Immediate Effect of Masticatory Muscle Activity with Transcutaneous Electrical Nerve Stimulation in Muscle Pain of Temporomandibular Disorders Patients
Abstract
1. Introduction
2. Experimental Section
2.1. Participants
2.2. Pretreatment and Posttreatment Examinations
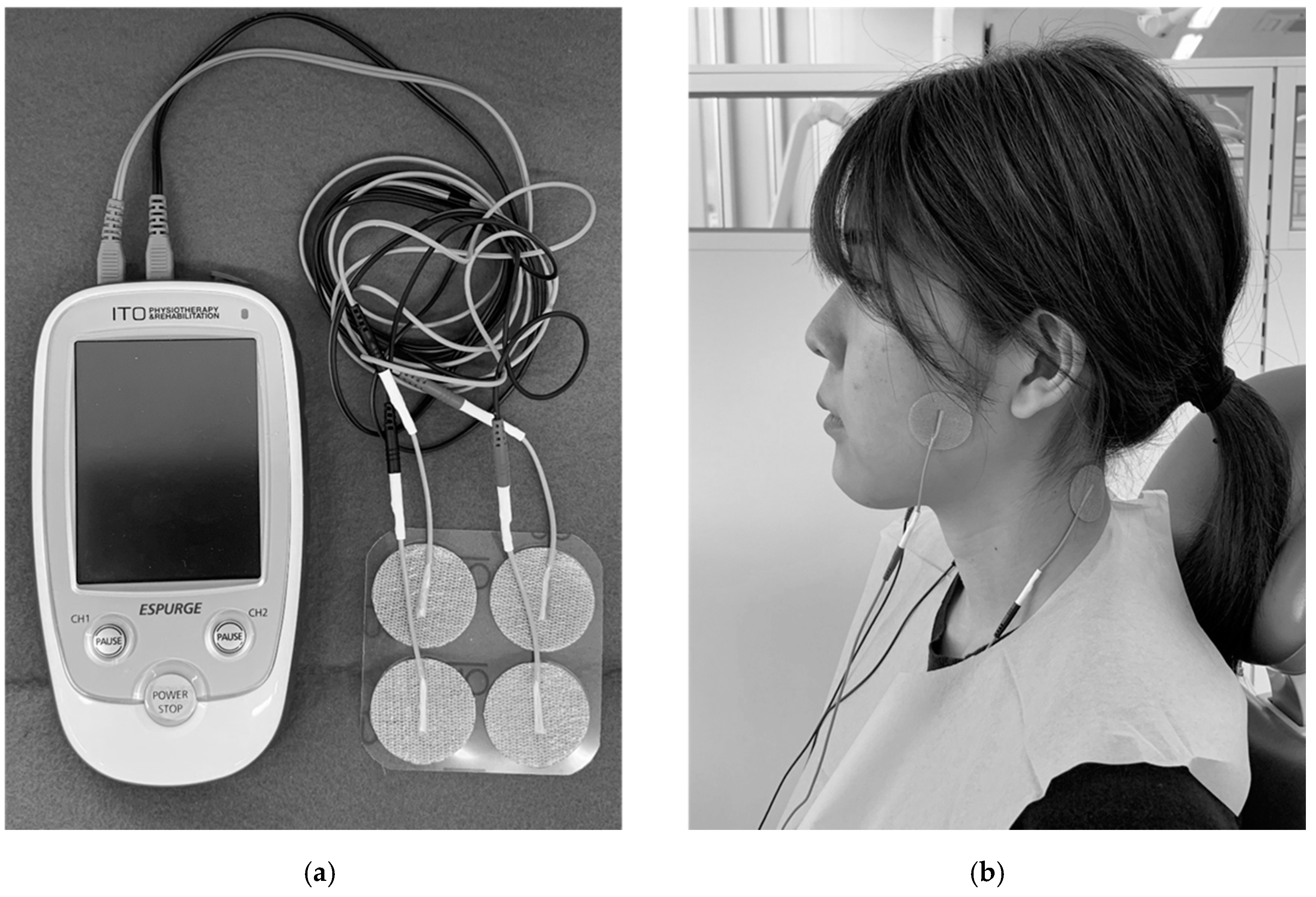
2.3. TENS Treatment
2.4. Statistical Analysis
3. Results
3.1. Participants
3.2. Pain Intensity
3.3. Objective Evaluation
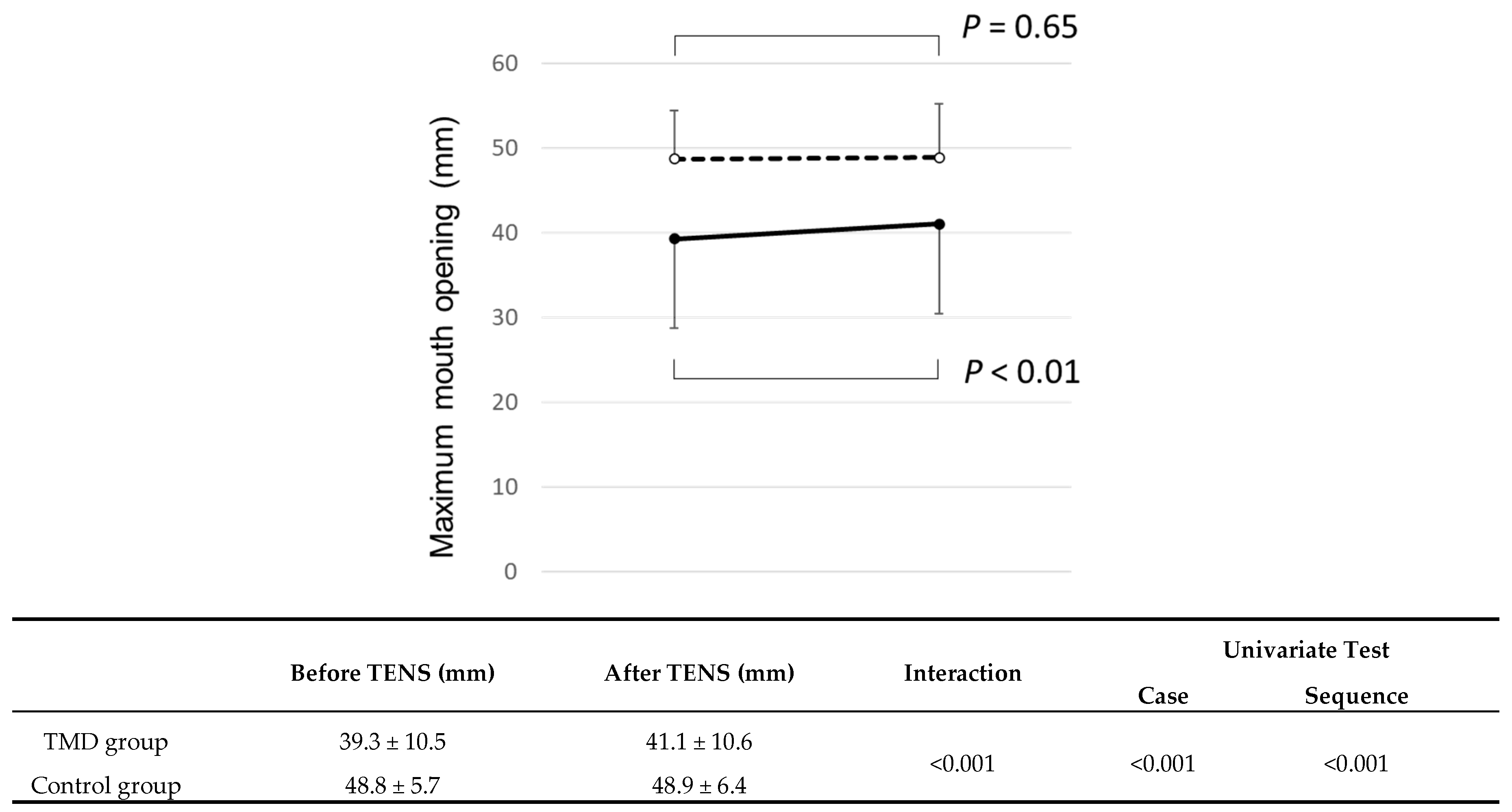
3.3.1. Maximum Mouth Opening without Pain
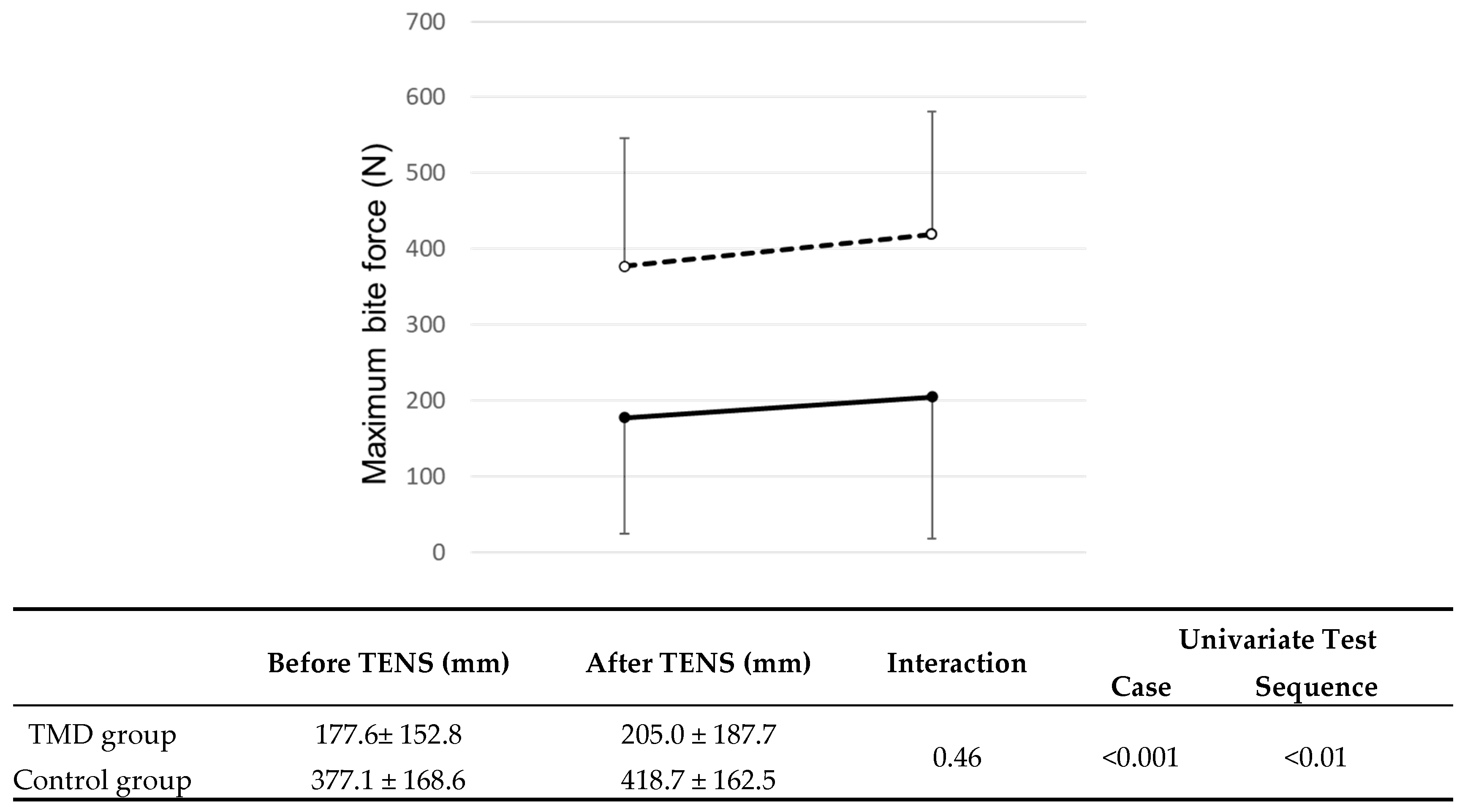
3.3.2. Maximum Bite Force
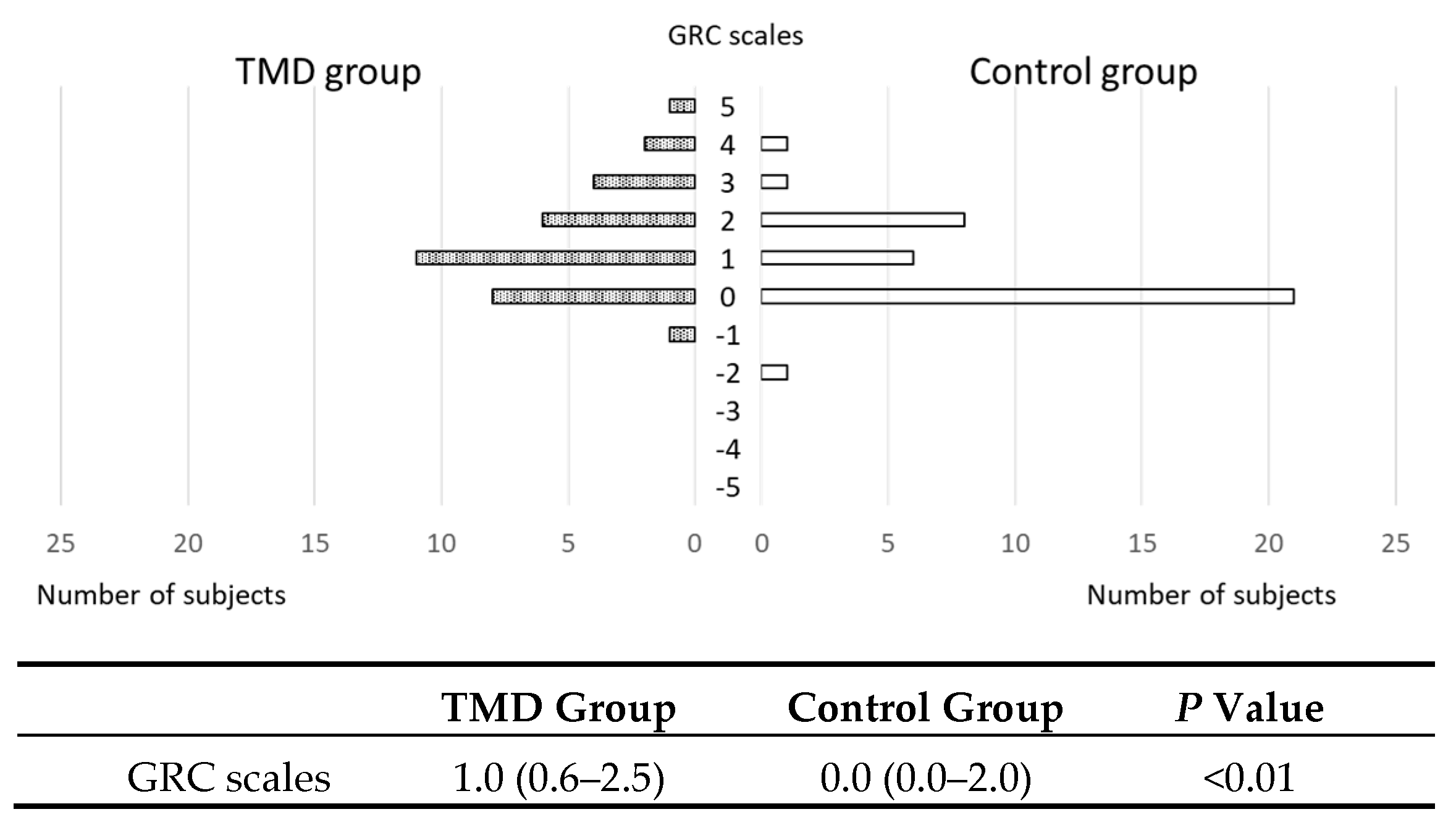
3.4. GRC Scales
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Carlsson, G.E. Epidemiology and treatment need for temporomandibular disorders. J. Orofac. Pain 1999, 13, 232–237. [Google Scholar]
- Magnusson, T.; Egermark, I.; Carlsson, G.E. A longitudinal epidemiologic study of signs and symptoms of temporomandibular disorders from 15 to 35 years of age. J. Orofac. Pain 2000, 14, 310–319. [Google Scholar]
- Goulet, J.P.; Lavigne, G.J.; Lund, J.P. Jaw pain prevalence among French-speaking Canadians in Québec and related symptoms of temporomandibular disorders. J. Dent. Res. 1995, 74, 1738–1744. [Google Scholar] [CrossRef] [PubMed]
- Macfarlane, T.V.; Blinkhorn, A.S.; Davies, R.M.; Ryan, P.; Worthington, H.V.; Macfarlane, G.J. Orofacial pain: Just another chronic pain? Results from a population-based survey. Pain 2002, 99, 453–458. [Google Scholar] [CrossRef]
- Nilsson, I.M.; List, T.; Drangsholt, M. Prevalence of temporomandibular pain and subsequent dental treatment in Swedish adolescents. J. Orofac. Pain 2005, 19, 144–150. [Google Scholar] [PubMed]
- Matsuka, Y.; Yatani, H.; Kuboki, T.; Yamashita, A. Temporomandibular disorders in the adult population of Okayama City, Japan. CRANIO 1996, 14, 158–162. [Google Scholar] [CrossRef] [PubMed]
- LeResche, L. Epidemiology of temporomandibular disorders: Implications for the investigation of etiologic factors. Crit. Rev. Oral Biol. Med. 1997, 8, 291–305. [Google Scholar] [CrossRef] [PubMed]
- Gesch, D.; Bernhardt, O.; Alte, D.; Kocher, T.; John, U.; Hensel, E. Malocclusions and clinical signs or subjective symptoms of temporomandibular disorders (TMD) in adults. Results of the population-based Study of Health in Pomerania (SHIP). J. Orofac. Orthop. 2004, 65, 88–103. [Google Scholar] [CrossRef] [PubMed]
- Kamisaka, M.; Yatani, H.; Kuboki, T.; Matsuka, Y.; Minakuchi, H. Four-year longitudinal course of TMD symptoms in an adult population and the estimation of risk factors in relation to symptoms. J. Orofac. Pain 2000, 14, 224–232. [Google Scholar] [PubMed]
- Katz, J.; Heft, M. The epidemiology of self-reported TMJ sounds and pain in young adults in Israel. J. Public Health Dent. 2002, 62, 177–179. [Google Scholar] [CrossRef]
- Cioffi, I.; Landino, D.; Donnarumma, V.; Castroflorio, T.; Lobbezoo, F.; Michelotti, A. Frequency of daytime tooth clenching episodes in individuals affected by masticatory muscle pain and pain-free controls during standardized ability tasks. Clin. Oral Investig. 2017, 21, 1139–1148. [Google Scholar] [CrossRef] [PubMed]
- Muzalev, K.; Lobbezoo, F.; Janal, M.N.; Raphael, K.G. Interepisode sleep bruxism intervals and myofascial face pain. Sleep 2017, 40, 78. [Google Scholar] [CrossRef][Green Version]
- Tanaka, E.; Detamore, M.S.; Mercuri, L.G. Degenerative disorders of the temporomandibular joint: Etiology, diagnosis, and treatment. J. Dent. Res. 2008, 87, 296–307. [Google Scholar] [CrossRef] [PubMed]
- Almoznino, G.; Zini, A.; Zakuto, A.; Sharav, Y.; Haviv, Y.; Hadad, A.; Chweidan, H.; Yarom, N.; Benoliel, R. Oral Health-Related Quality of Life in Patients with Temporomandibular Disorders. J. Oral Facial Pain Headache 2015, 29, 231–241. [Google Scholar] [CrossRef] [PubMed]
- Tjakkes, G.H.; Reinders, J.J.; Tenvergert, E.M.; Stegenga, B. TMD pain: The effect on health related quality of life and the influence of pain duration. Health Qual Life Outcomes 2010, 8, 46. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, G.; Gonçalves, D.A.G.; Conti, P. Musculoskeletal disorders. Dent. Clin. N. Am. 2018, 62, 553–564. [Google Scholar] [CrossRef]
- Gauer, R.L.; Semidey, M.J. Diagnosis and treatment of temporomandibular disorders. Am. Fam. Physician 2015, 91, 378–386. [Google Scholar]
- Harvey, J.; Tanner, S. Low back pain in young athletes. A practical approach. Sports Med. 1991, 12, 394–406. [Google Scholar] [CrossRef]
- Wall, P.D.; Sweet, W.H. Temporary abolition of pain in man. Science 1967, 155, 108–109. [Google Scholar] [CrossRef]
- Vance, C.G.; Dailey, D.L.; Rakel, B.A.; Sluka, K.A. Using TENS for pain control: The state of the evidence. Pain Manag. 2014, 4, 197–209. [Google Scholar] [CrossRef]
- Kato, M.T.; Kogawa, E.M.; Santos, C.N.; Conti, P.C. TENS and low-level laser therapy in the management of temporomandibular disorders. J. Appl. Oral. Sci. 2006, 14, 130–135. [Google Scholar] [CrossRef]
- Rai, S.; Ranjan, V.; Misra, D.; Panjwani, S. Management of myofascial pain by therapeutic ultrasound and transcutaneous electrical nerve stimulation: A comparative study. Eur. J. Dent. 2016, 10, 46–53. [Google Scholar] [CrossRef]
- Patil, S.; Iyengar, A.R.; Kotni, R.M.; Subash, B.V.; Joshi, R.K. Evaluation of Efficacy of Ultrasonography in the Assessment of Transcutaneous Electrical Nerve Stimulation in Subjects with Myositis and Myofascial Pain. Korean J. Pain. 2016, 29, 12–17. [Google Scholar] [CrossRef] [PubMed]
- Esclassan, R.; Rumerio, A.; Monsarrat, P.; Combadazou, J.C.; Champion, J.; Destruhaut, F.; Ghrenassia, C. Optimal duration of ultra-low frequency-transcutaneous electrical nerve stimulation (ULF-TENS) therapy for muscular relaxation in neuromuscular occlusion: A preliminary clinical study. CRANIO 2017, 35, 175–179. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, A.P.; Costa, D.R.; Oliveira, A.I.; Carvalho, E.A.; Conti, P.C.; Costa, Y.M.; Bonjardim, L.R. Short-term transcutaneous electrical nerve stimulation reduces pain and improves the masticatory muscle activity in temporomandibular disorder patients: A randomized controlled trial. J. Appl. Oral. Sci. 2017, 25, 112–120. [Google Scholar] [CrossRef]
- Schiffman, E.; Ohrbach, R.; Truelove, E.; Look, J.; Anderson, G.; Goulet, J.P.; List, T.; Svensson, P.; Gonzalez, Y.; Lobbezoo, F.; et al. Diagnostic Criteria for Temporomandibular Disorders (DC/TMD) for Clinical and Research Applications: Recommendations of the International RDC/TMD Consortium Network and Orofacial Pain Special Interest Group. J. Oral Facial Pain Headache 2014, 28, 6–27. [Google Scholar] [CrossRef] [PubMed]
- Tang, Z.; Chen, Y.; Zhou, W.; Zhang, J.; Wang, R.; Wang, K.; Svensson, P. Reliability of mechanical sensitivity mapping in the orofacial region of healthy Chinese individuals: Towards standardized assessment of somatosensory function. J. Oral Facial Pain Headache 2018, 32, 400–408. [Google Scholar] [CrossRef]
- Carlsson, A.M. Assessment of chronic pain, I. Aspects of the reliability and validity of the visual analogue scale. Pain 1983, 16, 87–101. [Google Scholar] [CrossRef]
- Katch, E.M. Application of transcutaneous electrical nerve stimulation in dentistry. Anesth. Prog. 1986, 33, 156–160. [Google Scholar]
- Kamper, S.J.; Maher, C.G.; Mackay, G. Global rating of change scales: A review of strengths and weaknesses and considerations for design. J. Man. Manip. Ther. 2009, 17, 163–170. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J. A power primer. Psychol. Bull. 1992, 112, 155–159. [Google Scholar] [CrossRef]
- Han, J.S.; Chen, X.H.; Sun, S.L.; Xu, X.J.; Yuan, Y.; Yan, S.C.; Hao, J.X.; Terenius, L. Effect of low- and high-frequency TENS on Met-enkephalin-Arg-Phe and dynorphin A immunoreactivity in human lumbar CSF. Pain 1991, 47, 295–298. [Google Scholar] [CrossRef]
- Fertout, A.; Manière-Ezvan, A.; Lupi, L.; Ehrmann, E. Management of temporomandibular disorders with transcutaneous electrical nerve stimulation: A systematic review. CRANIO 2019, 9, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Menezes, M.A.; Pereira, T.A.B.; Tavares, L.M.; Leite, B.T.Q.; Neto, A.G.R.; Chaves, L.M.S.; Lima, L.V.; Da Silva-Grigolleto, M.E.; DeSantana, J.M. Immediate effects of transcutaneous electrical nerve stimulation (TENS) administered during resistance exercise on pain intensity and physical performance of healthy subjects: A randomized clinical trial. Eur. J. Appl. Physiol. 2018, 118, 1941–1958. [Google Scholar] [CrossRef] [PubMed]
- Jung, K.S.; Jung, J.H.; In, T.S.; Cho, H.Y. Effects of Weight-shifting Exercise Combined with Transcutaneous Electrical Nerve Stimulation on Muscle Activity and Trunk Control in Patients with Stroke. Occup. Ther. Int. 2016, 23, 436–443. [Google Scholar] [CrossRef]
- Jung, K.S.; In, T.S.; Cho, H.Y. Effects of sit-to-stand training combined with transcutaneous electrical stimulation on spasticity, muscle strength and balance ability in patients with stroke: A randomized controlled study. Gait Posture 2017, 54, 183–187. [Google Scholar] [CrossRef]
- Konishi, Y.; McNair, P.J.; Rice, D.A. TENS Alleviates Muscle Weakness Attributable to Attenuation of Ia Afferents. Int. J. Sports Med. 2017, 38, 253–257. [Google Scholar] [CrossRef] [PubMed]
- Seifi, M.; Ebadifar, A.; Kabiri, S.; Badiee, M.R.; Abdolazimi, Z.; Amdjadi, P. Comparative effectiveness of Low Level Laser therapy and Transcutaneous Electric Nerve Stimulation on Temporomandibular Joint Disorders. J. Lasers Med. Sci. 2017, 8, S27–S31. [Google Scholar] [CrossRef] [PubMed]
- Monaco, A.; Sgolastra, F.; Ciarrocchi, I.; Cattaneo, R. Effects of transcutaneous electrical nervous stimulation on electromyographic and kinesiographic activity of patients with temporomandibular disorders: A placebo-controlled study. J. Electromyogr. Kinesiol. 2012, 22, 463–468. [Google Scholar] [CrossRef]

| TMD Patients | Controls | p Value | |
|---|---|---|---|
| Number of Male | 9 | 17 | 0.09 ** |
| Number of Female | 27 | 22 | |
| Age * | 56.8 ± 17.2 | 52.5 ± 17.6 | 0.82 |
| Male | 51.3 ± 25.3 | 55.23 ± 15.7 | 0.67 |
| Female | 58.6 ± 13.7 | 50.3 ± 19.0 | 0.09 |
| Output Power | 13.8 (11.0–17.0) | 15.0 (12.0–17.0) | 0.28 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abe, S.; Miyagi, A.; Yoshinaga, K.; Matsuka, Y.; Matsumoto, F.; Uyama, E.; Suzuki, Y.; Oshima, M.; Okura, K.; Tanaka, E. Immediate Effect of Masticatory Muscle Activity with Transcutaneous Electrical Nerve Stimulation in Muscle Pain of Temporomandibular Disorders Patients. J. Clin. Med. 2020, 9, 3330. https://doi.org/10.3390/jcm9103330
Abe S, Miyagi A, Yoshinaga K, Matsuka Y, Matsumoto F, Uyama E, Suzuki Y, Oshima M, Okura K, Tanaka E. Immediate Effect of Masticatory Muscle Activity with Transcutaneous Electrical Nerve Stimulation in Muscle Pain of Temporomandibular Disorders Patients. Journal of Clinical Medicine. 2020; 9(10):3330. https://doi.org/10.3390/jcm9103330
Chicago/Turabian StyleAbe, Susumu, Akane Miyagi, Kaoru Yoshinaga, Yoshizo Matsuka, Fumihiro Matsumoto, Emi Uyama, Yoshitaka Suzuki, Masamitsu Oshima, Kazuo Okura, and Eiji Tanaka. 2020. "Immediate Effect of Masticatory Muscle Activity with Transcutaneous Electrical Nerve Stimulation in Muscle Pain of Temporomandibular Disorders Patients" Journal of Clinical Medicine 9, no. 10: 3330. https://doi.org/10.3390/jcm9103330
APA StyleAbe, S., Miyagi, A., Yoshinaga, K., Matsuka, Y., Matsumoto, F., Uyama, E., Suzuki, Y., Oshima, M., Okura, K., & Tanaka, E. (2020). Immediate Effect of Masticatory Muscle Activity with Transcutaneous Electrical Nerve Stimulation in Muscle Pain of Temporomandibular Disorders Patients. Journal of Clinical Medicine, 9(10), 3330. https://doi.org/10.3390/jcm9103330








