Profound Hypotension before Aortic Clamping Can Exacerbate Spinal Cord Ischemic Injury after Aortic Surgery in Rats
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Care
2.2. Experimental Group Assignment
2.3. Anesthesia and Surgical Preparation
2.4. Experimental Protocol
2.5. Mean Arterial Pressure Monitoring
2.6. Evaluation of Neurobehavioral Outcomes
2.7. Histopathology
2.8. Evaluation of the Oxidative Stress and Inflammation
2.9. Statistical Analysis
3. Results
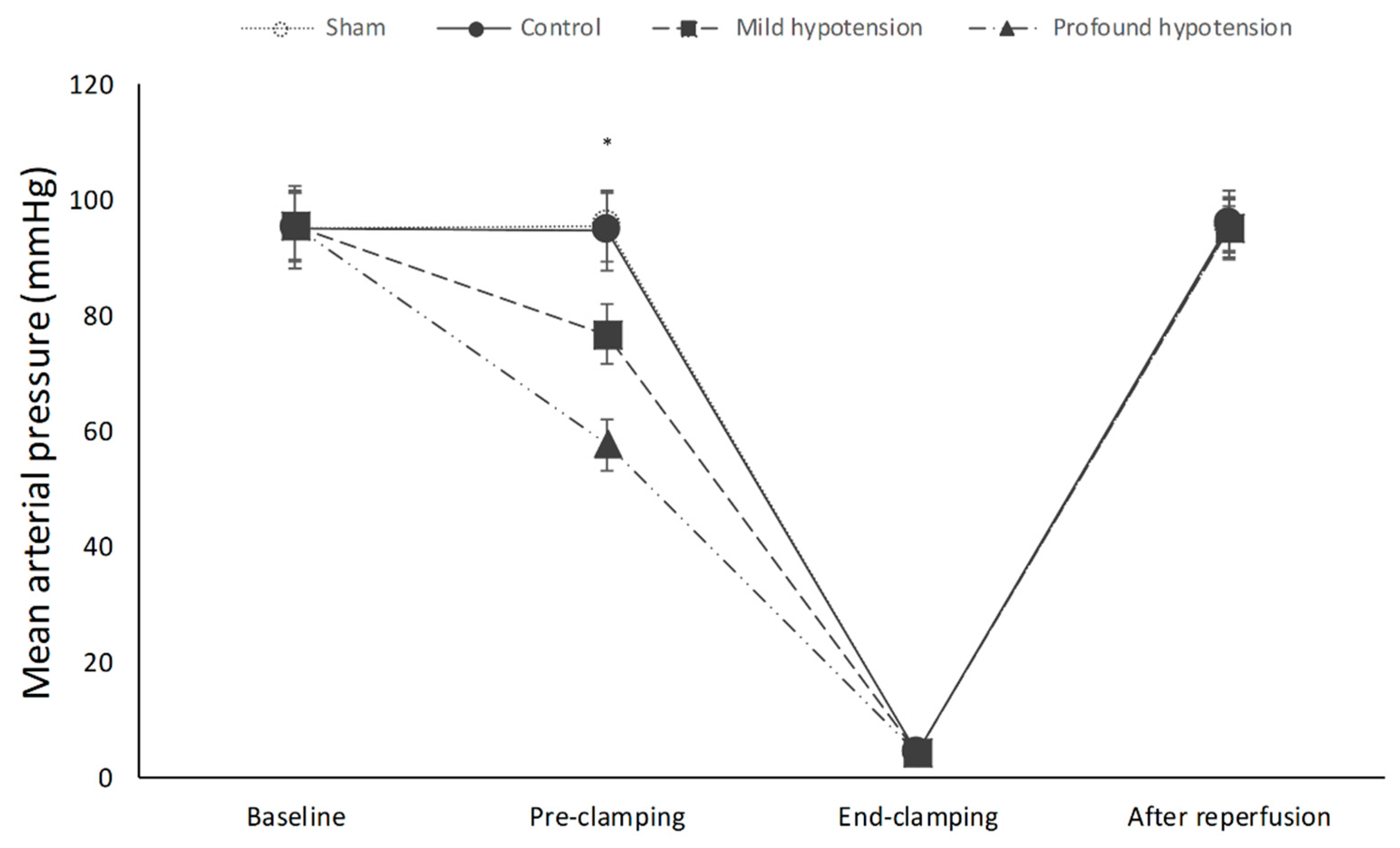
3.1. Mean Arterial Pressure
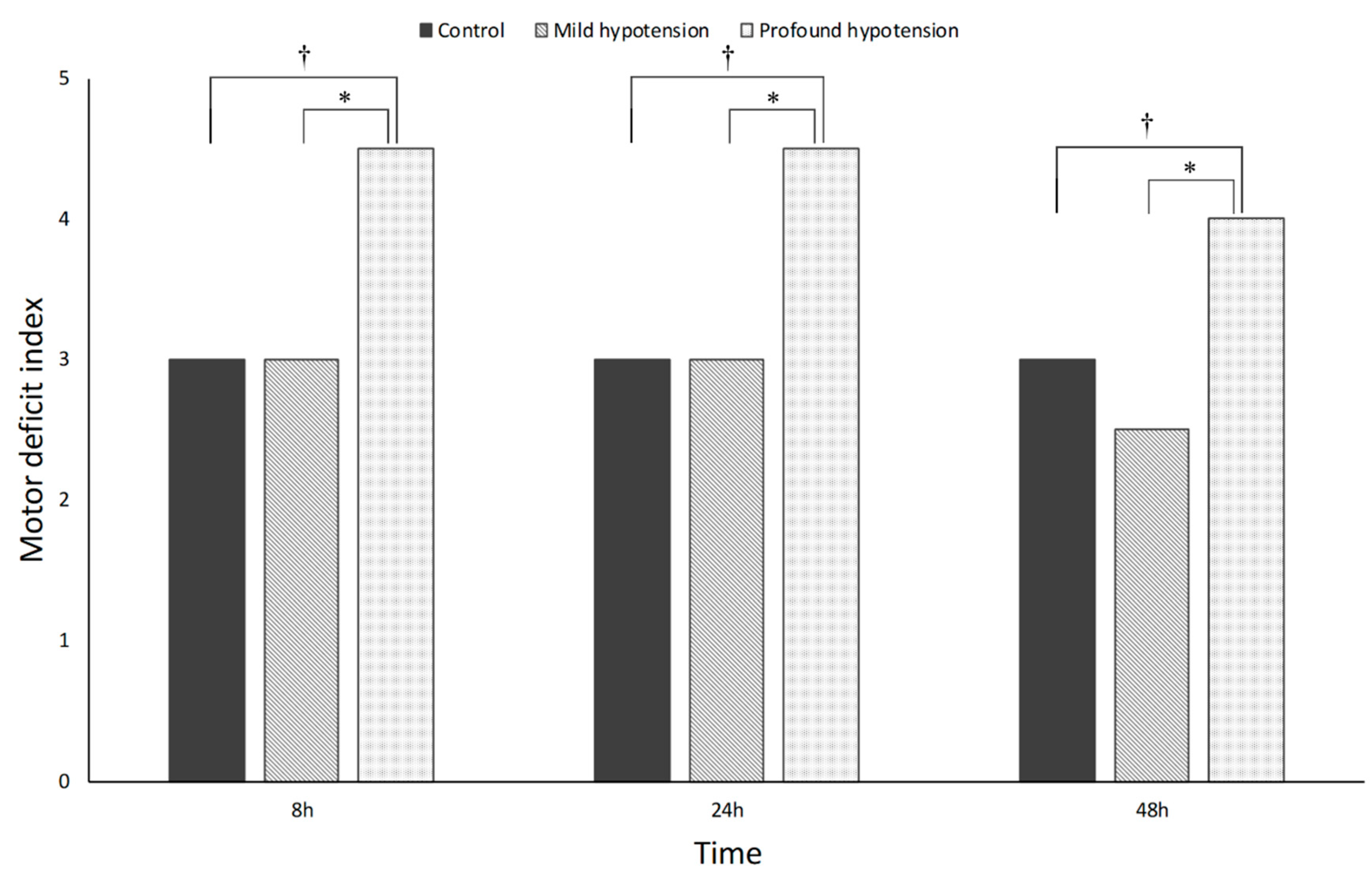
3.2. Neurobehavioral Outcomes
3.3. Histopathology
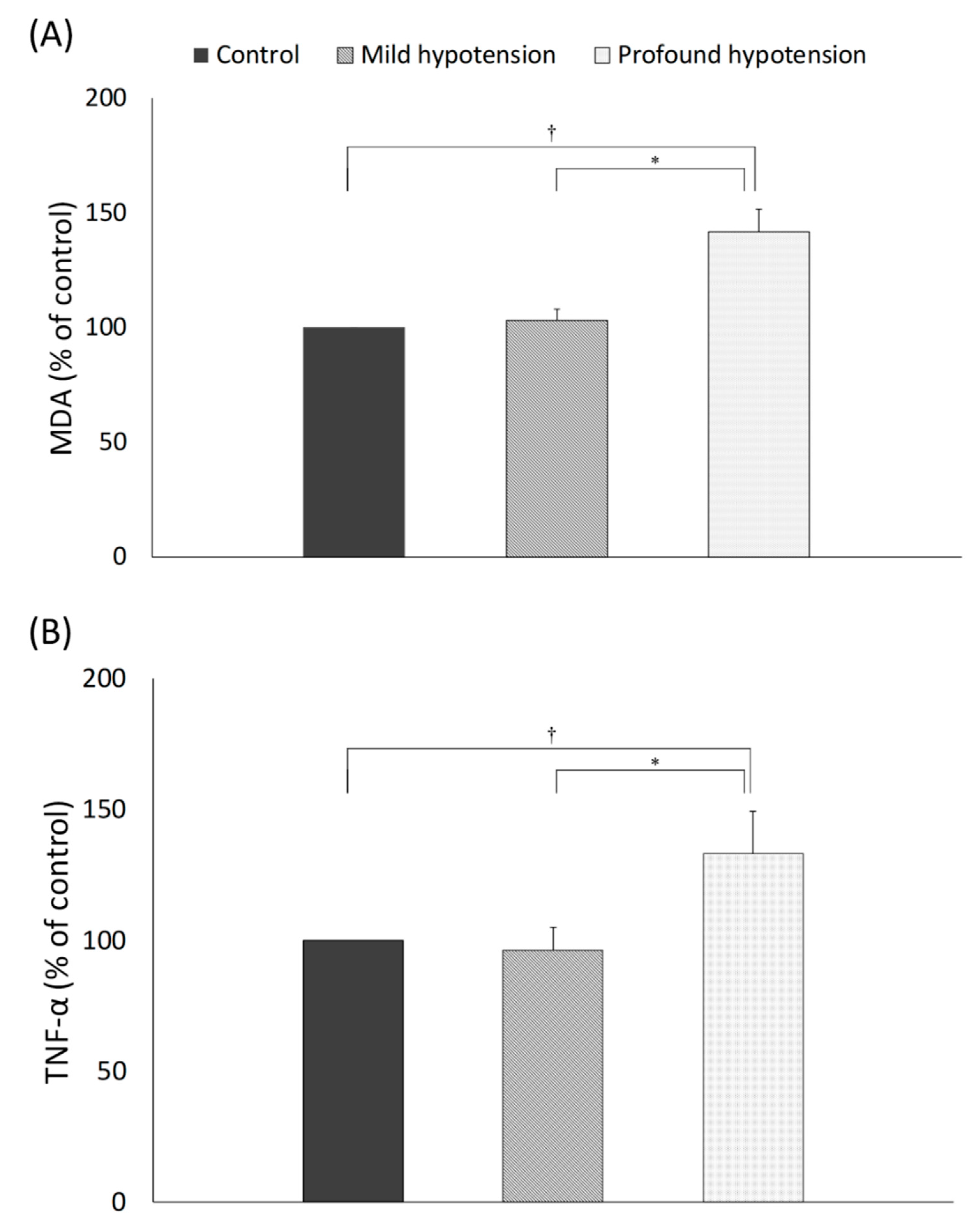
3.4. Oxidative Stress and Inflammatory Reaction
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Coselli, J.S.; Green, S.Y.; Price, M.D.; Zhang, Q.; Preventza, O.; de la Cruz, K.I.; Whitlock, R.; Amarasekara, H.S.; Woodside, S.J.; Perez-Orozco, A.; et al. Spinal cord deficit after 1114 extent II open thoracoabdominal aortic aneurysm repairs. J. Thorac. Cardiovasc. Surg. 2020, 159, 1–13. [Google Scholar] [CrossRef]
- Kamman, A.V.; de Beaufort, H.W.; van Bogerijen, G.H.; Nauta, F.J.; Heijmen, R.H.; Moll, F.L.; van Herwaarden, J.A.; Trimarchi, S. Contemporary management strategies for chronic type B aortic dissections: A systematic review. PLoS ONE 2016, 11, e0154930. [Google Scholar] [CrossRef] [PubMed]
- Wong, D.R.; Coselli, J.S.; Amerman, K.; Bozinovski, J.; Carter, S.A.; Vaughn, W.K.; LeMaire, S.A. Delayed spinal cord deficits after thoracoabdominal aortic aneurysm repair. Ann. Thorac. Surg. 2007, 83, 1345–1355. [Google Scholar] [CrossRef]
- Czerny, M.; Eggebrecht, H.; Sodeck, G.; Verzini, F.; Cao, P.; Maritati, G.; Riambau, V.; Beyersdorf, F.; Rylski, B.; Funovics, M.; et al. Mechanisms of symptomatic spinal cord ischemia after TEVAR: Insights from the European Registry of Endovascular Aortic Repair Complications (EuREC). J. Endovasc. Ther. 2012, 19, 37–43. [Google Scholar] [CrossRef]
- Izumi, S.; Okada, K.; Hasegawa, T.; Omura, A.; Munakata, H.; Matsumori, M.; Okita, Y. Augmentation of systemic blood pressure during spinal cord ischemia to prevent postoperative paraplegia after aortic surgery in a rabbit model. J. Thorac. Cardiovasc. Surg. 2010, 139, 1261–1268. [Google Scholar] [CrossRef]
- Toung, T.J.; Chang, Y.; Williams, M.; Crain, B.J.; Traystman, R.J.; Bhardwaj, A. Experimental spinal cord ischemia: Model characterization and improved outcome with arterial hypertension. Crit. Care Med. 2004, 32, 1346–1351. [Google Scholar] [CrossRef]
- Azizzadeh, A.; Huynh, T.T.; Miller, C.C., 3rd; Estrera, A.L.; Porat, E.E.; Sheinbaum, R.; Safi, H.J. Postoperative risk factors for delayed neurologic deficit after thoracic and thoracoabdominal aortic aneurysm repair: A case-control study. J. Vasc. Surg. 2003, 37, 750–754. [Google Scholar] [CrossRef][Green Version]
- Kawanishi, Y.; Okada, K.; Matsumori, M.; Tanaka, H.; Yamashita, T.; Nakagiri, K.; Okita, Y. Influence of perioperative hemodynamics on spinal cord ischemia in thoracoabdominal aortic repair. Ann. Thorac. Surg. 2007, 84, 488–492. [Google Scholar] [CrossRef]
- Hiratzka, L.F.; Bakris, G.L.; Beckman, J.A.; Bersin, R.M.; Carr, V.F.; Casey, D.E., Jr.; Eagle, K.A.; Hermann, L.K.; Isselbacher, E.M.; Kazerooni, E.A.; et al. 2010 ACCF/AHA/AATS/ACR/ASA/SCA/SCAI/SIR/STS/SVM guidelines for the diagnosis and management of patients with thoracic aortic disease: A report of the american college of cardiology foundation/american heart association task force on practice guidelines, american association for thoracic surgery, american college of radiology, american stroke association, society of cardiovascular anesthesiologists, society for cardiovascular angiography and interventions, society of interventional radiology, society of thoracic surgeons, and society for vascular medicine. Circulation 2010, 121, e266–e369. [Google Scholar]
- Sadove, M.S.; Wyant, G.M.; Gleave, G. Controlled hypotension; a study on arfonad (RO 2-2222). Anesthesia 1953, 8, 175–181. [Google Scholar] [CrossRef]
- van der Vliet, J.A.; van Aalst, D.L.; Schultze Kool, L.J.; Wever, J.J.; Blankensteijn, J.D. Hypotensive hemostatis (permissive hypotension) for ruptured abdominal aortic aneurysm: Are we really in control? Vascular 2007, 15, 197–200. [Google Scholar] [CrossRef] [PubMed]
- Boisvert, D.P.; Gelb, A.W.; Tang, C.; Lam, A.M.; Mielke, B.; Dowman, R. Brain tolerance to middle cerebral artery occlusion during hypotension in primates. Surg. Neurol. 1989, 31, 6–13. [Google Scholar] [CrossRef]
- Cole, D.J.; Drummond, J.C.; Shapiro, H.M.; Zornow, M.H. Influence of hypotension and hypotensive technique on the area of profound reduction in cerebral blood flow during focal cerebral ischaemia in the rat. Br. J. Anaesth. 1990, 64, 498–502. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.H.; Moon, I.S.; Park, J.S.; Koh, Y.B.; Ahn, H. Nicardipine hydrochloride injectable phase IV open-label clinical trial: Study on the anti-hypertensive effect and safety of nicardipine for acute aortic dissection. J. Int. Med. Res. 2002, 30, 337–345. [Google Scholar] [CrossRef]
- Wu, K.S.; Zhou, J.C.; Li, H.Y.; Gu, D.Y.; Pan, K.H.; Li, W.D.; Hu, Y.H. Antihypertensive therapy with nicardipine for patients with aortic disease is associated with more esmolol usage than urapidil. J. Thorac. Dis. 2014, 6, 1765–1771. [Google Scholar]
- Badner, N.H.; Nicolaou, G.; Clarke, C.F.; Forbes, T.L. Use of spinal near-infrared spectroscopy for monitoring spinal cord perfusion during endovascular thoracic aortic repairs. J. Cardiothorac. Vasc. Anesth. 2011, 25, 316–319. [Google Scholar] [CrossRef]
- LeMaire, S.A.; Ochoa, L.N.; Conklin, L.D.; Widman, R.A.; Clubb, F.J., Jr.; Undar, A.; Schmittling, Z.C.; Wang, X.L.; Fraser, C.D., Jr.; Coselli, J.S. Transcutaneous near-infrared spectroscopy for detection of regional spinal ischemia during intercostal artery ligation: Preliminary experimental results. J. Thorac. Cardiovasc. Surg. 2006, 132, 1150–1155. [Google Scholar] [CrossRef]
- Petersen, N.H.; Ortega-Gutierrez, S.; Wang, A.; Lopez, G.V.; Strander, S.; Kodali, S.; Silverman, A.; Zheng-Lin, B.; Dandapat, S.; Sansing, L.H.; et al. Decreases in blood pressure during thrombectomy are associated with larger infarct volumes and worse functional outcome. Stroke 2019, 50, 1797–1804. [Google Scholar] [CrossRef] [PubMed]
- Treurniet, K.M.; Berkhemer, O.A.; Immink, R.V.; Lingsma, H.F.; Ward-van der Stam, V.M.C.; Hollmann, M.W.; Vuyk, J.; van Zwam, W.H.; van der Lugt, A.; van Oostenbrugge, R.J.; et al. A decrease in blood pressure is associated with unfavorable outcome in patients undergoing thrombectomy under general anesthesia. J. Neurointerv. Surg. 2018, 10, 107–111. [Google Scholar] [CrossRef]
- Genovese, T.; Cuzzocrea, S. Role of free radicals and poly(ADP-ribose)polymerase-1 in the development of spinal cord injury: New potential therapeutic targets. Curr. Med. Chem. 2008, 15, 477–487. [Google Scholar]
- Peters, O.; Back, T.; Lindauer, U.; Busch, C.; Megow, D.; Dreier, J.; Dirnagl, U. Increased formation of reactive oxygen species after permanent and reversible middle cerebral artery occlusion in the rat. J. Cereb. Blood Flow Metab. 1998, 18, 196–205. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, F.; Bonacasa, B.; Fenoy, F.J.; Salom, M.G. Reactive oxygen and nitrogen species in the renal ischemia/reperfusion injury. Curr. Pharm. Des. 2013, 19, 2776–2794. [Google Scholar] [CrossRef] [PubMed]
- Koc, R.K.; Akdemir, H.; Kurtsoy, A.; Pasaoglu, H.; Kavuncu, I.; Pasaoglu, A.; Karakucuk, I. Lipid peroxidation in experimental spinal cord injury. Comparison of treatment with Ginkgo biloba, TRH and methylprednisolone. Res. Exp. Med. 1995, 195, 117–123. [Google Scholar] [CrossRef] [PubMed]
- Peker, S.; Abacioglu, U.; Sun, I.; Konya, D.; Yuksel, M.; Pamir, N.M. Prophylactic effects of magnesium and vitamin E in rat spinal cord radiation damage: Evaluation based on lipid peroxidation levels. Life Sci. 2004, 75, 1523–1530. [Google Scholar] [CrossRef]
- Toklu, H.Z.; Hakan, T.; Celik, H.; Biber, N.; Erzik, C.; Ogunc, A.V.; Akakin, D.; Cikler, E.; Cetinel, S.; Ersahin, M.; et al. Neuroprotective effects of alpha-lipoic acid in experimental spinal cord injury in rats. J. Spinal Cord Med. 2010, 33, 401–409. [Google Scholar] [CrossRef][Green Version]
- Holmin, S.; Mathiesen, T. Intracerebral administration of interleukin-1beta and induction of inflammation, apoptosis, and vasogenic edema. J. Neurosurg. 2000, 92, 108–120. [Google Scholar] [CrossRef]
- Nour, M.; Scalzo, F.; Liebeskind, D.S. Ischemia-reperfusion injury in stroke. Interv. Neurol. 2012, 1, 185–199. [Google Scholar] [CrossRef]
- Reece, T.B.; Okonkwo, D.O.; Ellman, P.I.; Warren, P.S.; Smith, R.L.; Hawkins, A.S.; Linden, J.; Kron, I.L.; Tribble, C.G.; Kern, J.A. The evolution of ischemic spinal cord injury in function, cytoarchitecture, and inflammation and the effects of adenosine A2A receptor activation. J. Thorac. Cardiovasc. Surg. 2004, 128, 925–932. [Google Scholar] [CrossRef][Green Version]
- Yazihan, N.; Uzuner, K.; Salman, B.; Vural, M.; Koken, T.; Arslantas, A. Erythropoietin improves oxidative stress following spinal cord trauma in rats. Injury 2008, 39, 1408–1413. [Google Scholar] [CrossRef]
- Coselli, J.S.; LeMaire, S.A.; Koksoy, C.; Schmittling, Z.C.; Curling, P.E. Cerebrospinal fluid drainage reduces paraplegia after thoracoabdominal aortic aneurysm repair: Results of a randomized clinical trial. J. Vasc. Surg. 2002, 35, 631–639. [Google Scholar] [CrossRef]
- Estrera, A.L.; Miller, C.C., 3rd; Huynh, T.T.; Porat, E.; Safi, H.J. Neurologic outcome after thoracic and thoracoabdominal aortic aneurysm repair. Ann. Thorac. Surg. 2001, 72, 1225–1230. [Google Scholar] [CrossRef]
- Maeda, S.; Miyamoto, T.; Murata, H.; Yamashita, K. Prevention of spinal cord ischemia by monitoring spinal cord perfusion pressure and somatosensory evoked potentials. J. Cardiovasc. Surg. (Torino) 1989, 30, 565–571. [Google Scholar]
| Sham Group | Control Group | Mild Hypotension Group | Profound Hypotension Group | p Value |
|---|---|---|---|---|
| 16.9 (1.3) † | 11.9 (1.7) | 11.3 (1.7) | 7.5 (1.7) ‡ | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Koo, C.-H.; Ryu, J.-H.; Hwang, J.-Y.; Kim, J.-H.; Shin, H.-J.; Han, S.-H. Profound Hypotension before Aortic Clamping Can Exacerbate Spinal Cord Ischemic Injury after Aortic Surgery in Rats. J. Clin. Med. 2020, 9, 3395. https://doi.org/10.3390/jcm9113395
Koo C-H, Ryu J-H, Hwang J-Y, Kim J-H, Shin H-J, Han S-H. Profound Hypotension before Aortic Clamping Can Exacerbate Spinal Cord Ischemic Injury after Aortic Surgery in Rats. Journal of Clinical Medicine. 2020; 9(11):3395. https://doi.org/10.3390/jcm9113395
Chicago/Turabian StyleKoo, Chang-Hoon, Jung-Hee Ryu, Jin-Young Hwang, Jin-Hee Kim, Hyun-Jung Shin, and Sung-Hee Han. 2020. "Profound Hypotension before Aortic Clamping Can Exacerbate Spinal Cord Ischemic Injury after Aortic Surgery in Rats" Journal of Clinical Medicine 9, no. 11: 3395. https://doi.org/10.3390/jcm9113395
APA StyleKoo, C.-H., Ryu, J.-H., Hwang, J.-Y., Kim, J.-H., Shin, H.-J., & Han, S.-H. (2020). Profound Hypotension before Aortic Clamping Can Exacerbate Spinal Cord Ischemic Injury after Aortic Surgery in Rats. Journal of Clinical Medicine, 9(11), 3395. https://doi.org/10.3390/jcm9113395





