Diagnosis and Treatment of Early Chronic Obstructive Lung Disease (COPD)
Abstract
1. Introduction
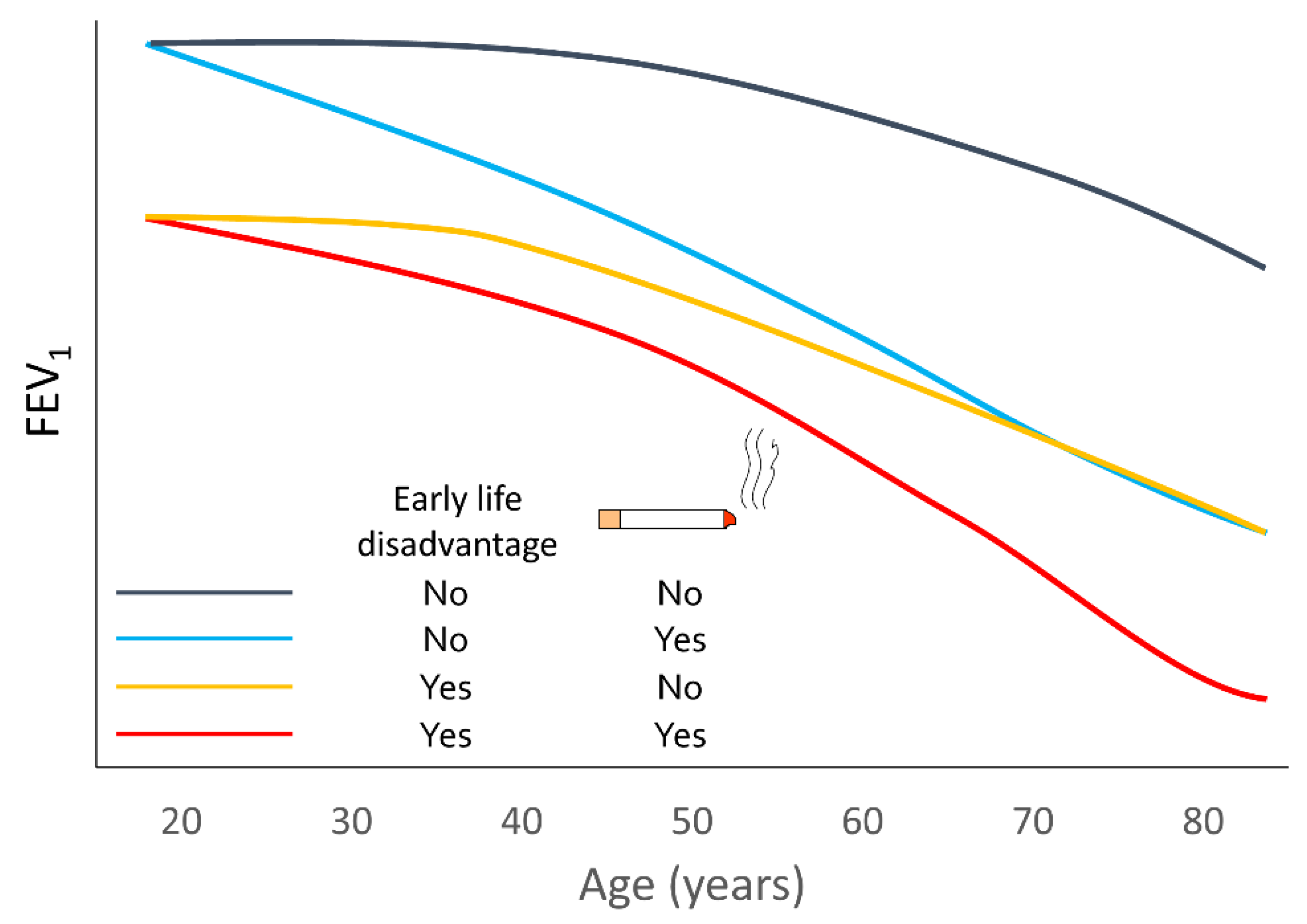
2. Variable Lung-Function Trajectories
3. Diagnosis
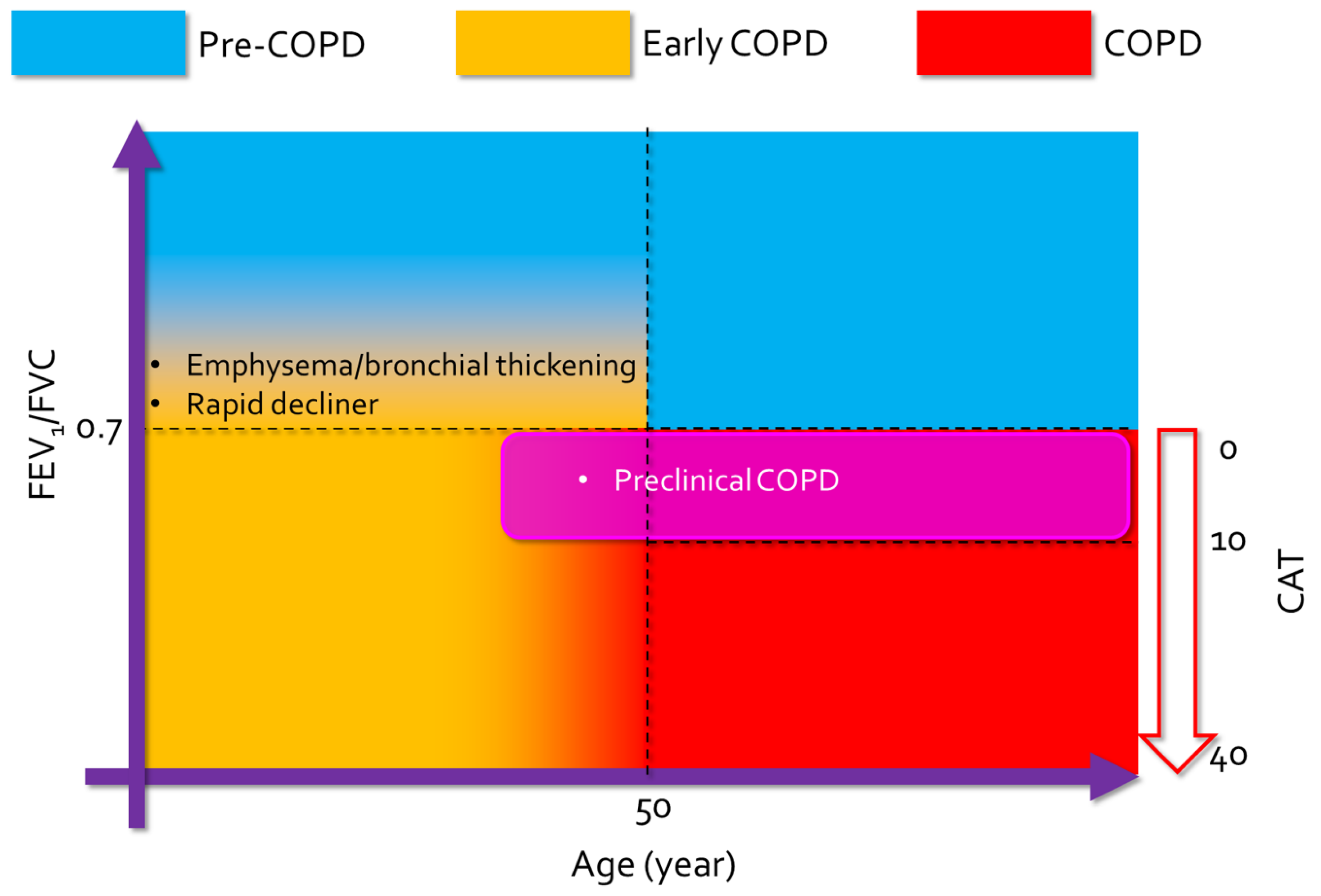
3.1. Definition
3.2. Diagnostic Tools for Early COPD
3.2.1. Identification of Risk Factors
3.2.2. Pulmonary Function Test and Other Physiological Tests
3.2.3. Imaging Studies
3.2.4. Clinical Features
3.2.5. Biomarkers of Disease Progression
3.3. Natural Course of Early COPD
3.4. Treatment of Early COPD
3.5. Treatment of Early-Onset COPD and Mild COPD
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Vos, T.; Flaxman, A.D.; Naghavi, M.; Lozano, R.; Michaud, C.; Ezzati, M.; Shibuya, K.; Salomon, J.A.; Abdalla, S.; Aboyans, V.; et al. Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990-2010: A systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012, 380, 2163–2196. [Google Scholar] [CrossRef]
- WHO. The Top 10 Causes of Death. Available online: http://www.who.int/mediacentre/factsheets/fs310/en/ (accessed on 14 April 2020).
- Anzueto, A.; Sethi, S.; Martinez, F.J. Exacerbations of chronic obstructive pulmonary disease. Proc. Am. Thorac. Soc. 2007, 4, 554–564. [Google Scholar] [CrossRef] [PubMed]
- Li, L.S.K.; Williams, M.T.; Johnston, K.N.; Frith, P.; Hypponen, E.; Paquet, C. Parental and life-course influences on symptomatic airflow obstruction. ERJ Open Res. 2020, 6. [Google Scholar] [CrossRef] [PubMed]
- Guerra, S.; Martinez, F.D. The complex beginnings of chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2020, 201, 641–642. [Google Scholar] [CrossRef] [PubMed]
- Petersen, H.; Sood, A.; Polverino, F.; Owen, C.A.; Pinto-Plata, V.; Celli, B.R.; Tesfaigzi, Y. The course of lung function in middle-aged heavy smokers: Incidence and time to early onset of chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2018, 198, 1449–1451. [Google Scholar] [CrossRef] [PubMed]
- Wijnant, S.R.A.; De Roos, E.; Kavousi, M.; Stricker, B.H.; Terzikhan, N.; Lahousse, L.; Brusselle, G.G. Trajectory and mortality of preserved ratio impaired spirometry: The Rotterdam Study. Eur. Respir. J. 2020, 55. [Google Scholar] [CrossRef] [PubMed]
- Grant, T.; Brigham, E.P.; McCormack, M.C. Childhood origins of adult lung disease as opportunities for prevention. J. Allergy Clin. Immunol. Pract. 2020, 8, 849–858. [Google Scholar] [CrossRef]
- Çolak, Y.; Nordestgaard, B.G.; Vestbo, J.; Lange, P.; Afzal, S. Prognostic significance of chronic respiratory symptoms in individuals with normal spirometry. Eur. Respir. J. 2019, 54, 1900734. [Google Scholar] [CrossRef]
- Woodruff, P.G.; Barr, R.G.; Bleecker, E.; Christenson, S.A.; Couper, D.; Curtis, J.L.; Gouskova, N.A.; Hansel, N.N.; Hoffman, E.A.; Kanner, R.E.; et al. Clinical significance of symptoms in smokers with preserved pulmonary function. N. Engl. J. Med. 2016, 374, 1811–1821. [Google Scholar] [CrossRef]
- Naya, I.P.; Tombs, L.; Lipson, D.A.; Compton, C. Preventing clinically important deterioration of COPD with addition of umeclidinium to inhaled corticosteroid/long-acting beta2-agonist therapy: An integrated post hoc analysis. Adv. Ther. 2018, 35, 1626–1638. [Google Scholar] [CrossRef]
- Colak, Y.; Afzal, S.; Nordestgaard, B.G.; Vestbo, J.; Lange, P. Prognosis of asymptomatic and symptomatic, undiagnosed COPD in the general population in Denmark: A prospective cohort study. Lancet Respir. Med. 2017, 5, 426–434. [Google Scholar] [CrossRef]
- Van Remoortel, H.; Hornikx, M.; Langer, D.; Burtin, C.; Everaerts, S.; Verhamme, P.; Boonen, S.; Gosselink, R.; Decramer, M.; Troosters, T.; et al. Risk factors and comorbidities in the preclinical stages of chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2014, 189, 30–38. [Google Scholar]
- Bui, D.S.; Lodge, C.J.; Burgess, J.A.; Lowe, A.J.; Perret, J.; Bui, M.Q.; Bowatte, G.; Gurrin, L.; Johns, D.P.; Thompson, B.R.; et al. Childhood predictors of lung function trajectories and future COPD risk: A prospective cohort study from the first to the sixth decade of life. Lancet Respir. Med. 2018, 6, 535–544. [Google Scholar] [CrossRef]
- Martinez, F.J.; Han, M.K.; Allinson, J.P.; Barr, R.G.; Boucher, R.C.; Calverley, P.M.A.; Celli, B.R.; Christenson, S.A.; Crystal, R.G.; Fageras, M.; et al. At the root: Defining and halting progression of early chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2018, 197, 1540–1551. [Google Scholar] [CrossRef]
- Rennard, S.I.; Drummond, M.B. Early chronic obstructive pulmonary disease: Definition, assessment, and prevention. Lancet 2015, 385, 1778–1788. [Google Scholar] [CrossRef]
- Washko, G.R.; Colangelo, L.A.; Estepar, R.S.J.; Ash, S.Y.; Bhatt, S.P.; Okajima, Y.; Liu, K.; Jacobs, D.R., Jr.; Iribarren, C.; Thyagarajan, B.; et al. Adult life-course trajectories of lung function and the development of emphysema: The CARDIA lung study. Am. J. Med. 2020, 133, 222–230. [Google Scholar] [CrossRef] [PubMed]
- Allinson, J.P.; Hardy, R.; Donaldson, G.C.; Shaheen, S.O.; Kuh, D.; Wedzicha, J.A. Combined impact of smoking and early-life exposures on adult lung function trajectories. Am. J. Respir. Crit. Care Med. 2017, 196, 1021–1030. [Google Scholar] [CrossRef]
- Lange, P.; Celli, B.; Agusti, A.; Boje Jensen, G.; Divo, M.; Faner, R.; Guerra, S.; Marott, J.L.; Martinez, F.D.; Martinez-Camblor, P.; et al. Lung-function trajectories leading to chronic obstructive pulmonary disease. N. Engl. J. Med. 2015, 373, 111–122. [Google Scholar] [CrossRef]
- Sansores, R.H.; Ramirez-Venegas, A. COPD in women: Susceptibility or vulnerability? Eur. Respir. J. 2016, 47, 19–22. [Google Scholar] [CrossRef]
- Mustonen, T.K.; Spencer, S.M.; Hoskinson, R.A.; Sachs, D.P.; Garvey, A.J. The influence of gender, race, and menthol content on tobacco exposure measures. Nicotine Tob. Res. 2005, 7, 581–590. [Google Scholar] [CrossRef] [PubMed]
- Anderson, D.O.; Ferris, B.G., Jr. Role of tobacco smoking in the causation of chronic respiratory disease. N. Engl. J. Med. 1962, 267, 787–794. [Google Scholar] [CrossRef] [PubMed]
- Fletcher, C.; Peto, R. The natural history of chronic airflow obstruction. Br. Med. J. 1977, 1, 1645–1648. [Google Scholar] [CrossRef] [PubMed]
- Gold, D.R.; Wang, X.; Wypij, D.; Speizer, F.E.; Ware, J.H.; Dockery, D.W. Effects of cigarette smoking on lung function in adolescent boys and girls. N. Engl. J. Med. 1996, 335, 931–937. [Google Scholar] [CrossRef]
- Pillai, S.G.; Kong, X.; Edwards, L.D.; Cho, M.H.; Anderson, W.H.; Coxson, H.O.; Lomas, D.A.; Silverman, E.K. Loci identified by genome-wide association studies influence different disease-related phenotypes in chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2010, 182, 1498–1505. [Google Scholar] [CrossRef]
- Svanes, C.; Sunyer, J.; Plana, E.; Dharmage, S.; Heinrich, J.; Jarvis, D.; de Marco, R.; Norback, D.; Raherison, C.; Villani, S.; et al. Early life origins of chronic obstructive pulmonary disease. Thorax 2010, 65, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Andresen, E.; Gunther, G.; Bullwinkel, J.; Lange, C.; Heine, H. Increased expression of beta-defensin 1 (DEFB1) in chronic obstructive pulmonary disease. PLoS ONE 2011, 6, e21898. [Google Scholar] [CrossRef]
- Yuan, C.; De Chang, G.L.; Deng, X. Genetic polymorphism and chronic obstructive pulmonary disease. Int. J. Chronic Obstruct. Pulm. Dis. 2017, 12, 1385–1393. [Google Scholar] [CrossRef]
- Shannon, J.M.; Wikenheiser-Brokamp, K.A.; Greenberf, J.M. Lung Growth and Development. In Textbook of Respiratory Medicine 6; Broaddus, V.C., Mason, R.J., Eds.; Elsevier: Philadelphia, PA, USA, 2016; pp. 22–31. [Google Scholar]
- Beyer, D.; Mitfessel, H.; Gillissen, A. Maternal smoking promotes chronic obstructive lung disease in the offspring as adults. Eur. J. Med. Res. 2009, 14, 27–31. [Google Scholar] [CrossRef]
- McEvoy, C.T.; Spindel, E.R. Pulmonary effects of maternal smoking on the fetus and child: Effects on lung development, respiratory morbidities, and life long lung health. Paediatr. Respir. Rev. 2017, 21, 27–33. [Google Scholar] [CrossRef]
- Balte, P.; Karmaus, W.; Roberts, G.; Kurukulaaratchy, R.; Mitchell, F.; Arshad, H. Relationship between birth weight, maternal smoking during pregnancy and childhood and adolescent lung function: A path analysis. Respir. Med. 2016, 121, 13–20. [Google Scholar] [CrossRef]
- Barker, D.J.; Godfrey, K.M.; Fall, C.; Osmond, C.; Winter, P.D.; Shaheen, S.O. Relation of birth weight and childhood respiratory infection to adult lung function and death from chronic obstructive airways disease. BMJ 1991, 303, 671–675. [Google Scholar] [CrossRef]
- Simpson, S.J.; Turkovic, L.; Wilson, A.C.; Verheggen, M.; Logie, K.M.; Pillow, J.J.; Hall, G.L. Lung function trajectories throughout childhood in survivors of very preterm birth: A longitudinal cohort study. Lancet Child Adolesc. Health 2018, 2, 350–359. [Google Scholar] [CrossRef]
- Kwinta, P.; Pietrzyk, J.J. Preterm birth and respiratory disease in later life. Expert Rev. Respir. Med. 2010, 4, 593–604. [Google Scholar] [CrossRef] [PubMed]
- Svanes, C.; Omenaas, E.; Jarvis, D.; Chinn, S.; Gulsvik, A.; Burney, P. Parental smoking in childhood and adult obstructive lung disease: Results from the European Community Respiratory Health Survey. Thorax 2004, 59, 295–302. [Google Scholar] [CrossRef] [PubMed]
- Lange, P.; Parner, J.; Vestbo, J.; Schnohr, P.; Jensen, G. A 15-year follow-up study of ventilatory function in adults with asthma. N. Engl. J. Med. 1998, 339, 1194–1200. [Google Scholar] [CrossRef] [PubMed]
- McGeachie, M.J.; Yates, K.P.; Zhou, X.; Guo, F.; Sternberg, A.L.; Van Natta, M.L.; Wise, R.A.; Szefler, S.J.; Sharma, S.; Kho, A.T.; et al. Patterns of growth and decline in lung function in persistent childhood asthma. N. Engl. J. Med. 2016, 374, 1842–1852. [Google Scholar] [CrossRef]
- Tagiyeva, N.; Devereux, G.; Fielding, S.; Turner, S.; Douglas, G. Outcomes of childhood asthma and wheezy bronchitis. A 50-year cohort study. Am. J. Respir. Crit. Care Med. 2016, 193, 23–30. [Google Scholar] [CrossRef]
- Chan, J.Y.; Stern, D.A.; Guerra, S.; Wright, A.L.; Morgan, W.J.; Martinez, F.D. Pneumonia in childhood and impaired lung function in adults: A longitudinal study. Pediatrics 2015, 135, 607–616. [Google Scholar] [CrossRef]
- De Marco, R.; Accordini, S.; Cerveri, I.; Corsico, A.; Anto, J.M.; Kunzli, N.; Janson, C.; Sunyer, J.; Jarvis, D.; Chinn, S.; et al. Incidence of chronic obstructive pulmonary disease in a cohort of young adults according to the presence of chronic cough and phlegm. Am. J. Respir. Crit. Care Med. 2007, 175, 32–39. [Google Scholar] [CrossRef]
- Guerra, S.; Sherrill, D.L.; Venker, C.; Ceccato, C.M.; Halonen, M.; Martinez, F.D. Chronic bronchitis before age 50 years predicts incident airflow limitation and mortality risk. Thorax 2009, 64, 894–900. [Google Scholar] [CrossRef]
- Stern, D.A.; Morgan, W.J.; Wright, A.L.; Guerra, S.; Martinez, F.D. Poor airway function in early infancy and lung function by age 22 years: A non-selective longitudinal cohort study. Lancet 2007, 370, 758–764. [Google Scholar] [CrossRef]
- Guerra, S.; Stern, D.A.; Zhou, M.; Sherrill, D.L.; Wright, A.L.; Morgan, W.J.; Martinez, F.D. Combined effects of parental and active smoking on early lung function deficits: A prospective study from birth to age 26 years. Thorax 2013, 68, 1021–1028. [Google Scholar] [CrossRef] [PubMed]
- Tager, I.B.; Segal, M.R.; Speizer, F.E.; Weiss, S.T. The natural history of forced expiratory volumes. Effect of cigarette smoking and respiratory symptoms. Am. Rev. Respir. Dis. 1988, 138, 837–849. [Google Scholar] [CrossRef] [PubMed]
- Sherrill, D.L.; Lebowitz, M.D.; Knudson, R.J.; Burrows, B. Continuous longitudinal regression equations for pulmonary function measures. Eur. Respir. J. 1992, 5, 452–462. [Google Scholar] [PubMed]
- Park, B.; Koo, S.M.; An, J.; Lee, M.; Kang, H.Y.; Qiao, D.; Cho, M.H.; Sung, J.; Silverman, E.K.; Yang, H.J.; et al. Genome-wide assessment of gene-by-smoking interactions in COPD. Sci. Rep. 2018, 8, 9319. [Google Scholar] [CrossRef] [PubMed]
- Molfino, N.A. Genetic predisposition to accelerated decline of lung function in COPD. Int. J. Chronic Obstruct. Pulm. Dis. 2007, 2, 117–119. [Google Scholar]
- Leem, A.Y.; Park, B.; Kim, Y.S.; Chang, J.; Won, S.; Jung, J.Y. Longitudinal decline in lung function: A community-based cohort study in Korea. Sci. Rep. 2019, 9, 13614. [Google Scholar] [CrossRef]
- Perez-Padilla, R.; Fernandez-Plata, R.; Montes de Oca, M.; Lopez-Varela, M.V.; Jardim, J.R.; Muino, A.; Valdivia, G.; Menezes, A.M.B. Lung function decline in subjects with and without COPD in a population-based cohort in Latin-America. PLoS ONE 2017, 12, e0177032. [Google Scholar] [CrossRef]
- Omori, H.; Nonami, Y.; Morimoto, Y. Effect of smoking on FEV decline in a cross-sectional and longitudinal study of a large cohort of Japanese males. Respirology 2005, 10, 464–469. [Google Scholar] [CrossRef]
- Dransfield, M.T.; Davis, J.J.; Gerald, L.B.; Bailey, W.C. Racial and gender differences in susceptibility to tobacco smoke among patients with chronic obstructive pulmonary disease. Respir. Med. 2006, 100, 1110–1116. [Google Scholar] [CrossRef]
- Terzikhan, N.; Verhamme, K.M.; Hofman, A.; Stricker, B.H.; Brusselle, G.G.; Lahousse, L. Prevalence and incidence of COPD in smokers and non-smokers: The Rotterdam Study. Eur. J. Epidemiol. 2016, 31, 785–792. [Google Scholar] [CrossRef] [PubMed]
- Jindal, S.K. Chronic obstructive pulmonary disease in non-smokers—Is it a different phenotype? Indian J. Med. Res. 2018, 147, 337–339. [Google Scholar] [CrossRef]
- Salvi, S. Tobacco smoking and environmental risk factors for chronic obstructive pulmonary disease. Clin. Chest Med. 2014, 35, 17–27. [Google Scholar] [CrossRef]
- Liao, S.Y.; Lin, X.; Christiani, D.C. Occupational exposures and longitudinal lung function decline. Am. J. Ind. Med. 2015, 58, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Harber, P.; Tashkin, D.P.; Simmons, M.; Crawford, L.; Hnizdo, E.; Connett, J. Effect of occupational exposures on decline of lung function in early chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2007, 176, 994–1000. [Google Scholar] [CrossRef]
- Dransfield, M.T.; Kunisaki, K.M.; Strand, M.J.; Anzueto, A.; Bhatt, S.P.; Bowler, R.P.; Criner, G.J.; Curtis, J.L.; Hanania, N.A.; Nath, H.; et al. Acute exacerbations and lung function loss in smokers with and without chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2017, 195, 324–330. [Google Scholar] [PubMed]
- Donaldson, G.C.; Seemungal, T.A.; Bhowmik, A.; Wedzicha, J.A. Relationship between exacerbation frequency and lung function decline in chronic obstructive pulmonary disease. Thorax 2002, 57, 847–852. [Google Scholar] [CrossRef]
- Drummond, M.B.; Hansel, N.N.; Connett, J.E.; Scanlon, P.D.; Tashkin, D.P.; Wise, R.A. Spirometric predictors of lung function decline and mortality in early chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2012, 185, 1301–1306. [Google Scholar] [CrossRef]
- Lee, J.Y.; Rhee, C.K.; Jung, K.S.; Yoo, K.H. Strategies for management of the early chronic obstructive lung disease. Tuberc. Respir. Dis. 2016, 79, 121–126. [Google Scholar] [CrossRef]
- Choi, H.S.; Rhee, C.K.; Park, Y.B.; Yoo, K.H.; Lim, S.Y. Metabolic syndrome in early chronic obstructive pulmonary disease: Gender differences and impact on exacerbation and medical costs. Int. J. Chronic Obstruct. Pulm. Dis. 2019, 14, 2873–2883. [Google Scholar] [CrossRef]
- Siafakas, N.; Bizymi, N.; Mathioudakis, A.; Corlateanu, A. EARLY versus MILD chronic obstructive pulmonary disease (COPD). Respir. Med. 2018, 140, 127–131. [Google Scholar] [CrossRef] [PubMed]
- Varkey, A.B. Chronic obstructive pulmonary disease in women: Exploring gender differences. Curr. Opin. Pulm. Med. 2004, 10, 98–103. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Hwang, E.D.; Lim, J.E.; Moon, S.; Kang, Y.A.; Jung, J.Y.; Park, M.S.; Kim, S.K.; Chang, J.; Kim, Y.S.; et al. The risk factors and characteristics of COPD among nonsmokers in Korea: An analysis of KNHANES IV and V. Lung 2016, 194, 353–361. [Google Scholar] [CrossRef]
- Vestbo, J.; Lange, P. Can GOLD Stage 0 provide information of prognostic value in chronic obstructive pulmonary disease? Am. J. Respir. Crit. Care Med. 2002, 166, 329–332. [Google Scholar] [CrossRef]
- McDonough, J.E.; Yuan, R.; Suzuki, M.; Seyednejad, N.; Elliott, W.M.; Sanchez, P.G.; Wright, A.C.; Gefter, W.B.; Litzky, L.; Coxson, H.O.; et al. Small-airway obstruction and emphysema in chronic obstructive pulmonary disease. N. Engl. J. Med. 2011, 365, 1567–1575. [Google Scholar] [CrossRef]
- Koo, H.K.; Vasilescu, D.M.; Booth, S.; Hsieh, A.; Katsamenis, O.L.; Fishbane, N.; Elliott, W.M.; Kirby, M.; Lackie, P.; Sinclair, I.; et al. Small airways disease in mild and moderate chronic obstructive pulmonary disease: A cross-sectional study. Lancet Respir. Med. 2018, 6, 591–602. [Google Scholar] [CrossRef]
- Global Strategy for Prevention, Diagnosis and Managment of Chronic Obstructive Pulmonary Disease. 2020. Available online: https://goldcopd.org/gold-reports/ (accessed on 30 June 2020).
- Sansores, R.H.; Velazquez-Uncal, M.; Perez-Bautista, O.; Villalba-Caloca, J.; Falfan-Valencia, R.; Ramirez-Venegas, A. Prevalence of chronic obstructive pulmonary disease in asymptomatic smokers. Int. J. Chronic Obstruct. Pulm. Dis. 2015, 10, 2357–2363. [Google Scholar] [CrossRef][Green Version]
- Kaplan, A.; Thomas, M. Screening for COPD: The gap between logic and evidence. Eur. Respir. Rev. 2017, 26. [Google Scholar] [CrossRef]
- Cunningham, J.; Dockery, D.W.; Speizer, F.E. Maternal smoking during pregnancy as a predictor of lung function in children. Am. J. Epidemiol. 1994, 139, 1139–1152. [Google Scholar] [CrossRef]
- Hayatbakhsh, M.R.; Sadasivam, S.; Mamun, A.A.; Najman, J.M.; Williams, G.M.; O’Callaghan, M.J. Maternal smoking during and after pregnancy and lung function in early adulthood: A prospective study. Thorax 2009, 64, 810–814. [Google Scholar] [CrossRef]
- Jaakkola, M.S.; Jaakkola, J.J.; Becklake, M.R.; Ernst, P. Passive smoking and evolution of lung function in young adults. An 8-year longitudinal study. J. Clin. Epidemiol. 1995, 48, 317–327. [Google Scholar] [CrossRef]
- Jaakkola, M.S.; Ernst, P.; Jaakkola, J.J.; Becklake, M.R. Effect of cigarette smoking on evolution of ventilatory lung function in young adults: An eight year longitudinal study. Thorax 1991, 46, 907–913. [Google Scholar] [CrossRef] [PubMed]
- Salvi, S.S.; Barnes, P.J. Chronic obstructive pulmonary disease in non-smokers. Lancet 2009, 374, 733–743. [Google Scholar] [CrossRef]
- Hayden, L.P.; Hobbs, B.D.; Cohen, R.T.; Wise, R.A.; Checkley, W.; Crapo, J.D.; Hersh, C.P.; COPDGene Investigators. Childhood pneumonia increases risk for chronic obstructive pulmonary disease: The COPDGene study. Respir. Res. 2015, 16, 115. [Google Scholar] [CrossRef]
- Johnston, I.D.; Strachan, D.P.; Anderson, H.R. Effect of pneumonia and whooping cough in childhood on adult lung function. N. Engl. J. Med. 1998, 338, 581–587. [Google Scholar] [CrossRef]
- Aanerud, M.; Carsin, A.E.; Sunyer, J.; Dratva, J.; Gislason, T.; Jarvis, D.; deMarco, R.; Raherison, C.; Wjst, M.; Dharmage, S.C.; et al. Interaction between asthma and smoking increases the risk of adult airway obstruction. Eur. Respir. J. 2015, 45, 635–643. [Google Scholar] [CrossRef]
- De Marco, R.; Accordini, S.; Marcon, A.; Cerveri, I.; Anto, J.M.; Gislason, T.; Heinrich, J.; Janson, C.; Jarvis, D.; Kuenzli, N.; et al. Risk factors for chronic obstructive pulmonary disease in a European cohort of young adults. Am. J. Respir. Crit. Care Med. 2011, 183, 891–897. [Google Scholar] [CrossRef]
- Jo, Y.S.; Hwang, Y.I.; Yoo, K.H.; Kim, T.H.; Lee, M.G.; Lee, S.H.; Shin, K.C.; In, K.H.; Yoon, H.K.; Rhee, C.K. Comparing the different diagnostic criteria of Asthma-COPD overlap. Allergy 2019, 74, 186–189. [Google Scholar] [CrossRef]
- Nielsen, M.; Bårnes, C.B.; Ulrik, C.S. Clinical characteristics of the asthma-COPD overlap syndrome—A systematic review. Int. J. Chronic Obstruct. Pulm. Dis. 2015, 10, 1443–1454. [Google Scholar]
- Bai, J.W.; Mao, B.; Yang, W.L.; Liang, S.; Lu, H.W.; Xu, J.F. Asthma-COPD overlap syndrome showed more exacerbations however lower mortality than COPD. QJM 2017, 110, 431–436. [Google Scholar] [CrossRef]
- Ramírez-Venegas, A.; Sansores, R.H.; Pérez-Padilla, R.; Regalado, J.; Velázquez, A.; Sánchez, C.; Mayar, M.E. Survival of patients with chronic obstructive pulmonary disease due to biomass smoke and tobacco. Am. J. Respir. Crit. Care Med. 2006, 173, 393–397. [Google Scholar] [CrossRef] [PubMed]
- Camp, P.G.; Ramirez-Venegas, A.; Sansores, R.H.; Alva, L.F.; McDougall, J.E.; Sin, D.D.; Pare, P.D.; Muller, N.L.; Silva, C.I.; Rojas, C.E.; et al. COPD phenotypes in biomass smoke- versus tobacco smoke-exposed Mexican women. Eur. Respir. J. 2014, 43, 725–734. [Google Scholar] [CrossRef] [PubMed]
- Moran-Mendoza, O.; Perez-Padilla, J.R.; Salazar-Flores, M.; Vazquez-Alfaro, F. Wood smoke-associated lung disease: A clinical, functional, radiological and pathological description. Int. J. Tuberc. Lung Dis. 2008, 12, 1092–1098. [Google Scholar]
- Viegi, G.; Di Pede, C. Chronic obstructive lung diseases and occupational exposure. Curr. Opin. Allergy Clin. Immunol. 2002, 2, 115–121. [Google Scholar] [CrossRef]
- Kan, H.; Heiss, G.; Rose, K.M.; Whitsel, E.; Lurmann, F.; London, S.J. Traffic exposure and lung function in adults: The Atherosclerosis Risk in Communities study. Thorax 2007, 62, 873–879. [Google Scholar] [CrossRef]
- Schikowski, T.; Adam, M.; Marcon, A.; Cai, Y.; Vierkötter, A.; Carsin, A.E.; Jacquemin, B.; Al Kanani, Z.; Beelen, R.; Birk, M.; et al. Association of ambient air pollution with the prevalence and incidence of COPD. Eur. Respir. J. 2014, 44, 614–626. [Google Scholar] [CrossRef]
- To, T.; Zhu, J.; Villeneuve, P.J.; Simatovic, J.; Feldman, L.; Gao, C.; Williams, D.; Chen, H.; Weichenthal, S.; Wall, C.; et al. Chronic disease prevalence in women and air pollution—A 30-year longitudinal cohort study. Environ. Int. 2015, 80, 26–32. [Google Scholar] [CrossRef]
- Gauderman, W.J.; Avol, E.; Gilliland, F.; Vora, H.; Thomas, D.; Berhane, K.; McConnell, R.; Kuenzli, N.; Lurmann, F.; Rappaport, E.; et al. The effect of air pollution on lung development from 10 to 18 years of age. N. Engl. J. Med. 2004, 351, 1057–1067. [Google Scholar] [CrossRef] [PubMed]
- Eisner, M.D.; Anthonisen, N.; Coultas, D.; Kuenzli, N.; Perez-Padilla, R.; Postma, D.; Romieu, I.; Silverman, E.K.; Balmes, J.R. An official American Thoracic Society public policy statement: Novel risk factors and the global burden of chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2010, 182, 693–718. [Google Scholar] [CrossRef] [PubMed]
- Arshad, S.H.; Hodgekiss, C.; Holloway, J.W.; Kurukulaaratchy, R.; Karmaus, W.; Zhang, H.; Roberts, G. Association of asthma and smoking with lung function impairment in adolescence and early adulthood: The Isle of Wight Birth Cohort Study. Eur. Respir. J. 2020, 55. [Google Scholar] [CrossRef]
- Scichilone, N.; Battaglia, S.; La Sala, A.; Bellia, V. Clinical implications of airway hyperresponsiveness in COPD. Int. J. Chronic Obstruct. Pulm. Dis. 2006, 1, 49–60. [Google Scholar]
- Deesomchok, A.; Webb, K.A.; Forkert, L.; Lam, Y.M.; Ofir, D.; Jensen, D.; O’Donnell, D.E. Lung hyperinflation and its reversibility in patients with airway obstruction of varying severity. COPD 2010, 7, 428–437. [Google Scholar] [CrossRef] [PubMed]
- Nagelmann, A.; Tonnov, Ä.; Laks, T.; Sepper, R.; Prikk, K. Lung dysfunction of chronic smokers with no signs of COPD. COPD 2011, 8, 189–195. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Jian, W.; Gao, Y.; Xie, Y.; Song, Y.; Zheng, J. Early COPD patients with lung hyperinflation associated with poorer lung function but better bronchodilator responsiveness. Int. J. Chronic Obstruct. Pulm. Dis. 2016, 11, 2519–2526. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Soumagne, T.; Laveneziana, P.; Veil-Picard, M.; Guillien, A.; Claude, F.; Puyraveau, M.; Annesi-Maesano, I.; Roche, N.; Dalphin, J.C.; Degano, B. Asymptomatic subjects with airway obstruction have significant impairment at exercise. Thorax 2016, 71, 804–811. [Google Scholar] [CrossRef] [PubMed]
- Matheson, M.C.; Raven, J.; Johns, D.P.; Abramson, M.J.; Walters, E.H. Associations between reduced diffusing capacity and airflow obstruction in community-based subjects. Respir. Med. 2007, 101, 1730–1737. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Harvey, B.G.; Strulovici-Barel, Y.; Kaner, R.J.; Sanders, A.; Vincent, T.L.; Mezey, J.G.; Crystal, R.G. Risk of COPD with obstruction in active smokers with normal spirometry and reduced diffusion capacity. Eur. Respir. J. 2015, 46, 1589–1597. [Google Scholar] [CrossRef]
- Zaigham, S.; Wollmer, P.; Engström, G. The association of lung clearance index with COPD and FEV(1) reduction in ‘men born in 1914’. COPD J. Chronic Obstr. Pulm. Dis. 2017, 14, 324–329. [Google Scholar] [CrossRef]
- Frantz, S.; Nihlén, U.; Dencker, M.; Engström, G.; Löfdahl, C.G.; Wollmer, P. Impulse oscillometry may be of value in detecting early manifestations of COPD. Respir. Med. 2012, 106, 1116–1123. [Google Scholar] [CrossRef]
- Kolsum, U.; Borrill, Z.; Roy, K.; Starkey, C.; Vestbo, J.; Houghton, C.; Singh, D. Impulse oscillometry in COPD: Identification of measurements related to airway obstruction, airway conductance and lung volumes. Respir. Med. 2009, 103, 136–143. [Google Scholar] [CrossRef]
- Gong, S.G.; Yang, W.L.; Liu, J.M.; Liu, W.Z.; Zheng, W. Change in pulmonary function in chronic obstructive pulmonary disease stage 0 patients. Int. J. Clin. Exp. Med. 2015, 8, 21400–21406. [Google Scholar]
- Omori, H.; Nakashima, R.; Otsuka, N.; Mishima, Y.; Tomiguchi, S.; Narimatsu, A.; Nonami, Y.; Mihara, S.; Koyama, W.; Marubayashi, T.; et al. Emphysema detected by lung cancer screening with low-dose spiral CT: Prevalence, and correlation with smoking habits and pulmonary function in Japanese male subjects. Respirology 2006, 11, 205–210. [Google Scholar] [CrossRef] [PubMed]
- McAllister, D.A.; Ahmed, F.S.; Austin, J.H.; Henschke, C.I.; Keller, B.M.; Lemeshow, A.; Reeves, A.P.; Mesia-Vela, S.; Pearson, G.D.; Shiau, M.C.; et al. Emphysema predicts hospitalisation and incident airflow obstruction among older smokers: A prospective cohort study. PLoS ONE 2014, 9, e93221. [Google Scholar] [CrossRef] [PubMed]
- Vestbo, J.; Edwards, L.D.; Scanlon, P.D.; Yates, J.C.; Agusti, A.; Bakke, P.; Calverley, P.M.; Celli, B.; Coxson, H.O.; Crim, C.; et al. Changes in forced expiratory volume in 1 second over time in COPD. N. Engl. J. Med. 2011, 365, 1184–1192. [Google Scholar] [CrossRef] [PubMed]
- Koo, H.K.; Jin, K.N.; Kim, D.K.; Chung, H.S.; Lee, C.H. Association of incidental emphysema with annual lung function decline and future development of airflow limitation. Int. J. Chronic Obstruct. Pulm. Dis. 2016, 11, 161–166. [Google Scholar] [CrossRef]
- Bhatt, S.P. Imaging small airway disease: Probabilities and possibilities. Ann. Am. Thorac. Soc. 2019, 16, 975–977. [Google Scholar] [CrossRef]
- Galban, C.J.; Han, M.K.; Boes, J.L.; Chughtai, K.A.; Meyer, C.R.; Johnson, T.D.; Galban, S.; Rehemtulla, A.; Kazerooni, E.A.; Martinez, F.J.; et al. Computed tomography-based biomarker provides unique signature for diagnosis of COPD phenotypes and disease progression. Nat. Med. 2012, 18, 1711–1715. [Google Scholar] [CrossRef]
- Bhatt, S.P.; Soler, X.; Wang, X.; Murray, S.; Anzueto, A.R.; Beaty, T.H.; Boriek, A.M.; Casaburi, R.; Criner, G.J.; Diaz, A.A.; et al. Association between functional small airway disease and FEV1 decline in chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2016, 194, 178–184. [Google Scholar] [CrossRef]
- Kirby, M.; Tanabe, N.; Tan, W.C.; Zhou, G.; Obeidat, M.; Hague, C.J.; Leipsic, J.; Bourbeau, J.; Sin, D.D.; Hogg, J.C.; et al. Total airway count on computed tomography and the risk of chronic obstructive pulmonary disease progression. Findings from a population-based study. Am. J. Respir. Crit. Care Med. 2018, 197, 56–65. [Google Scholar] [CrossRef]
- Mohamed Hoesein, F.A.; de Jong, P.A.; Lammers, J.W.; Mali, W.P.; Schmidt, M.; de Koning, H.J.; van der Aalst, C.; Oudkerk, M.; Vliegenthart, R.; Groen, H.J.; et al. Airway wall thickness associated with forced expiratory volume in 1 second decline and development of airflow limitation. Eur. Respir. J. 2015, 45, 644–651. [Google Scholar] [CrossRef]
- Zeng, S.; Tham, A.; Bos, B.; Jin, J.; Giang, B.; Arjomandi, M. Lung volume indices predict morbidity in smokers with preserved spirometry. Thorax 2019, 74, 114–124. [Google Scholar] [CrossRef] [PubMed]
- Arjomandi, M.; Zeng, S.; Barjaktarevic, I.; Barr, R.G.; Bleecker, E.R.; Bowler, R.P.; Buhr, R.G.; Criner, G.J.; Comellas, A.P.; Cooper, C.B.; et al. Radiographic lung volumes predict progression to COPD in smokers with preserved spirometry in SPIROMICS. Eur. Respir. J. 2019, 54. [Google Scholar] [CrossRef]
- Ruppert, K.; Qing, K.; Patrie, J.T.; Altes, T.A.; Mugler, J.P., 3rd. Using hyperpolarized xenon-129 MRI to quantify early-stage lung disease in smokers. Acad Radiol. 2019, 26, 355–366. [Google Scholar] [CrossRef] [PubMed]
- Polverino, F.; Hysinger, E.B.; Gupta, N.; Willmering, M.; Olin, T.; Abman, S.H.; Woods, J.C. Lung MRI as a potential complementary diagnostic tool for early COPD. Am. J. Med. 2020, 133, 757–760. [Google Scholar] [CrossRef]
- Fan, L.; Xia, Y.; Guan, Y.; Yu, H.; Zhang, T.F.; Liu, S.Y.; Li, B. Capability of differentiating smokers with normal pulmonary function from COPD patients: A comparison of CT pulmonary volume analysis and MR perfusion imaging. Eur. Radiol. 2013, 23, 1234–1241. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Guan, Y.; Fan, L.; Liu, S.Y.; Yu, H.; Zhao, L.M.; Li, B. Dynamic contrast enhanced magnetic resonance perfusion imaging in high-risk smokers and smoking-related COPD: Correlations with pulmonary function tests and quantitative computed tomography. COPD J. Chronic Obstr. Pulm. Dis. 2014, 11, 510–520. [Google Scholar] [CrossRef]
- Kim, V.; Han, M.K.; Vance, G.B.; Make, B.J.; Newell, J.D.; Hokanson, J.E.; Hersh, C.P.; Stinson, D.; Silverman, E.K.; Criner, G.J. The chronic bronchitic phenotype of COPD: An analysis of the COPDGene Study. Chest 2011, 140, 626–633. [Google Scholar] [CrossRef]
- Meek, P.M.; Petersen, H.; Washko, G.R.; Diaz, A.A.; Klm, V.; Sood, A.; Tesfaigzi, Y. Chronic bronchitis is associated with worse symptoms and quality of life than chronic airflow obstruction. Chest 2015, 148, 408–416. [Google Scholar] [CrossRef]
- Kania, A.; Krenke, R.; Kuziemski, K.; Czajkowska-Malinowska, M.; Celejewska-Wojcik, N.; Kuznar-Kaminska, B.; Farnik, M.; Bokiej, J.; Miszczuk, M.; Damps-Konstanska, I.; et al. Distribution and characteristics of COPD phenotypes results from the Polish sub-cohort of the POPE study. Int. J. Chronic Obstruct. Pulm. Dis. 2018, 13, 1613–1621. [Google Scholar] [CrossRef]
- Choi, J.Y.; Yoon, H.K.; Park, S.J.; Park, Y.B.; Shin, K.C.; Na, J.O.; Yoo, K.H.; Jung, K.S.; Kim, Y.K.; Rhee, C.K. Chronic bronchitis is an independently associated factor for more symptom and high-risk groups. Int. J. Chronic Obstruct. Pulm. Dis. 2016, 11, 1335–1341. [Google Scholar]
- Ferris, B.G. Epidemiology Standardization Project (American Thoracic Society). Am. Rev. Respir. Dis. 1978, 118, 1–120. [Google Scholar] [PubMed]
- Choi, J.Y.; Yoon, H.K.; Shin, K.C.; Park, S.Y.; Lee, C.Y.; Ra, S.W.; Jung, K.S.; Yoo, K.H.; Lee, C.H.; Rhee, C.K. CAT Score and SGRQ Definitions of chronic bronchitis as an alternative to the classical definition. Int. J. Chronic Obstruct. Pulm. Dis. 2019, 14, 3043–3052. [Google Scholar] [CrossRef] [PubMed]
- Kim, V.; Zhao, H.; Regan, E.; Han, M.K.; Make, B.J.; Crapo, J.D.; Jones, P.W.; Curtis, J.L.; Silverman, E.K.; Criner, G.J.; et al. The St. George’s Respiratory Questionnaire definition of chronic bronchitis may be a better predictor of COPD exacerbations compared with the classic definition. Chest 2019, 156, 685–695. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.U.; Lee, J.H.; Kim, T.H.; Lee, J.S.; Lee, S.D.; Oh, Y.M.; Rhee, C.K. Alternative definitions of chronic bronchitis and their correlation with CT parameters. Int. J. Chronic Obstruct. Pulm. Dis. 2018, 13, 1893–1899. [Google Scholar] [CrossRef]
- Kim, V.; Oros, M.; Durra, H.; Kelsen, S.; Aksoy, M.; Cornwell, W.D.; Rogers, T.J.; Criner, G.J. Chronic bronchitis and current smoking are associated with more goblet cells in moderate to severe COPD and smokers without airflow obstruction. PLoS ONE 2015, 10, e0116108. [Google Scholar] [CrossRef]
- Holm, M.; Toren, K.; Andersson, E. Incidence of chronic bronchitis: A prospective study in a large general population. Int. J. Tuberc. Lung Dis. 2014, 18, 870–875. [Google Scholar] [CrossRef]
- Mejza, F.; Gnatiuc, L.; Buist, A.S.; Vollmer, W.M.; Lamprecht, B.; Obaseki, D.O.; Nastalek, P.; Nizankowska-Mogilnicka, E.; Burney, P.G.J. Prevalence and burden of chronic bronchitis symptoms: Results from the BOLD study. Eur. Respir. J. 2017, 50. [Google Scholar] [CrossRef]
- Vestbo, J.; Prescott, E.; Lange, P. Association of chronic mucus hypersecretion with FEV1 decline and chronic obstructive pulmonary disease morbidity. Copenhagen City Heart Study Group. Am. J. Respir. Crit. Care Med. 1996, 153, 1530–1535. [Google Scholar] [CrossRef]
- Allinson, J.P.; Hardy, R.; Donaldson, G.C.; Shaheen, S.O.; Kuh, D.; Wedzicha, J.A. The presence of chronic mucus hypersecretion across adult life in relation to chronic obstructive pulmonary disease development. Am. J. Respir. Crit. Care Med. 2016, 193, 662–672. [Google Scholar] [CrossRef]
- Morice, A.H.; Celli, B.; Kesten, S.; Lystig, T.; Tashkin, D.; Decramer, M. COPD in young patients: A pre-specified analysis of the four-year trial of tiotropium (UPLIFT). Respir. Med. 2010, 104, 1659–1667. [Google Scholar] [CrossRef]
- Wang, Y.; Liao, J.; Zhong, Y.; Zhang, C.; Li, X.; Wang, G. Predictive value of combining inflammatory biomarkers and rapid decline of FEV(1) for COPD in Chinese population: A prospective cohort study. Int. J. Chronic Obstruct. Pulm. Dis. 2019, 14, 2825–2833. [Google Scholar] [CrossRef] [PubMed]
- Zemans, R.L.; Jacobson, S.; Keene, J.; Kechris, K.; Miller, B.E.; Tal-Singer, R.; Bowler, R.P. Multiple biomarkers predict disease severity, progression and mortality in COPD. Respir. Res. 2017, 18, 117. [Google Scholar] [CrossRef] [PubMed]
- Baralla, A.; Fois, A.G.; Sotgiu, E.; Zinellu, E.; Mangoni, A.A.; Sotgia, S.; Zinellu, A.; Pirina, P.; Carru, C. Plasma proteomic signatures in early chronic obstructive pulmonary disease. Proteom. Clin. Appl. 2018, 12, e1700088. [Google Scholar] [CrossRef] [PubMed]
- Rhee, C.K.; Kim, K.; Yoon, H.K.; Kim, J.A.; Kim, S.H.; Lee, S.H.; Park, Y.B.; Jung, K.S.; Yoo, K.H.; Hwang, Y.I. Natural course of early COPD. Int. J. Chronic Obstruct. Pulm. Dis. 2017, 12, 663–668. [Google Scholar] [CrossRef]
- Colak, Y.; Afzal, S.; Nordestgaard, B.G.; Vestbo, J.; Lange, P. Prevalence, characteristics, and prognosis of early chronic obstructive pulmonary disease. The Copenhagen General Population Study. Am. J. Respir. Crit. Care Med. 2020, 201, 671–680. [Google Scholar] [CrossRef]
- Mapel, D.W.; Dalal, A.A.; Blanchette, C.M.; Petersen, H.; Ferguson, G.T. Severity of COPD at initial spirometry-confirmed diagnosis: Data from medical charts and administrative claims. Int. J. Chronic Obstruct. Pulm. Dis. 2011, 6, 573–581. [Google Scholar]
- Price, D.; Freeman, D.; Cleland, J.; Kaplan, A.; Cerasoli, F. Earlier diagnosis and earlier treatment of COPD in primary care. Prim. Care Respir. J. 2011, 20, 15–22. [Google Scholar] [CrossRef]
- Maltais, F.; Dennis, N.; Chan, C.K. Rationale for earlier treatment in COPD: A systematic review of published literature in mild-to-moderate COPD. COPD J. Chronic Obstruct. Pulm. Dis. 2013, 10, 79–103. [Google Scholar] [CrossRef]
- Celli, B.R.; Agustí, A. COPD: Time to improve its taxonomy? ERJ Open Res. 2018, 4. [Google Scholar] [CrossRef]
- Anthonisen, N.R.; Connett, J.E.; Kiley, J.P.; Altose, M.D.; Bailey, W.C.; Buist, A.S.; Conway, W.A., Jr.; Enright, P.L.; Kanner, R.E.; O’Hara, P.; et al. Effects of smoking intervention and the use of an inhaled anticholinergic bronchodilator on the rate of decline of FEV1. The Lung Health Study. JAMA 1994, 272, 1497–1505. [Google Scholar] [CrossRef]
- Scanlon, P.D.; Connett, J.E.; Waller, L.A.; Altose, M.D.; Bailey, W.C.; Buist, A.S.; Tashkin, D.P. Smoking cessation and lung function in mild-to-moderate chronic obstructive pulmonary disease. The Lung Health Study. Am. J. Respir. Crit. Care Med. 2000, 161, 381–390. [Google Scholar] [CrossRef] [PubMed]
- Kanner, R.E.; Connett, J.E.; Williams, D.E.; Buist, A.S. Effects of randomized assignment to a smoking cessation intervention and changes in smoking habits on respiratory symptoms in smokers with early chronic obstructive pulmonary disease: The Lung Health Study. Am. J. Med. 1999, 106, 410–416. [Google Scholar] [CrossRef]
- Anthonisen, N.R.; Skeans, M.A.; Wise, R.A.; Manfreda, J.; Kanner, R.E.; Connett, J.E. The effects of a smoking cessation intervention on 14.5-year mortality: A randomized clinical trial. Ann. Intern. Med. 2005, 142, 233–239. [Google Scholar] [CrossRef]
- Chinn, S.; Jarvis, D.; Melotti, R.; Luczynska, C.; Ackermann-Liebrich, U.; Antó, J.M.; Cerveri, I.; de Marco, R.; Gislason, T.; Heinrich, J.; et al. Smoking cessation, lung function, and weight gain: A follow-up study. Lancet 2005, 365, 1629–1635. [Google Scholar] [CrossRef]
- Lee, P.N.; Fry, J.S. Systematic review of the evidence relating FEV1 decline to giving up smoking. BMC Med. 2010, 8, 84. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhong, N.S.; Li, X.; Chen, S.; Zheng, J.; Zhao, D.; Yao, W.; Zhi, R.; Wei, L.; He, B.; et al. Tiotropium in early-stage chronic obstructive pulmonary disease. N. Engl. J. Med. 2017, 377, 923–935. [Google Scholar] [CrossRef]
- Gagnon, P.; Saey, D.; Provencher, S.; Milot, J.; Bourbeau, J.; Tan, W.C.; Martel, S.; Maltais, F. Walking exercise response to bronchodilation in mild COPD: A randomized trial. Respir. Med. 2012, 106, 1695–1705. [Google Scholar] [CrossRef]
- Singh, D.; D’Urzo, A.D.; Donohue, J.F.; Kerwin, E.M. Weighing the evidence for pharmacological treatment interventions in mild COPD; a narrative perspective. Respir. Res. 2019, 20, 141. [Google Scholar] [CrossRef]
- Dusser, D.; Bravo, M.L.; Iacono, P. The effect of tiotropium on exacerbations and airflow in patients with COPD. Eur. Respir. J. 2006, 27, 547–555. [Google Scholar] [CrossRef]
- Decramer, M.; Celli, B.; Kesten, S.; Lystig, T.; Mehra, S.; Tashkin, D.P. Effect of tiotropium on outcomes in patients with moderate chronic obstructive pulmonary disease (UPLIFT): A prespecified subgroup analysis of a randomised controlled trial. Lancet 2009, 374, 1171–1178. [Google Scholar] [CrossRef]
- Tashkin, D.P.; Celli, B.; Senn, S.; Burkhart, D.; Kesten, S.; Menjoge, S.; Decramer, M. A 4-year trial of tiotropium in chronic obstructive pulmonary disease. N. Engl. J. Med. 2008, 359, 1543–1554. [Google Scholar] [CrossRef] [PubMed]
- Troosters, T.; Celli, B.; Lystig, T.; Kesten, S.; Mehra, S.; Tashkin, D.P.; Decramer, M. Tiotropium as a first maintenance drug in COPD: Secondary analysis of the UPLIFT trial. Eur. Respir. J. 2010, 36, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Ray, R.; Tombs, L.; Naya, I.; Compton, C.; Lipson, D.A.; Boucot, I. Efficacy and safety of the dual bronchodilator combination umeclidinium/vilanterol in COPD by age and airflow limitation severity: A pooled post hoc analysis of seven clinical trials. Pulm. Pharmacol. Ther. 2019, 57, 101802. [Google Scholar] [CrossRef]
- Jenkins, C.R.; Jones, P.W.; Calverley, P.M.; Celli, B.; Anderson, J.A.; Ferguson, G.T.; Yates, J.C.; Willits, L.R.; Vestbo, J. Efficacy of salmeterol/fluticasone propionate by GOLD stage of chronic obstructive pulmonary disease: Analysis from the randomised, placebo-controlled TORCH study. Respir. Res. 2009, 10, 59. [Google Scholar] [CrossRef]
- Pauwels, R.A.; Löfdahl, C.G.; Laitinen, L.A.; Schouten, J.P.; Postma, D.S.; Pride, N.B.; Ohlsson, S.V. Long-term treatment with inhaled budesonide in persons with mild chronic obstructive pulmonary disease who continue smoking. European Respiratory Society Study on Chronic Obstructive Pulmonary Disease. N. Engl. J. Med. 1999, 340, 1948–1953. [Google Scholar] [CrossRef] [PubMed]
- Calverley, P.M.A.; Anderson, J.A.; Brook, R.D.; Crim, C.; Gallot, N.; Kilbride, S.; Martinez, F.J.; Yates, J.; Newby, D.E.; Vestbo, J.; et al. Fluticasone furoate, vilanterol, and lung function decline in patients with moderate chronic obstructive pulmonary disease and heightened cardiovascular risk. Am. J. Respir. Crit. Care Med. 2018, 197, 47–55. [Google Scholar] [CrossRef]
- Vestbo, J.; Sorensen, T.; Lange, P.; Brix, A.; Torre, P.; Viskum, K. Long-term effect of inhaled budesonide in mild and moderate chronic obstructive pulmonary disease: A randomised controlled trial. Lancet 1999, 353, 1819–1823. [Google Scholar] [CrossRef]
- Jones, P.W.; Willits, L.R.; Burge, P.S.; Calverley, P.M. Disease severity and the effect of fluticasone propionate on chronic obstructive pulmonary disease exacerbations. Eur. Respir. J. 2003, 21, 68–73. [Google Scholar] [CrossRef]
- Van Grunsven, P.; Schermer, T.; Akkermans, R.; Albers, M.; van den Boom, G.; van Schayck, O.; van Herwaarden, C.; van Weel, C. Short- and long-term efficacy of fluticasone propionate in subjects with early signs and symptoms of chronic obstructive pulmonary disease. Results of the DIMCA study. Respir. Med. 2003, 97, 1303–1312. [Google Scholar] [CrossRef][Green Version]
- Wise, R.; Connett, J.; Weinmann, G.; Scanlon, P.; Skeans, M. Effect of inhaled triamcinolone on the decline in pulmonary function in chronic obstructive pulmonary disease. N. Engl. J. Med. 2000, 343, 1902–1909. [Google Scholar]
- Di Lorenzo, G.; Morici, G.; Drago, A.; Pellitteri, M.E.; Mansueto, P.; Melluso, M.; Norrito, F.; Squassante, L.; Fasolo, A. Efficacy, tolerability, and effects on quality of life of inhaled salmeterol and oral theophylline in patients with mild-to-moderate chronic obstructive pulmonary disease. SLMT02 Italian Study Group. Clin. Ther. 1998, 20, 1130–1148. [Google Scholar] [CrossRef]
- Hirai, D.M.; Jones, J.H.; Zelt, J.T.; da Silva, M.L.; Bentley, R.F.; Edgett, B.A.; Gurd, B.J.; Tschakovsky, M.E.; O’Donnell, D.E.; Neder, J.A. Oral N-acetylcysteine and exercise tolerance in mild chronic obstructive pulmonary disease. J. Appl. Physiol. 2017, 122, 1351–1361. [Google Scholar] [CrossRef] [PubMed]
- Decramer, M.; Rutten-van Mölken, M.; Dekhuijzen, P.N.; Troosters, T.; van Herwaarden, C.; Pellegrino, R.; van Schayck, C.P.; Olivieri, D.; Del Donno, M.; De Backer, W.; et al. Effects of N-acetylcysteine on outcomes in chronic obstructive pulmonary disease (Bronchitis Randomized on NAC Cost-Utility Study, broncus): A randomised placebo-controlled trial. Lancet 2005, 365, 1552–1560. [Google Scholar] [CrossRef]
| Category | Diagnostic Tools | Supplements |
|---|---|---|
| Identification of risk factors | Tobacco smoking | In utero Parental smoking Second-hand smoke Smoking |
| Childhood infection | ||
| Respiratory diseases (e.g., asthma) | ||
| Biomass smoke exposure | ||
| Air pollution | NO2, NOx, PM10, traffic indicators | |
| Occupational exposure | ||
| Genetic factors | AAT deficiency, cutis laxa, Marfan syndrome, Ehlers-Danlos syndrome | |
| Physiological tests | Accelerated FEV1 decline | Annual decline >60 mL |
| FEF25–75 | Small-airway obstruction | |
| Airway hyperresponsiveness | ||
| RV/TLC | Lung hyperinflation | |
| Cardiopulmonary exercise test | ||
| DLCO | Alveolar destruction | |
| Lung clearance index | Heterogeneity of small-airway function | |
| Impedance oscillometry | Airway resistance and capacitance | |
| Imaging studies | Chest CT | Distinguishing structural deformities Quantification of emphysema TAC Airway wall thickness Radiographically measured RV/TLC |
| Parametric Response Mapping (PRM) | Identification of small-airway disease | |
| Hyperpolarized MRI | Structural and functional abnormality of lung Regional ventilation Alveolar enlargement Gas diffusion | |
| Gadolinium-enhanced MRI | Early structural change of COPD | |
| Clinical features | Chronic bronchitis symptom | Cough, sputum in 3 months per year (≥2 consecutive years) |
| SGRQ score | Health-related QOL | |
| 6 MWT | Exercise function |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, J.Y.; Rhee, C.K. Diagnosis and Treatment of Early Chronic Obstructive Lung Disease (COPD). J. Clin. Med. 2020, 9, 3426. https://doi.org/10.3390/jcm9113426
Choi JY, Rhee CK. Diagnosis and Treatment of Early Chronic Obstructive Lung Disease (COPD). Journal of Clinical Medicine. 2020; 9(11):3426. https://doi.org/10.3390/jcm9113426
Chicago/Turabian StyleChoi, Joon Young, and Chin Kook Rhee. 2020. "Diagnosis and Treatment of Early Chronic Obstructive Lung Disease (COPD)" Journal of Clinical Medicine 9, no. 11: 3426. https://doi.org/10.3390/jcm9113426
APA StyleChoi, J. Y., & Rhee, C. K. (2020). Diagnosis and Treatment of Early Chronic Obstructive Lung Disease (COPD). Journal of Clinical Medicine, 9(11), 3426. https://doi.org/10.3390/jcm9113426






