harrold, L.R.; Harrington, J.T.; Curtis, J.R.; Furst, D.E.; Bentley, M.J.; Shan, Y.; Reed, G.; Kremer, J.; Greenberg, J.D. Prescribing practices in a US cohort of rheumatoid arthritis patients before and after publication of the American College of Rheumatology treatment recommendations. Arthritis Rheum. 2012, 64, 630–638. [Google Scholar] [CrossRef]
- Gvozdenovic, E.; Allaart, C.F.; van der Heijde, D.; Ferraccioli, G.; Smolen, J.S.; Huizinga, T.W.; Landewe, R. When rheumatologists report that they agree with a guideline, does this mean that they practise the guideline in clinical practice? Results of the International Recommendation Implementation Study (IRIS). RMD Open 2016, 2, e000221. [Google Scholar] [CrossRef]
- Yu, Z.; Lu, B.; Agosti, J.; Bitton, A.; Corrigan, C.; Fraenkel, L.; Harrold, L.R.; Losina, E.; Katz, J.N.; Solomon, D.H. Implementation of Treat-to-Target for Rheumatoid Arthritis in the US: Analysis of Baseline Data From a Randomized Controlled Trial. Arthritis Care Res. 2018, 70, 801–806. [Google Scholar] [CrossRef]
- Ranzolin, A.; Brenol, J.C.T.; Bredemeier, M.; Guarienti, J.; Rizzatti, M.; Feldman, D.; Xavier, R.M. Association of concomitant fibromyalgia with worse disease activity score in 28 joints, health assessment questionnaire, and short form 36 scores in patients with rheumatoid arthritis. Arthritis Rheum. 2009, 61, 794–800. [Google Scholar] [CrossRef]
- Uhlig, T.; Kvien, T.K.; Pincus, T. Test-retest reliability of disease activity core set measures and indices in rheumatoid arthritis. Ann. Rheum. Dis. 2009, 68, 972–975. [Google Scholar] [CrossRef] [PubMed]
- Chatziharalambous, D.; Lygirou, V.; Latosinska, A.; Stravodimos, K.; Vlahou, A.; Jankowski, V.; Zoidakis, J. Analytical Performance of ELISA Assays in Urine: One More Bottleneck towards Biomarker Validation and Clinical Implementation. PLoS ONE 2016, 11, e0149471. [Google Scholar] [CrossRef] [PubMed]
- Kay, J.; Morgacheva, O.; Messing, S.P.; Kremer, J.M.; Greenberg, J.D.; Reed, G.W.; Gravallese, E.M.; Furst, D.E. Clinical disease activity and acute phase reactant levels are discordant among patients with active rheumatoid arthritis: Acute phase reactant levels contribute separately to predicting outcome at one year. Arthritis Res. Ther. 2014, 16, R40. [Google Scholar] [CrossRef] [PubMed]
- Nikiphorou, E.; Radner, H.; Chatzidionysiou, K.; Desthieux, C.; Zabalan, C.; van Eijk-Hustings, Y.; Dixon, W.G.; Hyrich, K.L.; Askling, J.; Gossec, L. Patient global assessment in measuring disease activity in rheumatoid arthritis: A review of the literature. Arthritis Res. Ther. 2016, 18, 251. [Google Scholar] [CrossRef] [PubMed]
- Bataille, R.; Klein, B. C-reactive protein levels as a direct indicator of interleukin-6 levels in humans in vivo. Arthritis Rheum. 1992, 35, 982–984. [Google Scholar] [CrossRef]
- Lindsay, K.; Ibrahim, G.; Sokoll, K.; Tripathi, M.; Melsom, R.D.; Helliwell, P.S. The composite DAS Score is impractical to use in daily practice: Evidence that physicians use the objective component of the DAS in decision making. J. Clin. Rheumatol. 2009, 15, 223–225. [Google Scholar] [CrossRef] [PubMed]
| DAS28ESR | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Baseline Visit | 6 Month Visit | Combination (Baseline and 6 Month) | |||||||
| Simplified DASCD14 | Low | Moderate | High | Low | Moderate | High | Low | Moderate | High |
| Low | 7 | 0 | 0 | 38 | 9 | 0 | 45 | 14 | 0 |
| Moderate | 9 | 21 | 6 | 5 | 6 | 2 | 9 | 27 | 6 |
| High | 0 | 6 | 13 | 0 | 0 | 2 | 0 | 8 | 15 |
| κ (95% CI) | 0.457 (0.270–0.645) | 0.387 (0.151–0.625) | 0.525 (0.397–0.652) | ||||||
| Weighted κ (95% CI) | 0.541 (0.375–0.707) | 0.476 (0.245–0.707) | 0.622 (0.514–0.730) | ||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Koh, J.H.; Lee, S.; Kim, H.-S.; Lee, K.; Lee, C.S.; Yoo, S.-A.; Lee, N.; Kim, W.-U. Development of Monitoring System for Assessing Rheumatoid Arthritis within 5 Minutes Using a Drop of Bio-Fluids. J. Clin. Med. 2020, 9, 3499. https://doi.org/10.3390/jcm9113499
Koh JH, Lee S, Kim H-S, Lee K, Lee CS, Yoo S-A, Lee N, Kim W-U. Development of Monitoring System for Assessing Rheumatoid Arthritis within 5 Minutes Using a Drop of Bio-Fluids. Journal of Clinical Medicine. 2020; 9(11):3499. https://doi.org/10.3390/jcm9113499
Chicago/Turabian StyleKoh, Jung Hee, Saseong Lee, Hyun-Sook Kim, Kyuheon Lee, Chang Seop Lee, Seung-Ah Yoo, Naeun Lee, and Wan-Uk Kim. 2020. "Development of Monitoring System for Assessing Rheumatoid Arthritis within 5 Minutes Using a Drop of Bio-Fluids" Journal of Clinical Medicine 9, no. 11: 3499. https://doi.org/10.3390/jcm9113499
APA StyleKoh, J. H., Lee, S., Kim, H.-S., Lee, K., Lee, C. S., Yoo, S.-A., Lee, N., & Kim, W.-U. (2020). Development of Monitoring System for Assessing Rheumatoid Arthritis within 5 Minutes Using a Drop of Bio-Fluids. Journal of Clinical Medicine, 9(11), 3499. https://doi.org/10.3390/jcm9113499





