Effect of Periodontal Disease on Diabetic Retinopathy in Type 2 Diabetic Patients: A Cross-Sectional Pilot Study
Abstract
1. Introduction
2. Methods
2.1. Study Population
2.2. Medical Examination
2.3. Periodontal Examination
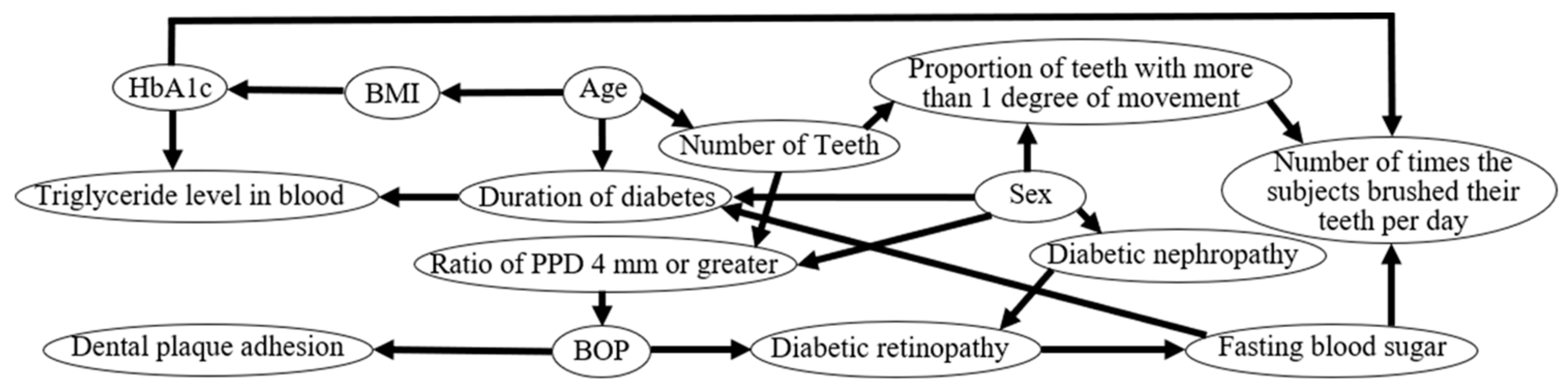
2.4. Bayesian Network
2.5. Statistical Analysis
3. Results
3.1. Subject Characteristics
3.2. Hemoglobin A1c in Type 2 Diabetic Patients with Adjacent Dental Plaque Attached to the Tooth Surface
3.3. Fasting Blood Sugar of Type 2 Diabetic Patients Who Brushed Their Teeth more than Twice a Day
3.4. Bleeding on Probing in Type 2 Diabetic Patients with Diabetic Retinopathy (1)
3.5. Bleeding on Probing in Type 2 Diabetic Patients with Diabetic Retinopathy (2)
3.6. Determination of Causal Effects Using Bayesian Network Analysis
4. Discussion
4.1. Effect of Bleeding on Probing on Diabetic Retinopathy
4.2. Relationship between Glycemic Control and Oral Hygiene Behavior
4.3. Relationship Between Periodontal Inflammation and Fasting Blood Sugar
4.4. Considerations
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Doğan, Ş.B.; Ballı, U.; Dede, F.Ö.; Sertoğlu, E.; Tazegül, K. Chemerin as a novel crevicular fluid marker of patients with periodontitis and type 2 diabetes mellitus. J. Periodontol. 2016, 87, 923–933. [Google Scholar] [CrossRef] [PubMed]
- Jourdain, M.L.; Velard, F.; Pierrard, L.; Sergheraert, J.; Gangloff, S.C.; Braux, J. Cationic antimicrobial peptides and periodontal physiopathology: A systematic review. J. Periodontal Res. 2019, 54, 589–600. [Google Scholar] [CrossRef] [PubMed]
- Sanz, M.; Ceriello, A.; Buysschaert, M.; Chapple, I.; Demmer, R.T.; Graziani, F.; Herrera, D.; Jepsen, S.; Lione, L.; Madianos, P.; et al. Scientific evidence on the links between periodontal diseases and diabetes: Consensus report and guidelines of the joint workshop on periodontal diseases and diabetes by the International Diabetes Federation and the European Federation of Periodontology. J. Clin. Periodontol. 2018, 45, 138–149. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, H.G.; Idris, S.B.; Mustafa, M.; Ahmed, M.F.; Åstrøm, A.N.; Mustafa, K.; Ibrahim, S.O. Impact of chronic periodontitis on levels of glucoregulatory biomarkers in gingival crevicular fluid of adults with and without type 2 diabetes. PLoS ONE 2015, 10, e0127660. [Google Scholar] [CrossRef] [PubMed]
- Casanova, L.; Hughes, F.J.; Preshaw, P.M. Diabetes and periodontal disease: A two-way relationship. Br. Dent. J. 2014, 217, 433–437. [Google Scholar] [CrossRef] [PubMed]
- Mealey, B.L.; Ocampo, G.L. Diabetes mellitus and periodontal disease. Periodontol. 2000 2007, 44, 127–153. [Google Scholar] [CrossRef]
- Taylor, G.W.; Burt, B.A.; Becker, M.P.; Genco, R.J.; Shlossman, M.; Knowler, W.C.; Pettitt, D.J. Severe periodontitis and risk for poor glycemic control in patients with non-insulin dependent diabetes mellitus. J. Periodontol. 1996, 67, 1085–1093. [Google Scholar] [CrossRef] [PubMed]
- Madianos, P.N.; Koromantzos, P.A. An update of the evidence on the potential impact of periodontal therapy on diabetes outcomes. J. Clin. Periodontol. 2018, 45, 188–195. [Google Scholar] [CrossRef]
- Darré, L.; Vergnes, J.N.; Gourdy, P.; Sixou, M. Efficacy of periodontal treatment on glycaemic control in diabetic patients: A meta-analysis of interventional studies. Diabet. Metab. 2008, 34, 497–506. [Google Scholar] [CrossRef]
- Choi, S.E.; Sima, C.; Pandya, A. Impact of treating oral disease on preventing vascular diseases: A model-based cost-effectiveness analysis of periodontal treatment among patients with type 2 diabetes. Diabet. Care 2020, 43, 563–571. [Google Scholar] [CrossRef]
- Katagiri, S.; Nitta, H.; Nagasawa, T.; Izumi, Y.; Kanazawa, M.; Matsuo, A.; Chiba, H.; Fukui, M.; Nakamura, N.; Oseko, F.; et al. Effect of glycemic control on periodontitis in type 2 diabetic patients with periodontal disease. J. Diabet. Investig. 2013, 4, 320–325. [Google Scholar] [CrossRef] [PubMed]
- Sonnenschein, S.K.; Meyle, J. Local inflammatory reactions in patients with diabetes and periodontitis. Periodontol. 2000 2015, 69, 221–254. [Google Scholar] [CrossRef] [PubMed]
- Merino, J.; Leong, A.; Liu, C.T.; Porneala, B.; Walford, G.A.; von Grotthuss, M.; Wang, T.J.; Flannick, J.; Dupuis, J.; Levy, D.; et al. Metabolomics insights into early type 2 diabetes pathogenesis and detection in individuals with normal fasting glucose. Diabetologia 2018, 61, 1315–1324. [Google Scholar] [CrossRef] [PubMed]
- Forbes, J.M.; Cooper, M.E. Mechanisms of diabetic complications. Physiol. Rev. 2013, 93, 137–188. [Google Scholar] [CrossRef] [PubMed]
- Deshpande, A.D.; Harris-Hayes, M.; Schootman, M. Epidemiology of diabetes and diabetes-related complications. Phys. Ther. 2008, 88, 1254–1264. [Google Scholar] [CrossRef]
- Sinclair, S.H.; Schwartz, S.S. Diabetic retinopathy-an underdiagnosed and undertreated inflammatory, neuro-vascular complication of diabetes. Front. Endocrinol. (Lausanne) 2019, 10, 843. [Google Scholar] [CrossRef]
- Veena, H.R.; Natesh, S.; Patil, S.R. Association between diabetic retinopathy and chronic periodontitis—A cross-sectional study. Med. Sci. 2018, 6, 104. [Google Scholar]
- Song, S.J.; Lee, S.S.; Han, K.; Park, J.B. Periodontitis is associated with diabetic retinopathy in non-obese adults. Endocrine 2017, 56, 82–89. [Google Scholar] [CrossRef]
- Commisso, L.; Monami, M.; Mannucci, E. Periodontal disease and oral hygiene habits in a type 2 diabetic population. Int. J. Dent. Hyg. 2011, 9, 68–73. [Google Scholar] [CrossRef]
- Mizutani, S.; Ekuni, D.; Tomofuji, T.; Irie, K.; Azuma, T.; Iwasaki, Y.; Morita, M. Self-efficacy and progression of periodontal disease: A prospective cohort study. J. Clin. Periodontol. 2015, 42, 1083–1089. [Google Scholar] [CrossRef]
- Tanaka, K.; Kawai, T.; Saisho, Y.; Meguro, S.; Harada, K.; Satoh, Y.; Kobayashi, K.; Mizushima, K.; Abe, T.; Itoh, H. Relationship between stage of diabetic retinopathy and pulse wave velocity in Japanese patients with type 2 diabetes. J. Diabet. Res. 2013, 2013, 193514. [Google Scholar] [CrossRef] [PubMed]
- Miller, P.D. A classification of marginal tissue recession. Int. J. Periodont. Restorat. Dent. 1985, 5, 8–13. [Google Scholar]
- Maglogiannis, I.; Zafiropoulos, E.; Platis, A.; Lambrinoudakis, C. Risk analysis of a patient monitoring system using Bayesian Network modeling. J. Biomed. Inform. 2006, 39, 637–647. [Google Scholar] [CrossRef] [PubMed]
- Tsuruta, M.; Takahashi, T.; Tokunaga, M.; Iwasaki, M.; Kataoka, S.; Kakuta, S.; Soh, I.; Awano, S.; Hirata, H.; Kagawa, M.; et al. Relationships between pathologic subjective halitosis, olfactory reference syndrome, and social anxiety in young Japanese women. BMC Psychol. 2017, 5, 7. [Google Scholar] [CrossRef]
- Killeen, A.C.; Harn, J.A.; Erickson, L.M.; Yu, F.; Reinhardt, R.A. Local minocycline effect on inflammation and clinical attachment during periodontal maintenance: Randomized clinical trial. J. Periodont. 2016, 87, 1149–1157. [Google Scholar] [CrossRef]
- Huck, O.; Mulhall, H.; Rubin, G.; Kizelnik, Z.; Iyer, R.; Perpich, J.D.; Haque, N.; Cani, P.D.; de Vos, W.M.; Amar, S. Akkermansia muciniphila reduces Porphyromonas gingivalis-induced inflammation and periodontal bone destruction. J. Clin. Periodont. 2020, 47, 202–212. [Google Scholar] [CrossRef] [PubMed]
- Yang, N.Y.; Zhang, Q.; Li, J.L.; Yang, S.H.; Shi, Q. Progression of periodontal inflammation in adolescents is associated with increased number of Porphyromonas gingivalis, Prevotella intermedia, Tannerella forsythensis, and Fusobacterium nucleatum. Int. J. Paediatr. Dent. 2014, 24, 226–233. [Google Scholar] [CrossRef] [PubMed]
- Sugiyama, S.; Takahashi, S.S.; Tokutomi, F.A.; Yoshida, A.; Kobayashi, K.; Yoshino, F.; Wada-Takahashi, S.; Toyama, T.; Watanabe, K.; Hamada, N.; et al. Gingival vascular functions are altered in type 2 diabetes mellitus model and/or periodontitis model. J. Clin. Biochem. Nutr. 2012, 51, 108–113. [Google Scholar] [CrossRef]
- Tóthová, L.; Celec, P. Oxidative stress and antioxidants in the diagnosis and therapy of periodontitis. Front. Physiol. 2017, 8, 1055. [Google Scholar] [CrossRef]
- Wright, H.J.; Chapple, I.L.; Matthews, J.B.; Cooper, P.R. Fusobacterium nucleatum regulation of neutrophil transcription. J. Periodont. Res. 2011, 46, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Żukowski, P.; Maciejczyk, M.; Waszkiel, D. Sources of free radicals and oxidative stress in the oral cavity. Arch. Oral Biol. 2018, 92, 8–17. [Google Scholar] [CrossRef] [PubMed]
- Suzumura, A.; Kaneko, H.; Funahashi, Y.; Takayama, K.; Nagaya, M.; Ito, S.; Okuno, T.; Hirakata, T.; Nonobe, N.; Kataoka, K.; et al. n-3 fatty acid and its metabolite 18-HEPE ameliorate retinal neuronal cell dysfunction by enhancing Müller BDNF in diabetic retinopathy. Diabetes 2020, 69, 724–735. [Google Scholar] [CrossRef] [PubMed]
- Cecilia, O.M.; José Alberto, C.G.; José, N.P.; Ernesto Germán, C.M.; Ana Karen, L.C.; Luis Miguel, R.P.; Ricardo Raúl, R.R.; Adolfo Daniel, R.C. Oxidative stress as the main target in diabetic retinopathy pathophysiology. J. Diabet. Res. 2019, 2019, 8562408. [Google Scholar] [CrossRef] [PubMed]
- Ren, S.; Hu, J.; Chen, Y.; Yuan, T.; Hu, H.; Li, S. Human umbilical cord derived mesenchymal stem cells promote interleukin-17 production from human peripheral blood mononuclear cells of healthy donors and systemic lupus erythematosus patients. Clin. Exp. Immunol. 2016, 183, 389–396. [Google Scholar] [CrossRef] [PubMed]
- Cheng, W.C.; Hughes, F.J.; Taams, L.S. The presence, function and regulation of IL-17 and Th17 cells in periodontitis. J. Clin. Periodont. 2014, 41, 541–549. [Google Scholar] [CrossRef] [PubMed]
- Apatzidou, D.A.; Iskas, A.; Konstantinidis, A.; Alghamdi, A.M.; Tumelty, M.; Lappin, D.F.; Nile, C.J. Clinical associations between acetylcholine levels and cholinesterase activity in saliva and gingival crevicular fluid and periodontal diseases. J. Clin. Periodont. 2018, 45, 1173–1183. [Google Scholar] [CrossRef]
- Chen, X.T.; Chen, L.L.; Tan, J.Y.; Shi, D.H.; Ke, T.; Lei, L.H. Th17 and Th1 lymphocytes are correlated with chronic periodontitis. Immunol. Investig. 2016, 45, 243–254. [Google Scholar] [CrossRef]
- Duarte, P.M.; da Rocha, M.; Sampaio, E.; Mestnik, M.J.; Feres, M.; Figueiredo, L.C.; Bastos, M.F.; Faveri, M. Serum levels of cytokines in subjects with generalized chronic and aggressive periodontitis before and after non-surgical periodontal therapy: A pilot study. J. Periodont. 2010, 81, 1056–1063. [Google Scholar] [CrossRef] [PubMed]
- Qiu, A.W.; Liu, Q.H.; Wang, J.L. Blocking IL-17A alleviates diabetic retinopathy in rodents. Cell Physiol. Biochem. 2017, 41, 960–972. [Google Scholar] [CrossRef]
- Sigurdardottir, S.; Zapadka, T.E.; Lindstrom, S.I.; Liu, H.; Taylor, B.E.; Lee, C.A.; Kern, T.S.; Taylor, P.R. Diabetes-mediated IL-17A enhances retinal inflammation, oxidative stress, and vascular permeability. Cell Immunol. 2019, 341, 103921. [Google Scholar] [CrossRef]
- Kinane, D.F.; Zhang, P.; Benakanakere, M.; Singleton, J.; Biesbrock, A.; Nonnenmacher, C.; He, T. Experimental gingivitis, bacteremia and systemic biomarkers: A randomized clinical trial. J. Periodont. Res. 2015, 50, 864–869. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Zhang, Y.; Lin, B.; Mei, Y.; Ping, Z.; Zhang, Z. The urban-rural disparity in the status and risk factors of health literacy: A cross-sectional survey in Central China. Int. J. Environ. Res. Public Health 2020, 17, 3848. [Google Scholar] [CrossRef] [PubMed]
- Tsiligianni, I.; Sifaki-Pistolla, D.; Gergianaki, I.; Kampouraki, M.; Papadokostakis, P.; Poulonirakis, I.; Gialamas, I.; Bempi, V.; Ierodiakonou, D. Associations of sense of coherence and self-efficacy with health status and disease severity in COPD. NPJ Prim. Care Respir. Med. 2020, 30, 27. [Google Scholar] [CrossRef] [PubMed]
- Robat-Sarpooshi, D.; Mahdizadeh, M.; Alizadeh Siuki, H.; Haddadi, M.; Robatsarpooshi, H.; Peyman, N. The relationship between health literacy level and self-care behaviors in patients with diabetes. Pat. Relat. Outcome Meas. 2020, 11, 129–135. [Google Scholar] [CrossRef] [PubMed]
- Saeed, H.; Saleem, Z.; Naeem, R.; Shahzadi, I.; Islam, M. Impact of health literacy on diabetes outcomes: A cross-sectional study from Lahore, Pakistan. Public Health 2018, 156, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Cohen, M.; Kanter, Y. Relation between sense of coherence and glycemic control in type 1 and type 2 diabetes. Behav. Med. 2004, 29, 175–183. [Google Scholar] [CrossRef]
- Odajima, Y.; Sumi, N. Factors related to sense of coherence in adult patients with Type 2 diabetes. Nagoya J. Med. Sci. 2018, 80, 61–71. [Google Scholar]
- Cepova, E.; Cicvakova, M.; Kolarcik, P.; Markovska, N.; Geckova, A.M. Associations of multidimensional health literacy with reported oral health promoting behaviour among Slovak adults: A cross-sectional study. BMC Oral Health 2018, 18, 44. [Google Scholar] [CrossRef]
- Bernabé, E.; Kivimäki, M.; Tsakos, G.; Suominen-Taipale, A.L.; Nordblad, A.; Savolainen, J.; Uutela, A.; Sheiham, A.; Watt, R.G. The relationship among sense of coherence, socio-economic status, and oral health-related behaviours among Finnish dentate adults. Eur. J. Oral. Sci. 2009, 117, 413–418. [Google Scholar] [CrossRef]
- Javed, F.; Al Amri, M.D.; Al-Kheraif, A.A.; Qadri, T.; Ahmed, A.; Ghanem, A.; Calvo-Guirado, J.L.; Romanos, G.E. Efficacy of non-surgical periodontal therapy with adjunct Nd:YAG laser therapy in the treatment of periodontal inflammation among patients with and without type 2 diabetes mellitus: A short-term pilot study. J. Photochem. Photobiol. B 2015, 149, 230–234. [Google Scholar] [CrossRef]
- Jung, Y.S.; Shin, M.H.; Kweon, S.S.; Lee, Y.H.; Kim, O.J.; Kim, Y.J.; Chung, H.J.; Kim, O.S. Periodontal disease associated with blood glucose levels in urban Koreans aged 50 years and older: The Dong-gu study. Gerodontology 2015, 32, 267–273. [Google Scholar] [CrossRef] [PubMed]
- Joshipura, K.J.; Muñoz-Torres, F.J.; Dye, B.A.; Leroux, B.G.; Ramírez-Vick, M.; Pérez, C.M. Longitudinal association between periodontitis and development of diabetes. Diabet. Res. Clin. Pract. 2018, 141, 284293. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.H.; McKeown, R.E.; Mayer-Davis, E.J.; Liese, A.D.; Song, K.B.; Merchant, A.T. Association between periodontitis and impaired fasting glucose and diabetes. Diabet. Care 2011, 34, 381–386. [Google Scholar] [CrossRef] [PubMed]
- Akutagawa, K.; Fujita, T.; Ouhara, K.; Takemura, T.; Tari, M.; Kajiya, M.; Matsuda, S.; Kuramitsu, S.; Mizuno, N.; Shiba, H.; et al. Glycyrrhizic acid suppresses inflammation and reduces the increased glucose levels induced by the combination of Porphyromonas gulae and ligature placement in diabetic model mice. Int. Immunopharmacol. 2019, 68, 30–38. [Google Scholar] [CrossRef] [PubMed]
- Colombo, N.H.; Shirakashi, D.J.; Chiba, F.Y.; Coutinho, M.S.; Ervolino, E.; Garbin, C.A.; Machado, U.F.; Sumida, D.H. Periodontal disease decreases insulin sensitivity and insulin signaling. J. Periodontol. 2012, 83, 864–870. [Google Scholar] [CrossRef] [PubMed]
| Total number | n = 104 |
| Sex (n): Female/male | 59/45 |
| Age (year): Mean ± SEM | 70.0 ± 1.22 |
| Age (year): Minimum/maximum | 23/86 |
| Number of teeth: Mean ± SEM | 21.4 ± 0.735 |
| BOP (%) | 27.3 ± 1.74 |
| Ratio of PPD, 4 mm or greater (%) | 13.2 ± 1.61 |
| Percentage of teeth with more than 1 degree of movement (%) | 9.90 ± 1.74 |
| Plaque adhesion on a surface that is adjacent to the tooth (n): Yes/no | 69/35 |
| Brushing the teeth 2 or more times a day: Yes/no | 65/39 |
| SPT in the dental clinic: Yes/no | 38/66 |
| Duration of diabetes (years): Mean ± SEM | 13.6 ± 1.02 |
| BMI (%): Mean ± SEM | 29.4 ± 0.467 |
| FBS (mg/dL): Mean ± SEM | 147 ± 4.95 |
| HbA1c (%): Mean ± SEM | 7.15 ± 0.0948 |
| Serum creatinine (mg/dL) | 1.08 ± 0.178 |
| Diabetic nephropathy: Yes/no | 12/92 |
| Diabetic retinopathy: Yes/no | 36/68 |
| Diabetic retinopathy: PDR/total number | 30/36 |
| Diabetic retinopathy: pre-PDR/total number | 4/36 |
| Diabetic retinopathy: PDR/total number | 2/36 |
| Diabetic neuropathy: Yes/no | 2/102 |
| Serum LDL cholesterol (mg/dL): Mean ± SEM | 113 ± 3.09 |
| Serum HDL cholesterol (mg/dL): Mean ± SEM | 57.3 ± 1.48 |
| Serum triglyceride (mg/dL): Mean ± SEM | 161 ± 1.48 |
| Variable | Adjacent Dental Plaque Attachment (n = 69) | No Adjacent Dental Plaque Attachment (n = 35) | p-Value * |
|---|---|---|---|
| Duration of diabetes (years) | 14.3 ± 8.44 | 12.3 ± 13.4 | 0.4 |
| BMI (%) | 29.5 ± 4.69 | 29.1 ± 4.97 | 0.7 |
| FBS (mg/dL) | 154 ± 49.1 | 134 ± 51.2 | 0.06 |
| HbA1c (%) | 7.27 ± 1.01 | 6.90 ± 0.835 | <0.05 |
| Creatinine (mg/dL) | 1.25 ± 2.21 | 0.735 ± 0.190 | 0.06 |
| LDL cholesterol (mg/dL) | 112 ± 32.5 | 113 ± 29.7 | 0.9 |
| HDL cholesterol (mg/dL) | 55.6 ± 14.1 | 60.5 ± 11.6 | 0.1 |
| Triglyceride (mg/dL) | 164 ± 88.4 | 156 ± 112 | 0.8 |
| Variable | Group Who Brushed Their Teeth ≥ 2 Times per Day (n = 65) | Group Who Brushed Their Teeth < 1 Time per Day (n = 39) | p-Value * |
|---|---|---|---|
| Duration of diabetes (years) | 12.7 ± 10.7 | 15.1 ± 9.70 | 0.2 |
| BMI (%) | 28.9 ± 4.66 | 30.3 ± 4.88 | 0.2 |
| FBS (mg/dL) | 128 ± 31.4 | 179 ± 60.0 | <0.0001 |
| HbA1c (%) | 7.00 ± 0.906 | 7.39 ± 1.03 | 0.05 |
| Creatinine (mg/dL) | 0.945 ± 1.56 | 1.31 ± 2.17 | 0.4 |
| LDL cholesterol (mg/dL) | 115 ± 32.0 | 108 ± 30.4 | 0.3 |
| HDL cholesterol (mg/dL) | 59.1 ± 13.8 | 54.2 ± 16.8 | 0.1 |
| Triglyceride (mg/dL) | 153 ± 93.2 | 173 ± 102 | 0.3 |
| Variable | Diabetic Retinopathy (n = 36) | No Diabetic Retinopathy (n = 68) | p-Value * |
|---|---|---|---|
| Number of teeth | 20.7 ± 8.50 | 21.7 ± 6.94 | 0.5 |
| BOP (%) | 34.1 ± 18.1 | 23.7 ± 16.6 | 0.006 |
| Ratio of PPD, 4 mm or greater (%) | 17.2 ± 19.0 | 11.1 ± 14.6 | 0.09 |
| Proportion of teeth with more than 1 degree of movement (%) | 12.0 ± 24.6 | 8.76 ± 12.7 | 0.5 |
| Number of times the subjects brushed their teeth per day | 1.53 ± 0.774 | 1.78 ± 0.666 | 0.1 |
| Variable | Fasting Blood Sugar (mg/dL) | ||
|---|---|---|---|
| rs * | p-Value | n | |
| Ratio of PPD 4 mm or greater (%) | 0.29 | 0.0029 | 104 |
| BOP (%) | 0.21 | 0.037 | 104 |
| Proportion of teeth with more than 1 degree of movement (%) | −0.041 | 0.68 | 104 |
| Number of teeth | 0.034 | 0.73 | 104 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yamamoto, Y.; Morozumi, T.; Hirata, T.; Takahashi, T.; Fuchida, S.; Toyoda, M.; Nakajima, S.; Minabe, M. Effect of Periodontal Disease on Diabetic Retinopathy in Type 2 Diabetic Patients: A Cross-Sectional Pilot Study. J. Clin. Med. 2020, 9, 3234. https://doi.org/10.3390/jcm9103234
Yamamoto Y, Morozumi T, Hirata T, Takahashi T, Fuchida S, Toyoda M, Nakajima S, Minabe M. Effect of Periodontal Disease on Diabetic Retinopathy in Type 2 Diabetic Patients: A Cross-Sectional Pilot Study. Journal of Clinical Medicine. 2020; 9(10):3234. https://doi.org/10.3390/jcm9103234
Chicago/Turabian StyleYamamoto, Yuko, Toshiya Morozumi, Takahisa Hirata, Toru Takahashi, Shinya Fuchida, Masami Toyoda, Shigeru Nakajima, and Masato Minabe. 2020. "Effect of Periodontal Disease on Diabetic Retinopathy in Type 2 Diabetic Patients: A Cross-Sectional Pilot Study" Journal of Clinical Medicine 9, no. 10: 3234. https://doi.org/10.3390/jcm9103234
APA StyleYamamoto, Y., Morozumi, T., Hirata, T., Takahashi, T., Fuchida, S., Toyoda, M., Nakajima, S., & Minabe, M. (2020). Effect of Periodontal Disease on Diabetic Retinopathy in Type 2 Diabetic Patients: A Cross-Sectional Pilot Study. Journal of Clinical Medicine, 9(10), 3234. https://doi.org/10.3390/jcm9103234






