Sirolimus Prolongs Survival after Living Donor Liver Transplantation for Hepatocellular Carcinoma Beyond Milan Criteria: A Prospective, Randomised, Open-Label, Multicentre Phase 2 Trial
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Sample Size Calculation
2.3. Treatment Protocol
2.4. Follow-up and Documentation
2.5. Study Endpoints
2.6. Statistical Analysis
3. Results
3.1. Patient Enrolment
3.2. Clinicopathological Findings
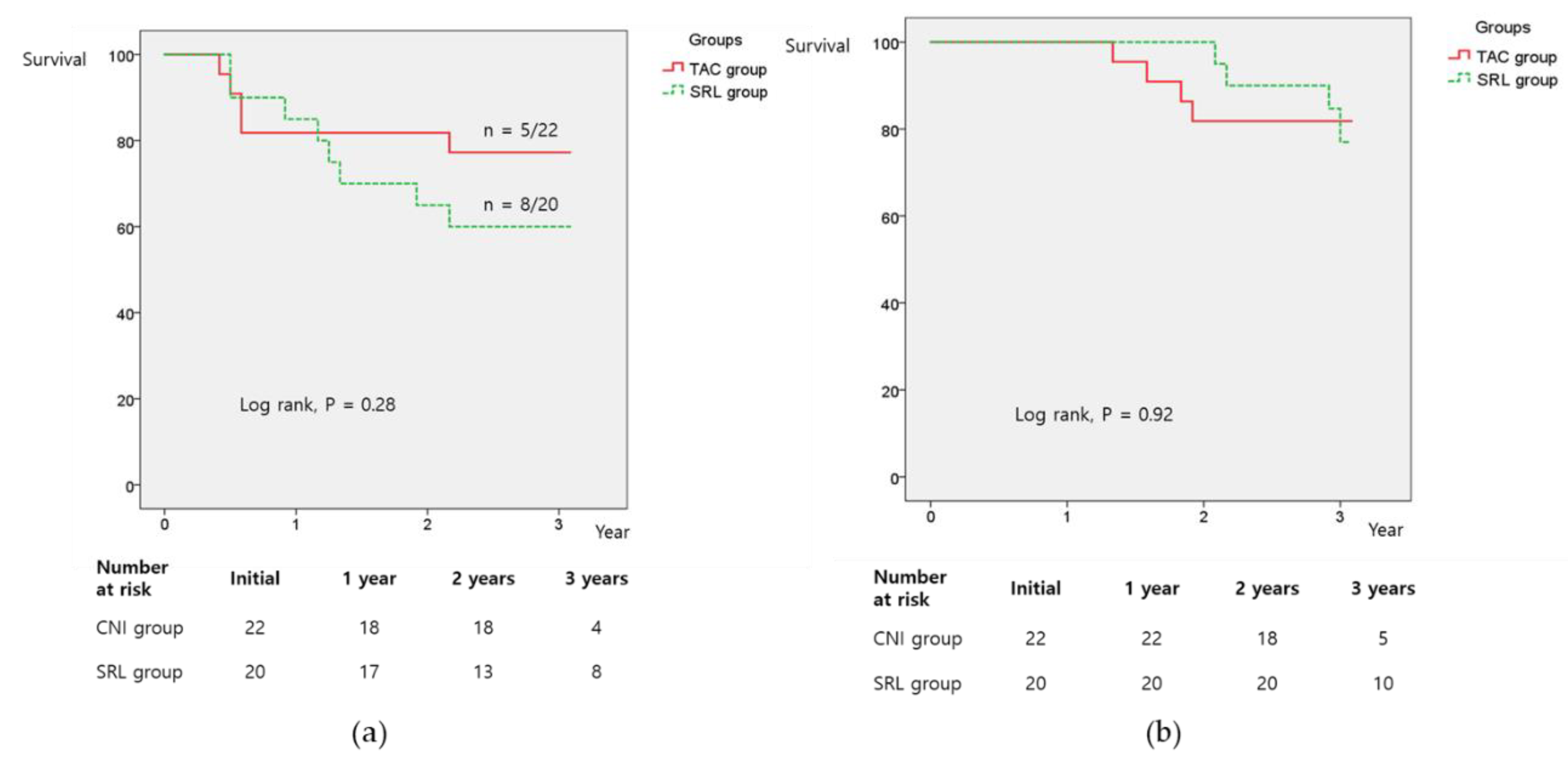
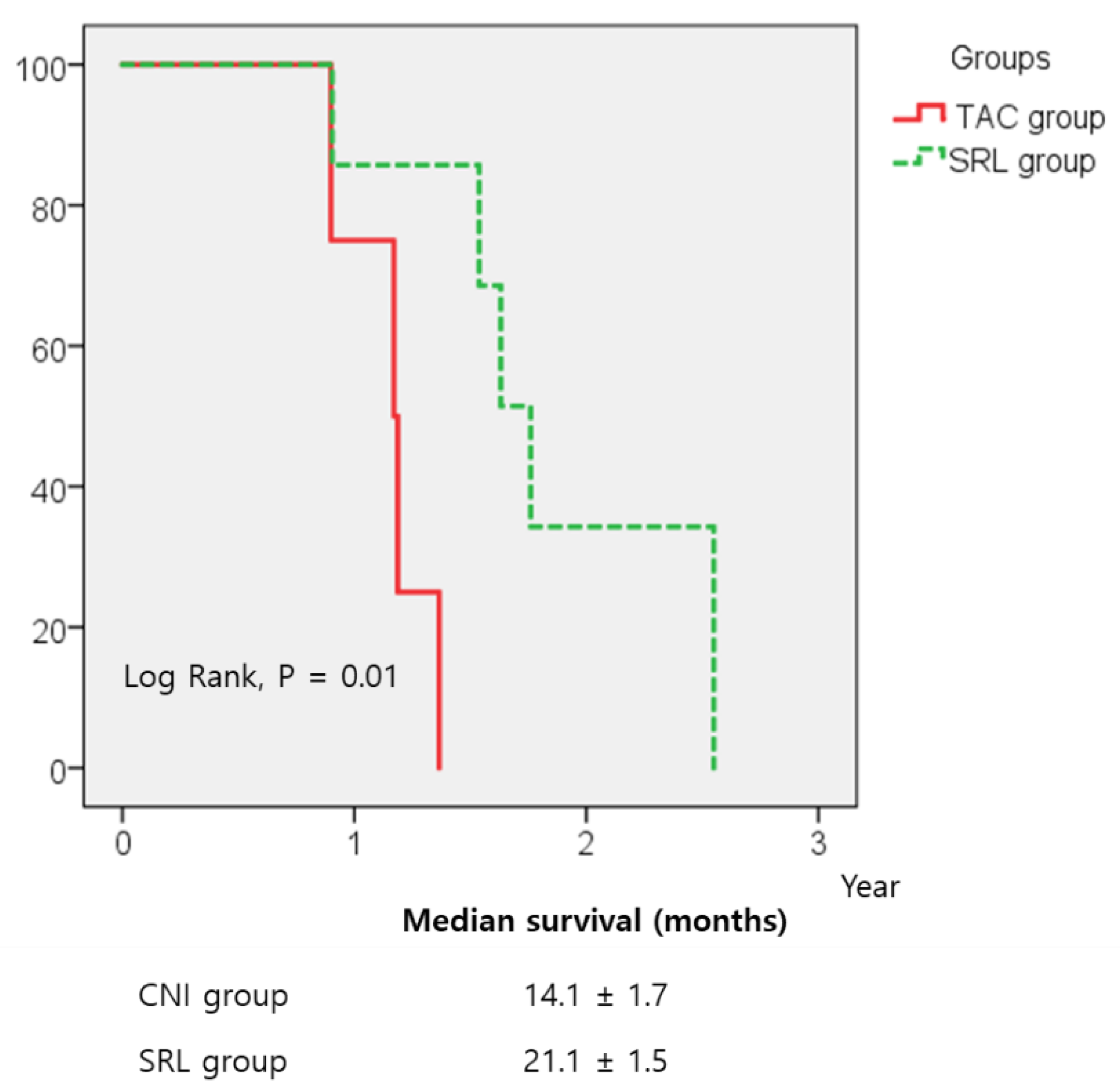
3.3. Recurrence-Free Survival (RFS) and Overall Survival (OS)
3.4. Risk Factors for Survival
3.5. Adverse Events and Complications
3.6. Changes in Estimated Glomerular Filtration Rate (eGFR)
4. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Hong, S.K.; Lee, K.W.; Kim, H.S.; Yoon, K.C.; Yi, N.J.; Suh, K.S. Living donor liver transplantation for hepatocellular carcinoma in Seoul National University. Hepatobiliary Surg. Nutr. 2016, 5, 453–460. [Google Scholar] [CrossRef]
- Hong, G.; Suh, K.S.; Suh, S.W.; Yoo, T.; Kim, H.; Park, M.S.; Choi, Y.; Paeng, J.C.; Yi, N.J.; Lee, K.W. Alpha-fetoprotein and (18)F-FDG positron emission tomography predict tumor recurrence better than Milan criteria in living donor liver transplantation. J. Hepatol. 2016, 64, 852–859. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Cho, Y.; Kim, H.Y.; Cho, E.J.; Lee, D.H.; Yu, S.J.; Lee, J.W.; Yi, N.J.; Lee, K.W.; Kim, S.H.; et al. Serum Tumor Markers Provide Refined Prognostication in Selecting Liver Transplantation Candidate for Hepatocellular Carcinoma Patients Beyond the Milan Criteria. Ann. Surg. 2016, 263, 842–850. [Google Scholar] [CrossRef] [PubMed]
- Vivarelli, M.; Cucchetti, A.; La Barba, G.; Ravaioli, M.; Del Gaudio, M.; Lauro, A.; Grazi, G.L.; Pinna, A.D. Liver transplantation for hepatocellular carcinoma under calcineurin inhibitors: Reassessment of risk factors for tumor recurrence. Ann. Surg. 2008, 248, 857–862. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.W.; Seo, Y.D.; Oh, S.C.; Suh, S.W.; Jeong, J.; Kim, H.; Yi, N.J.; Suh, K.S. What is the best immunosuppressant combination in terms of antitumor effect in hepatocellular carcinoma? Hepatol. Res. 2016, 46, 593–600. [Google Scholar] [CrossRef] [PubMed]
- Vivarelli, M.; Dazzi, A.; Zanello, M.; Cucchetti, A.; Cescon, M.; Ravaioli, M.; Del Gaudio, M.; Lauro, A.; Grazi, G.L.; Pinna, A.D. Effect of different immunosuppressive schedules on recurrence-free survival after liver transplantation for hepatocellular carcinoma. Transplantation 2010, 89, 227–231. [Google Scholar] [CrossRef]
- Grigg, S.E.; Sarri, G.L.; Gow, P.J.; Yeomans, N.D. Systematic review with meta-analysis: Sirolimus- or everolimus-based immunosuppression following liver transplantation for hepatocellular carcinoma. Aliment. Pharmacol. Ther. 2019, 49, 1260–1273. [Google Scholar] [CrossRef]
- Junge, G.; Saliba, F.; De Simone, P.; Fisher, L.; Dong, G.; Speziale, A.; Fung, J. Impact of Everolimus, an mTORC1 Inhibitor, on Hepatocellular Carcinoma Recurrence After Liver Transplantation: Results from the H2304 Study. Transplantation 2015, 99, 90. [Google Scholar]
- Duvoux, C.; Toso, C. mTOR inhibitor therapy: Does it prevent HCC recurrence after liver transplantation? Transplant. Rev. 2015, 29, 168–174. [Google Scholar] [CrossRef]
- Geissler, E.K.; Schnitzbauer, A.A.; Zulke, C.; Lamby, P.E.; Proneth, A.; Duvoux, C.; Burra, P.; Jauch, K.W.; Rentsch, M.; Ganten, T.M.; et al. Sirolimus Use in Liver Transplant Recipients With Hepatocellular Carcinoma: A Randomized, Multicenter, Open-Label Phase 3 Trial. Transplantation 2016, 100, 116–125. [Google Scholar] [CrossRef]
- Mazzaferro, V.; Chun, Y.S.; Poon, R.T.; Schwartz, M.E.; Yao, F.Y.; Marsh, J.W.; Bhoori, S.; Lee, S.G. Liver transplantation for hepatocellular carcinoma. Ann. Surg. Oncol. 2008, 15, 1001–1007. [Google Scholar] [CrossRef] [PubMed]
- Volk, M.L.; Vijan, S.; Marrero, J.A. A novel model measuring the harm of transplanting hepatocellular carcinoma exceeding Milan criteria. Am. J. Transplant. 2008, 8, 839–846. [Google Scholar] [CrossRef] [PubMed]
- Schulz, K.F.; Grimes, D.A. Sample size calculations in randomised trials: Mandatory and mystical. Lancet 2005, 365, 1348–1353. [Google Scholar] [CrossRef]
- Park, M.S.; Lee, K.W.; Yi, N.J.; Choi, Y.R.; Kim, H.; Hong, G.; Suh, K.S.; Kwon, C.H.; Joh, J.W.; Lee, S.K. Optimal tailored screening protocol after living donor liver transplantation for hepatocellular carcinoma. J. Korean Med. Sci. 2014, 29, 1360–1366. [Google Scholar] [CrossRef]
- Xu, S.L.; Zhang, Y.C.; Wang, G.Y.; Yang, Q.; Liu, B.; Zhang, J.; Li, H.; Wang, G.S.; Yang, Y.; Chen, G.H. Survival analysis of sirolimus-based immunosuppression in liver transplantation in patients with hepatocellular carcinoma. Clin. Res. Hepatol. Gastroenterol. 2016, 40, 674–681. [Google Scholar] [CrossRef]
- Ashworth, R.E.; Wu, J. Mammalian target of rapamycin inhibition in hepatocellular carcinoma. World J. Hepatol. 2014, 6, 776–782. [Google Scholar] [CrossRef]
- Bennett, J.; Cassidy, H.; Slattery, C.; Ryan, M.P.; McMorrow, T. Tacrolimus Modulates TGF-beta Signaling to Induce Epithelial-Mesenchymal Transition in Human Renal Proximal Tubule Epithelial Cells. J. Clin. Med. 2016, 5, 50. [Google Scholar] [CrossRef]
- Masola, V.; Carraro, A.; Zaza, G.; Bellin, G.; Montin, U.; Violi, P.; Lupo, A.; Tedeschi, U. Epithelial to mesenchymal transition in the liver field: The double face of Everolimus in vitro. BMC Gastroenterol. 2015, 15, 118. [Google Scholar] [CrossRef]
- Fischer, L.; Saliba, F.; Kaiser, G.M.; De Carlis, L.; Metselaar, H.J.; De Simone, P.; Duvoux, C.; Nevens, F.; Fung, J.J.; Dong, G.; et al. Three-year Outcomes in De Novo Liver Transplant Patients Receiving Everolimus With Reduced Tacrolimus: Follow-Up Results From a Randomized, Multicenter Study. Transplantation 2015, 99, 1455–1462. [Google Scholar] [CrossRef]
- Glover, T.E.; Watson, C.J.; Gibbs, P.; Bradley, J.A.; Ntzani, E.E.; Kosmoliaptsis, V. Conversion From Calcineurin to Mammalian Target of Rapamycin Inhibitors in Liver Transplantation: A Meta-Analysis of Randomized Controlled Trials. Transplantation 2016, 100, 621–629. [Google Scholar] [CrossRef]
- Park, M.S.; Lee, K.W.; Suh, S.W.; You, T.; Choi, Y.; Kim, H.; Hong, G.; Yi, N.J.; Kwon, C.H.; Joh, J.W.; et al. Living-donor liver transplantation associated with higher incidence of hepatocellular carcinoma recurrence than deceased-donor liver transplantation. Transplantation 2014, 97, 71–77. [Google Scholar] [CrossRef] [PubMed]

| Variable | Categorical Value | TAC (n = 22) | SRL (n = 20) | p Value |
|---|---|---|---|---|
| Preoperative factors | ||||
| Recipient Sex | Male | 21 (95%) | 19 (95%) | 1.00 |
| Age (Years) | Mean ± SD | 56.86 ± 6.40 | 51.95 ± 7.63 | 0.03 |
| Hypertension | n (%) | 5 (22.7) | 4 (20.0) | 1.00 |
| DM | n (%) | 6 (27.3) | 8 (40.0) | 0.52 |
| BMI (kg/m2) | Mean ± SD | 24.23 ± 3.05 | 25.04 ± 3.79 | 0.45 |
| MELD Score | Mean ± SD | 11.18 ± 9.10 | 8.95 ± 5.35 | 0.35 |
| CPT Score, n (%) | n (%) | 0.01 | ||
| A | 13 (59.1) | 10 (50.0) | ||
| B | 3 (13.6) | 10 (50.0) | ||
| C | 6 (27.3) | 0 (0) | ||
| Serum Creatinine (mg/dL) | Median | 0.93 (0.58–1.8) | 0.86 (0.57–1.3) | 0.27 |
| Donor Sex | Male | 13 (59.1%) | 14 (70%) | 0.53 |
| Donor Age | Median | 32 (18–53) | 25.5 (17–53) | 0.36 |
| Primary Liver Disease | n (%) | 0.40 | ||
| HBV | 17 (77.3) | 18 (90.0) | ||
| HCV | 1 (4.5) | 1 (4.2) | ||
| Alcoholism | 3 (13.6) | 0 (0) | ||
| Others | 1 (13.6) | 1 (4.2%) | ||
| Pre-LT Treatment | n (%) | |||
| TACE | 16 (72.7) | 15 (62.5) | 0.46 | |
| RFA | 2 (9.1) | 3 (15.0) | 0.66 | |
| PEIT | 2 (9.1) | 1 (8.3) | 1.00 | |
| Surgery | 3 (13.6) | 3 (12.5) | 1.00 | |
| AFP (ng/mL) | Mean ± SD | 317.93 ± 1274.23 | 706 ± 2916.97 | 0.57 |
| Median | 18.55 (2.7–6010) | 15.3 (2.4–14,070) | 0.49 | |
| ≤150 | n (%) | 19 (86.4) | 16 (80.0) | 0.69 |
| >150 | n (%) | 3 (13.6) | 4 (20.0) | |
| ≤400 | n (%) | 21 (95.5) | 17 (85%) | 0.27 |
| >400 | n (%) | 1 (4.5) | 3(15%) | |
| PET SUV ratio | Median | 1.02 (0.91–1.68) | 1.17 (0.82–3.39) | 0.35 |
| ≤1.15 | 13 (76.5) | 8 (50) | 0.16 | |
| >1.15 | 4 (23.5) | 8 (50) | ||
| Intraoperative factors | ||||
| GRWR | Mean ± SD | 1.18 ± 0.31 | 1.14 ± 0.28 | 0.69 |
| Cold Ischemia Time (min) | Mean ± SD | 63.3 ± 26.72 | 78.98 ± 21.29 | 0.05 |
| Warm Ischemia Time (min) | Mean ± SD | 33.33 ± 9.71 | 29.99 ± 8.02 | 0.23 |
| EBL (Ml) | Median | 1475 (400–23,000) | 1625 (500–12,000) | 0.68 |
| RBC Transfusion (Units) | Median | 0.5 (0–36) | 2 (0–24) | 0.61 |
| Pathologic Factors | ||||
| Tumour Number | Median | 3.5 (1–8) | 4.5 (1–11) | 0.33 |
| Tumour Maximum Size (cm) | Median | 3.8 (0.9–8) | 3.65 (2–10) | 0.55 |
| Tumour Sum Size (cm) | Mean ± SD | 7.59 ± 4.68 | 9.01 ± 3.44 | 0.25 |
| Microvascular Invasion | n (%) | 5 (22.7) | 5 (25.0) | 1.00 |
| Portal Vein Invasion | n (%) | 0 (0) | 0 (0) | NA |
| Bile Duct Involvement | n (%) | 1 (4.5) | 0 (0) | 1.00 |
| Mean tumour necrosis after TACE | Median | 61.6 (0–100) | 31 (0–99) | 0.26 |
| ES grade, n (%) | 0.13 | |||
| 1 | 1 (4.5) | 0 (0) | ||
| 2 | 4 (19.2) | 9 (45.0) | ||
| 3 | 12 (54.5) | 10 (50.0) | ||
| 4 | 5 (22.7) | 1 (5.0) | ||
| Immunosuppression Trough Level of TAC or SRL | Median | |||
| 3 Months | 5.1 (0.6–12.6) | 4.5 (1.6–15.1) | ||
| 6 Months | 8.5 (4.9–15.2) | 6.8 (2–30) | ||
| MMF dosage at 3 months | n (%) | |||
| ~500 mg/day | 0 | 1 (5.0) | ||
| 1000 mg/day | 17 (77.3) | 15 (75.0) | ||
| 1500 mg/day | 5 (22.7) | 4 (20.0) | ||
| HCC related outcome | ||||
| Time to LT | Median | 17 (0–107) | 2 (0–108) | 0.08 |
| Event of Recurrence | n (%) | 8 (36.4) | 5 (25) | 0.43 |
| Time to Recurrence | Median | 7 (5–31) | 15 (6–45) | 0.46 |
| Event of Death | n (%) | 7 (31.8) | 2 (10) | 0.14 |
| Variable | HR (95% CI) | p Value | |
|---|---|---|---|
| AFP (ng/mL) | ≤150 | ||
| >150 | 4.21 (1.26–14.08) | 0.02 | |
| PET Positivity (Tumour/Background SUV Ratio) | ≤1.15 | ||
| >1.15 | 7.13 (2.18–24.55) | 0.01 |
| Variable | HR (95% CI) | p Value | |
|---|---|---|---|
| AFP (ng/mL) | ≤150 | ||
| >150 | 35.23 (3.29–377.63) | <0.01 | |
| PET Positivity (Tumour/Background SUV Ratio) | ≤1.15 | ||
| >1.15 | 28.03 (2.67–293.95) | <0.01 | |
| Treatment Group | Sirolimus | ||
| Tacrolimus | 15.00 (1.30–172.85) | 0.03 |
| Adverse Event. | TAC (n = 22) | SRL (n = 20) | ||
|---|---|---|---|---|
| General | Weight Loss | 2 (9.1%) | 0 | 0.489 |
| Oedema of Both Legs | 0 | 1 (4.2%) | 0.476 | |
| Wound Complication | 0 | 2 (10%) | 0.221 | |
| Wound Lymphocele | 0 | 1 (4.2%) | 0.476 | |
| Umbilical Hernia | 0 | 1 (4.2%) | 0.476 | |
| Cardiovascular | Haemolytic Anaemia | 1 (4.5%) | 1 (4.2%) | 1.000 |
| HTN | 2 (9.1%) | 2 (10%) | 1.000 | |
| Dyslipidaemia | 0 | 3 (15%) | 0.099 | |
| Gastrointestinal | LFT Abnormality | 1 (4.5%) | 0 | 1.000 |
| Diffuse Fatty Liver | 1 (4.5%) | 0 | 1.000 | |
| Biliary Stricture | 2 (9.1%) | 2 (10%) | 1.000 | |
| Oral Mucositis | 0 | 2 (8.3%) | 0.221 | |
| Diarrhoea | 1 (4.5%) | 1 (4.2%) | 1.000 | |
| Dermatologic | Scaled Skin, Rash | 1 (4.5%) | 2 (8.3%) | 0.598 |
| Hyperpigmentation | 1 (4.5%) | 0 | 1.000 | |
| Infection | Herpes Zoster | 0 | 1 (5%) | 0.476 |
| Pulmonary Tb | 0 | 1 (5%) | 0.476 | |
| Radiation- and Nexavar-Related Symptom (Diarrhoea, Hand-Foot Syndrome) | 0 | 1 (5%) | 0.476 | |
| eGFR (MDRD) | ||||
| Initial | 92.3 ± 27.7 | 103.1 ± 30.1 | 0.24 | |
| 1 Year | 80.9 ± 28.9 | 95.8 ± 23.2 | 0.08 | |
| 2 Years | 80.5 ± 27.5 | 95.6 ± 26.7 | 0.09 | |
| 3 Years | 74.9 ± 25.0 | 90.2 ± 25.3 | 0.08 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, K.-W.; Kim, S.H.; Yoon, K.C.; Lee, J.-M.; Cho, J.-H.; Hong, S.K.; Yi, N.-J.; Han, S.-S.; Park, S.-J.; Suh, K.-S. Sirolimus Prolongs Survival after Living Donor Liver Transplantation for Hepatocellular Carcinoma Beyond Milan Criteria: A Prospective, Randomised, Open-Label, Multicentre Phase 2 Trial. J. Clin. Med. 2020, 9, 3264. https://doi.org/10.3390/jcm9103264
Lee K-W, Kim SH, Yoon KC, Lee J-M, Cho J-H, Hong SK, Yi N-J, Han S-S, Park S-J, Suh K-S. Sirolimus Prolongs Survival after Living Donor Liver Transplantation for Hepatocellular Carcinoma Beyond Milan Criteria: A Prospective, Randomised, Open-Label, Multicentre Phase 2 Trial. Journal of Clinical Medicine. 2020; 9(10):3264. https://doi.org/10.3390/jcm9103264
Chicago/Turabian StyleLee, Kwang-Woong, Seong Hoon Kim, Kyung Chul Yoon, Jeong-Moo Lee, Jae-Hyung Cho, Suk Kyun Hong, Nam-Joon Yi, Sung-Sik Han, Sang-Jae Park, and Kyung-Suk Suh. 2020. "Sirolimus Prolongs Survival after Living Donor Liver Transplantation for Hepatocellular Carcinoma Beyond Milan Criteria: A Prospective, Randomised, Open-Label, Multicentre Phase 2 Trial" Journal of Clinical Medicine 9, no. 10: 3264. https://doi.org/10.3390/jcm9103264
APA StyleLee, K.-W., Kim, S. H., Yoon, K. C., Lee, J.-M., Cho, J.-H., Hong, S. K., Yi, N.-J., Han, S.-S., Park, S.-J., & Suh, K.-S. (2020). Sirolimus Prolongs Survival after Living Donor Liver Transplantation for Hepatocellular Carcinoma Beyond Milan Criteria: A Prospective, Randomised, Open-Label, Multicentre Phase 2 Trial. Journal of Clinical Medicine, 9(10), 3264. https://doi.org/10.3390/jcm9103264





