Systemic Urate Deposition: An Unrecognized Complication of Gout?
Abstract
1. Introduction
2. Methods
3. Results
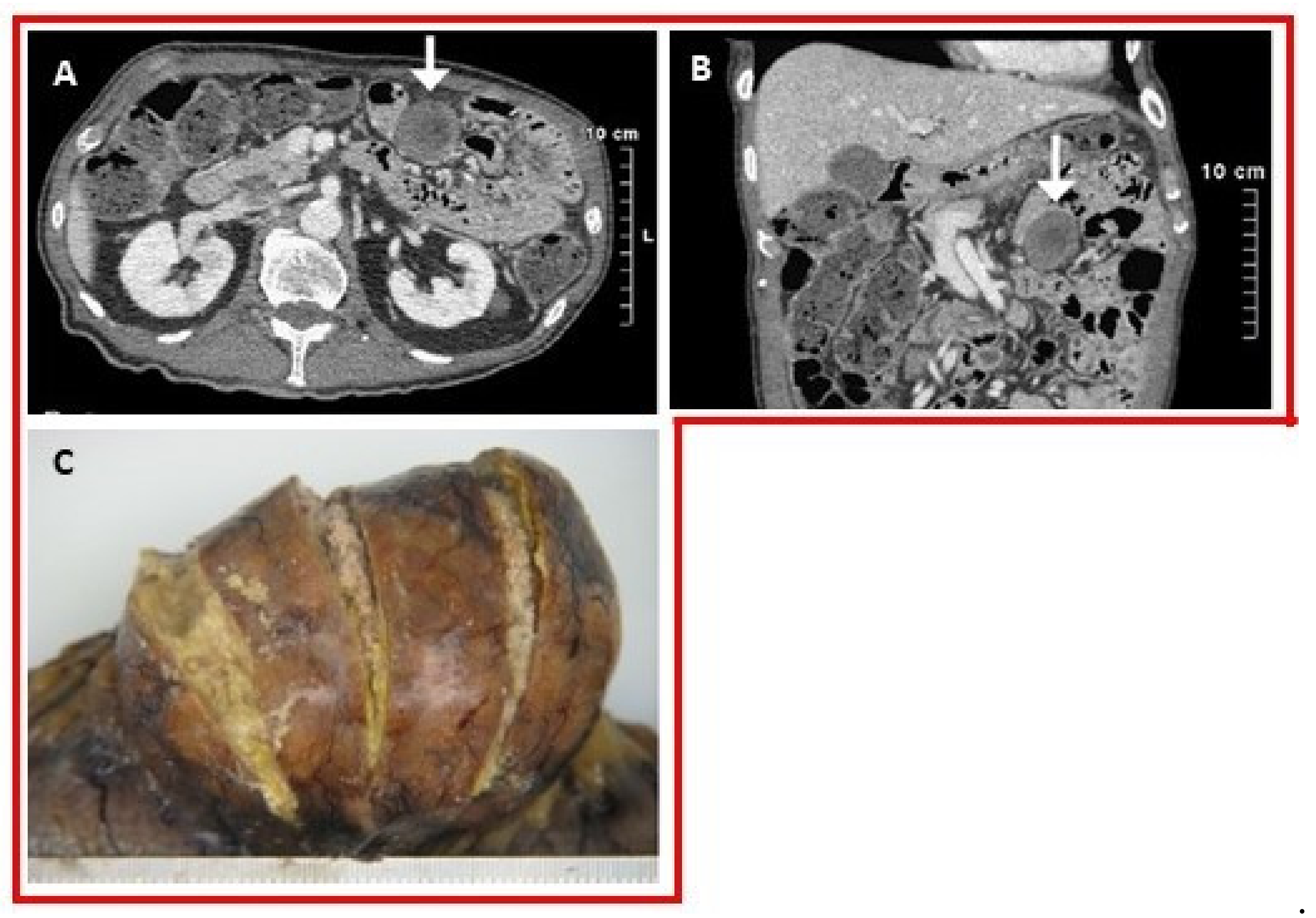
3.1. Cardiovascular
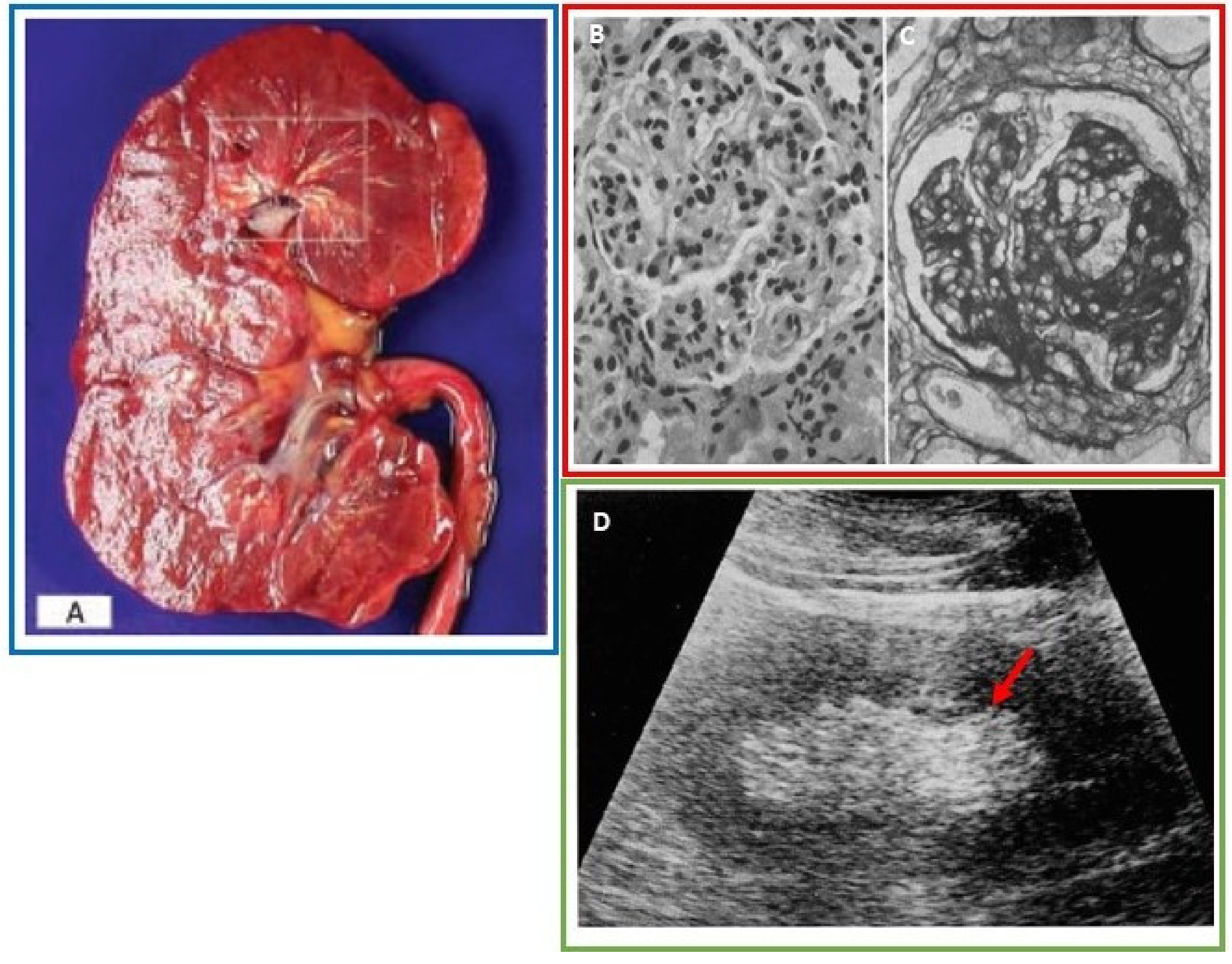
3.2. Renal
3.3. Spine
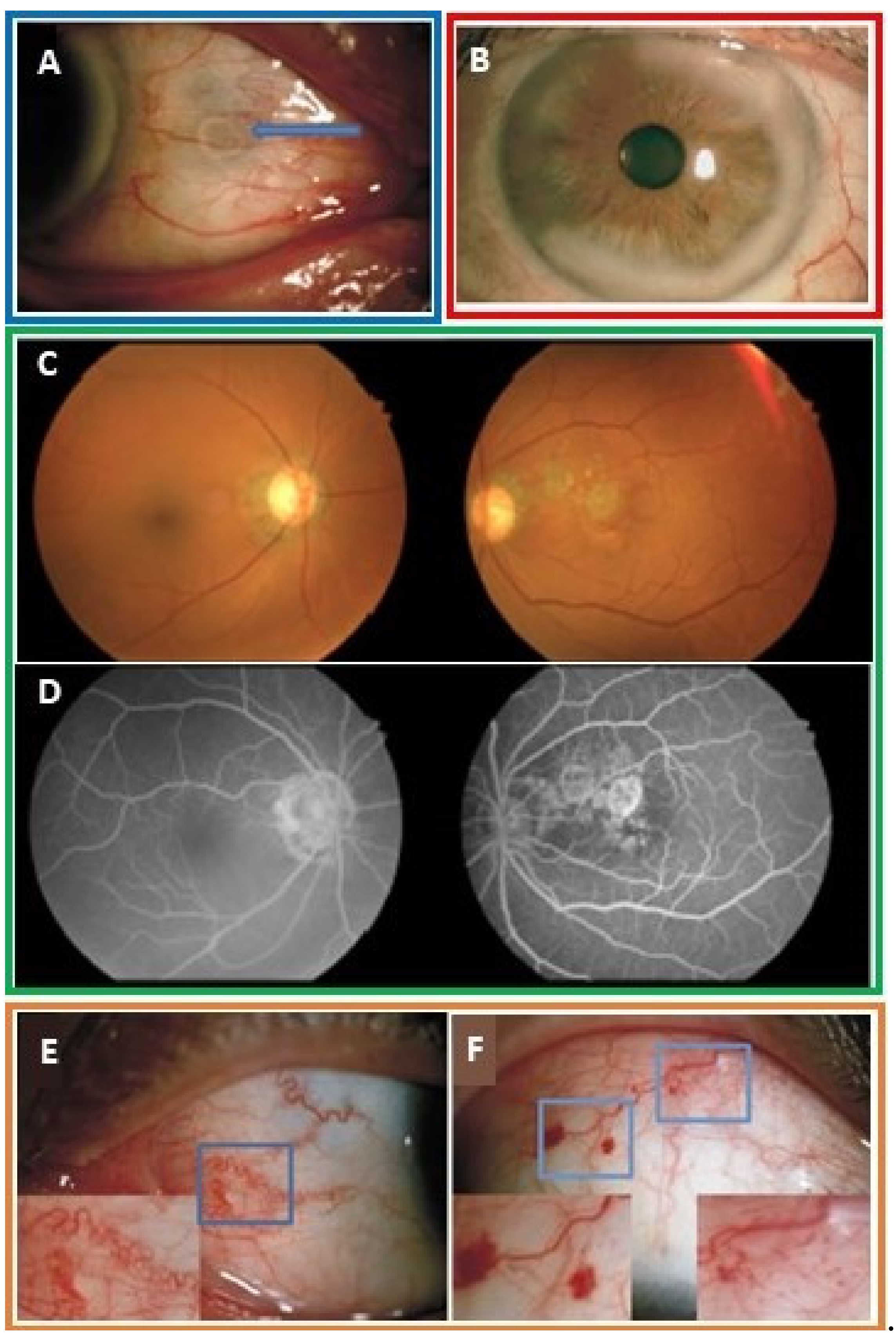
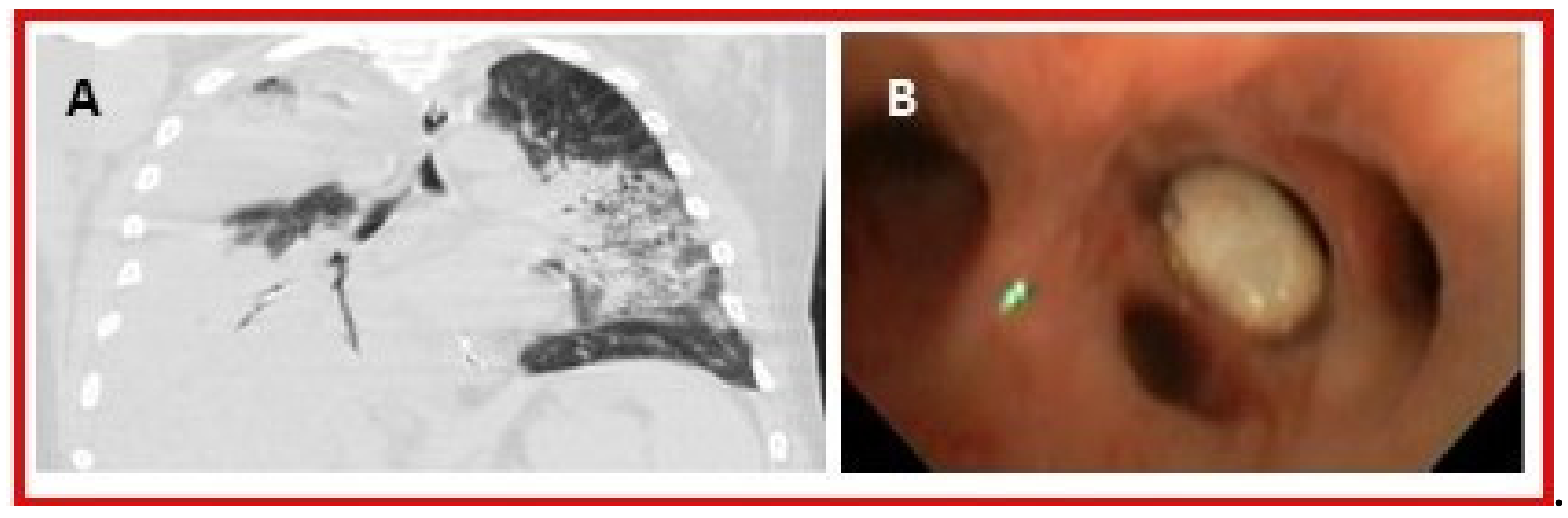
3.4. Ocular
3.5. Gastrointestinal
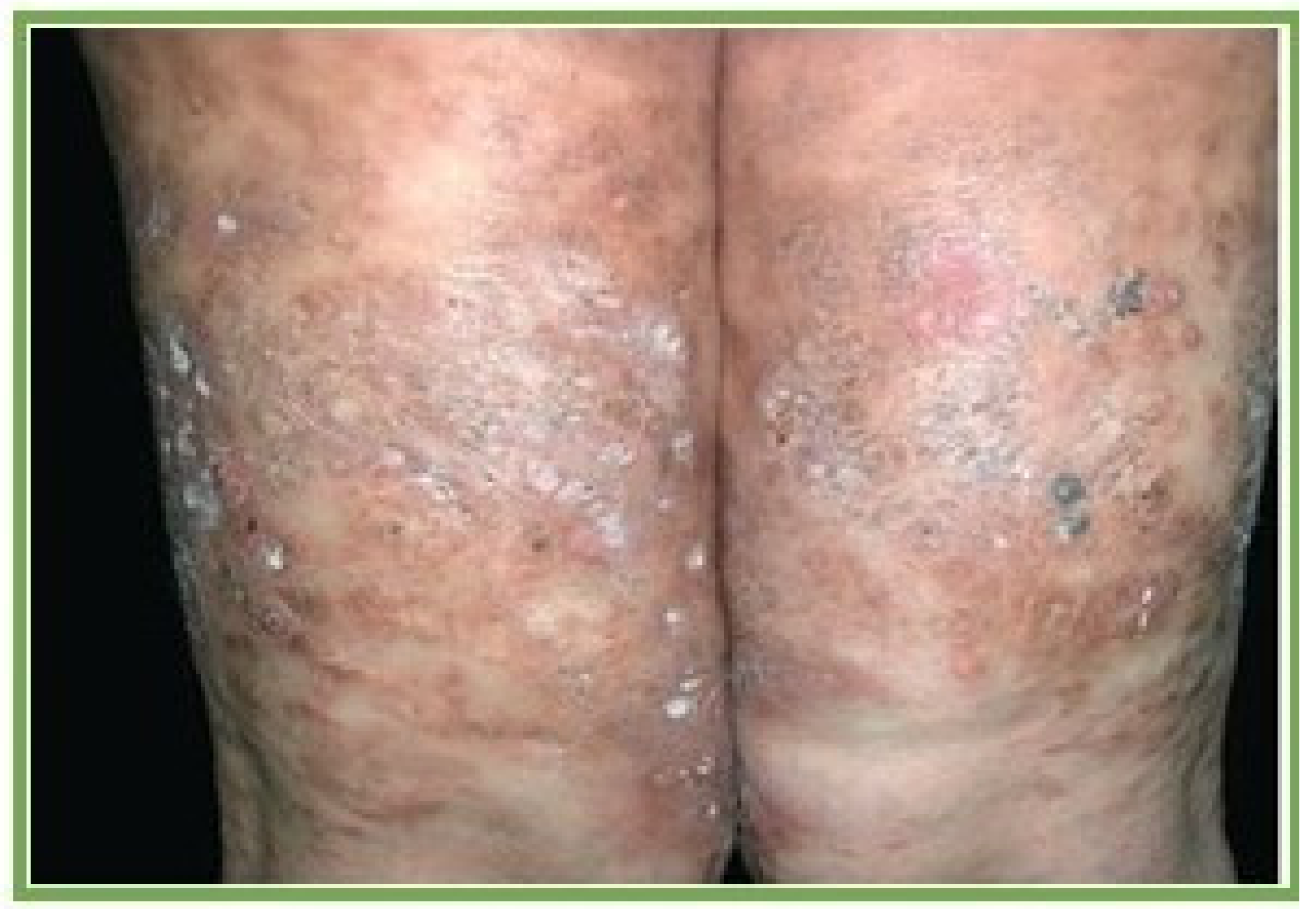
3.6. Integumentary
3.7. Head and Neck
3.8. Other
4. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Safiri, S.; Kolahi, A.A.; Cross, M.; Carson-Chahhoud, K.; Hoy, D.; Almasi-Hashiani, A.; Sepidarkish, M.; Ashrafi-Asgarabad, A.; Moradi-Lakeh, M.; Mansournia, M.A.; et al. Prevalence, incidence, and years lived with disability due to gout and its attributable risk factors for 195 countries and territories 1990-2017: A systematic analysis of the global burden of disease study 2017. Arthritis Rheumatol. 2020. (Online ahead of print). [Google Scholar] [CrossRef]
- Qaseem, A.; Harris, R.P.; Forciea, M.A.; Clinical Guidelines Committee of the American College of Physicians. Management of acute and recurrent gout: A clinical practice guideline from the American College of Physicians. Ann. Intern. Med. 2017, 166, 58–68. [Google Scholar] [CrossRef]
- Klauser, A.S.; Halpern, E.J.; Strobl, S.; Gruber, J.; Feuchtner, G.; Bellmann-Weiler, R.; Weiss, G.; Stofferin, H.; Jaschke, W. Dual-energy computed tomography detection of cardiovascular monosodium urate deposits in patients with gout. JAMA Cardiol. 2019, 4, 1019–1028. [Google Scholar] [CrossRef]
- Zhu, Y.; Pandya, B.J.; Choi, H.K. Comorbidities of gout and hyperuricemia in the US general population: NHANES 2007-2008. Am. J. Med. 2012, 125, 679–687. [Google Scholar] [CrossRef]
- Keenan, R.T.; O’Brien, W.R.; Lee, K.H.; Crittenden, D.B.; Fisher, M.C.; Goldfarb, D.S.; Krasnokutsky, S.; Oh, C.; Pillinger, M.H. Prevalence of contraindications and prescription of pharmacologic therapies for gout. Am. J. Med. 2011, 124, 155–163. [Google Scholar] [CrossRef] [PubMed]
- Pillinger, M.H.; Goldfarb, D.S.; Keenan, R.T. Gout and its comorbidities. Bull. NYU Hosp. Jt. Dis. 2010, 68, 199–203. [Google Scholar] [PubMed]
- Feig, D.I.; Kang, D.-H.; Johnson, R.J. Uric acid and cardiovascular risk. N. Engl. J. Med. 2008, 359, 1811–1821. [Google Scholar] [CrossRef] [PubMed]
- Vincent, Z.L.; Gamble, G.; House, M.; Knight, J.; Horne, A.; Taylor, W.J.; Dalbeth, N. Predictors of mortality in people with recent-onset gout: A prospective observational study. J. Rheumatol. 2016, 44, 368–373. [Google Scholar] [CrossRef]
- Lee, K.-A.; Ryu, S.-R.; Park, S.-J.; Kim, H.-R.; Lee, S.-H. Assessment of cardiovascular risk profile based on measurement of tophus volume in patients with gout. Clin. Rheumatol. 2018, 37, 1351–1358. [Google Scholar] [CrossRef]
- FitzGerald, J.D.; Dalbeth, N.; Mikuls, T.; Brignardello-Petersen, R.; Guyatt, G.; Abeles, A.M.; Gelber, A.C.; Harrold, L.R.; Khanna, D.; King, C.; et al. 2020 American College of Rheumatology guideline for the management of gout. Arthr. Care Res. 2020, 72, 744–760. [Google Scholar] [CrossRef]
- Singh, J.A.; Ramachandaran, R.; Yu, S.; Yang, S.; Xie, F.; Yun, H.; Zhang, J.; Curtis, J.R. Is gout a risk equivalent to diabetes for stroke and myocardial infarction? A retrospective claims database study. Arthr. Res. Ther. 2017, 19, 228. [Google Scholar] [CrossRef] [PubMed]
- Kuo, C.-F.; Grainge, M.J.; Mallen, C.; Zhang, W.; Doherty, M. Impact of gout on the risk of atrial fibrillation. Rheumatology (Oxford) 2016, 55, 721–728. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Pund, E.E.; Hawley, R.L.; McGee, H.J.; Blount, S.G. Gouty heart. N. Eng. J. Med. 1960, 263, 835–838. [Google Scholar] [CrossRef] [PubMed]
- Hench, P.S.; Darnall, C.M. A clinic on acute old-fashioned gout; with special reference to its inciting factors. Med. Clin. N. Amer. 1933, 16, 1371–1393. [Google Scholar]
- LaMoreaux, B.; Chandrasekaran, V. Gout causing urate cardiac vegetations: Summary of published cases. Ann. Rheum. Dis. 2019, 78 (Suppl. 2). [Google Scholar] [CrossRef]
- Frustaci, A.; Russo, M.A.; Sansone, L.; Francone, M.; Verardo, R.; Grande, C.; Alfarano, M.; Chimenti, C. Heart failure from gouty myocarditis: A case report. Ann. Intern. Med. 2020, 172, 363–365. [Google Scholar] [CrossRef]
- Dennstedt, F.E.; Weilbaecher, D.G. Tophaceous mitral value: Report of a case. Am. J. Surg. Pathol. 1982, 6, 79–81. [Google Scholar] [CrossRef]
- Curtiss, E.I.; Miller, T.R.; Shapiro, L.S. Pulmonic regurgitation due to valvular tophi. Circulation 1983, 67, 699–701. [Google Scholar] [CrossRef]
- Park, J.J.; Roudier, M.P.; Soman, D.; Mokadam, N.A.; Simkin, P.A. Prevalence of birefringent crystals in cardiac and prostatic tissues, an observational study. BMJ Open 2014, 4, e005308. [Google Scholar] [CrossRef]
- Patetsios, P.; Song, M.; Shutze, W.P.; Pappas, C.; Rodino, W.; Ramirez, J.A.; Panetta, T.F. Identification of uric acid and xanthine oxidase in atherosclerotic plaque. Am. J. Cardiol. 2001, 88, 188–191. [Google Scholar] [CrossRef]
- Patetsios, P.; Rodino, W.; Wisselink, W.; Bryan, D.; Kirwin, J.D.; Panetta, T.F. Identification of uric acid in aortic aneurysms and atherosclerotic artery. Ann. N. Y. Acad. Sci. 1996, 800, 243–245. [Google Scholar] [CrossRef] [PubMed]
- Barazani, S.; Chi, W.; Pyzik, R.; Jacobi, A.; O’Donnell, T.; Fayad, Z.; Mani, V.; Ali, Y. Detection of uric acid crystals in the vasculature of patients with gout using dual-energy computed tomography (abstract). Arthritis Rheum. 2018, 70 (Suppl. 9), 3584. [Google Scholar]
- Abdellatif, W.; Chow, B.; Nicolaou, S. Role of dual-energy CT as a screening tool for coronary gout. Ann. Rheum. Dis. 2019, 78 (Suppl. 2). [Google Scholar] [CrossRef]
- Ayoub, I.; Almaani, S.; Brodsky, S.; Nadasdy, T.; Prosek, J.; Hebert, L.; Rovin, B. revisiting medullary tophi: A link between uric acid and progressive chronic kidney disease? Clin. Nephrol. 2016, 85, 109–113. [Google Scholar] [CrossRef]
- Nickeleit, V.; Mihatsch, M.J. Uric acid nephropathy and end-stage renal disease--Review of a non-disease. Nephrol. Dial. Transplant. 1997, 12, 1832–1838. [Google Scholar] [CrossRef]
- Linnane, J.W.; Burry, A.F.; Emmerson, B.T. Urate deposits in the renal medulla. Prevalence and associations. Nephron 1981, 29, 216–222. [Google Scholar] [CrossRef]
- Lichtenstein, L.; Scott, H.W.; Levin, M.H. Pathologic changes in gout; Survey of eleven necropsied cases. Am. J. Pathol. 1956, 32, 871–895. [Google Scholar]
- Modern, F.W.S.; Meister, L. The kidney of gout, a clinical entity. Med. Clin. North. Am. 1952, 21, 941–951. [Google Scholar] [CrossRef]
- Fineberg, S.K.; Altschul, A. The nephropathy of gout. Ann. Intern. Med. 1956, 44, 1182–1194. [Google Scholar] [CrossRef]
- Brown, J.; Mallory, G.K. Renal changes in gout. N. Engl. J. Med. 1950, 243, 325–329. [Google Scholar] [CrossRef]
- Hsu, Y.-H. Chronic urate nephropathy. Incont. Pelvic Floor Dysfunct. 2012, 6, 89. [Google Scholar]
- Bluestone, R.; Waisman, J.; Klinenberg, J.R. The gouty kidney. Semin. Arthritis Rheum. 1977, 7, 97–113. [Google Scholar] [CrossRef]
- Talbott, J.H.; Terplan, K.L. The kidney in gout. Medicine (Baltimore) 1960, 39, 405–467. [Google Scholar] [CrossRef] [PubMed]
- Gonick, H.C.; Rubini, M.E.; Gleason, I.O.; Sommers, S.C. The renal lesion in gout. Ann. Intern. Med. 1965, 62, 667–674. [Google Scholar] [CrossRef] [PubMed]
- Ostberg, Y. Renal urate deposits in chronic renal insufficiency. Acta Med. Scand. 1968, 183, 197–201. [Google Scholar] [CrossRef] [PubMed]
- Greenbaum, D.; Ross, J.H.; Steinberg, V.L. Renal biopsy in gout. Br. Med. J. 1961, 1, 1502–1504. [Google Scholar] [CrossRef]
- Kim, M.Y.; Jeon, W.K.; Kim, H.K.; Kirn, Y.S.; Han, C.Y.; Kim, Y.T.; Han, S.T.; Lee, Y.W. Sonographic findings in gouty nephropathy. J. Korean. Radiol. Soc. 1994, 31, 523–527. [Google Scholar] [CrossRef][Green Version]
- Bardin, T.; Tran, K.M.; Nguyen, Q.D.; Sarfati, M.; Richette, P.; Vo, N.T.; Bousson, V.; Correas, J.-M. Renal medulla in severe gout: Typical findings on ultrasonography and dual-energy CT study in two patients. Ann. Rheum. Dis. 2019, 78, 433. [Google Scholar] [CrossRef]
- Toyoda, K.; Miyamoto, Y.; Ida, M.; Tada, S.; Utsunomiya, M. Hyperechoic medulla of the kidneys. Radiology 1989, 173, 431–434. [Google Scholar] [CrossRef]
- Wiederkehr, M.R.; Moe, O.W. Uric acid nephrolithiasis: A systemic metabolic disorder. Clin. Rev. Bone Miner. Metab. 2011, 9, 207–217. [Google Scholar] [CrossRef]
- Jepperson, M.A.; Cernigliaro, J.G.; Sella, D.; Ibrahim, E.; Thiel, D.D.; Leng, S.; Haley, W.E. Dual-energy CT for the evaluation of urinary calculi: Image interpretation, pitfalls and stone mimics. Clin. Radiol. 2013, 68, e707–e714. [Google Scholar] [CrossRef]
- Johnson, R.J.; Nakagawa, T.; Jalal, D.; Sánchez-Lozada, L.G.; Kang, D.-H.; Ritz, E. Uric acid and chronic kidney disease: Which is chasing which? Nephrol. Dial. Transplant. 2013, 28, 2221–2228. [Google Scholar] [CrossRef] [PubMed]
- Yü, T.F.; Berger, L. Impaired renal function gout: Its association with hypertensive vascular disease and intrinsic renal disease. Am. J. Med. 1982, 72, 95–100. [Google Scholar] [CrossRef]
- Kang, D.-H.; Nakagawa, T.; Feng, L.; Watanabe, S.; Han, L.; Mazzali, M.; Truong, L.; Harris, R.; Johnson, R.J. A role for uric acid in the progression of renal disease. J. Am. Soc. Nephrol. 2002, 13, 2888–2897. [Google Scholar] [CrossRef] [PubMed]
- Mazzali, M.; Hughes, J.; Kim, Y.G.; Jefferson, J.A.; Kang, D.H.; Gordon, KL.; Lan, H.Y.; Kivlighn, S.; Johnson, R.J. Elevated uric acid increases blood pressure in the rat by a novel crystal-independent mechanism. Hypertension 2001, 38, 1101–1106. [Google Scholar] [CrossRef]
- Kosugi, T.; Nakayama, T.; Heinig, M.; Zhang, L.; Yuzawa, Y.; Sanchez-Lozada, L.G.; Roncal, C.; Johnson, R.J.; Nakagawa, T. Effect of lowering uric acid on renal disease in the type 2 diabetic Db/Db mice. Am. J. Physiol. Renal Physiol. 2009, 297, F481–F488. [Google Scholar] [CrossRef]
- Kersley, G.D.; Mandel, L.; Jeffrey, M.R. Gout; an unusual case with softening and subluxation of the first cervical vertebra and splenomegaly. Ann. Rheum. Dis. 1950, 9, 282–304. [Google Scholar] [CrossRef]
- Yoon, J.-W.; Park, K.-B.; Park, H.; Kang, D.-H.; Lee, C.-H.; Hwang, S.-H.; Jung, J.-M.; Han, J.-W.; Park, I.S. Tophaceous gout of the spine causing neural compression. Korean J. Spine 2013, 10, 185–188. [Google Scholar] [CrossRef]
- Toprover, M.; Krasnokutsky, S.; Pillinger, M.H. Gout in the spine: Imaging, diagnosis, and outcomes. Curr Rheumatol. Rep. 2015, 17, 70. [Google Scholar] [CrossRef]
- Hasegawa, E.M.; de Mello, F.M.; Goldenstein-Schainberg, C.; Fuller, R. Gout in the spine. Rev. Bras. Reumatol. 2013, 53, 296–302. [Google Scholar] [CrossRef]
- Lu, H.; Sheng, J.; Dai, J.; Hu, X. Tophaceous gout causing lumbar stenosis: A case report. Medicine (Baltimore) 2017, 96, e7670. [Google Scholar] [CrossRef] [PubMed]
- Adler, S.; Seitz, M. The gouty spine: Old guy—new tricks. Rheumatology 2017, 56, 2243–2245. [Google Scholar] [CrossRef] [PubMed]
- Chotard, E.; Sverzut, J.; Lioté, F.; Bardin, T.; Ea, H.-K. Gout at the spine: A retrospective study with dual-energy computed tomography. Ann. Rheum. Dis. 2017, 76 (Suppl. 2). [Google Scholar] [CrossRef]
- Toprover, M.; Slobodnick, A.; Pike, C.; Oh, C.; Davis, C.; Mechlin, M.; Pillinger, M. Gout and serum urate levels are associated with lumbar spine monosodium urate deposition and chronic low back pain: A dual-energy CT study (abstract). Arthritis Rheum. 2019, 71 (Suppl. 10). [Google Scholar]
- De Monteynard, M.S.; Jacquier, J.; Adotti, F.; Bodard-Rickelman, E. Gouty tophus of the eyelid. Bull. Soc. Ophtalmol. Fr. 1986, 86, 53–54. [Google Scholar]
- Chu, Y.C.; Hsieh, Y.Y.; Ma, L. Medial canthal tophus associated with gout. Am. J. Ophthalmol. 2005, 140, 542–544. [Google Scholar] [CrossRef]
- Morris, W.R.; Fleming, J.C. Gouty tophus at the lateral canthus. Arch. Ophthalmol. 2003, 121, 1195–1197. [Google Scholar] [CrossRef]
- Lo, W.R.; Broocker, G.; Grossniklaus, H.E. Histopathologic examination of conjunctival tophi in gouty arthritis. Am. J. Ophthalmol. 2005, 140, 1152–1154. [Google Scholar] [CrossRef]
- Lin, J.; Zhao, G.-Q.; Che, C.-Y.; Yang, S.-S.; Wang, Q.; Li, C.-G. Characteristics of ocular abnormalities in gout patients. Int. J. Ophthalmol. 2013, 6, 307–311. [Google Scholar] [CrossRef]
- Bernad, B.; Narvaez, J.; Diaz-Torné, C.; Diez-Garcia, M.; Valverde, J. Clinical image: Corneal tophus deposition in gout. Arthritis Rheum. 2006, 54, 1025. [Google Scholar] [CrossRef]
- Coassin, M.; Piovanetti, O.; Stark, W.J.; Green, W.R. Urate deposition in the iris and anterior chamber. Ophthalmology 2006, 113, 462–465. [Google Scholar] [CrossRef] [PubMed]
- Topping, N.C.; Cassels-Brown, A.; Chakrabarty, A.; Cronin, P.; Ross, S.; Russell, J.; Tesha, P. Uric acid crystals presenting as an orbital mass. Eye 2003, 17, 427–429. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Brenner, J.E.; Foster, W.J. Retinal complications of gout: A case report and review of the literature. BMC Ophthalmol. 2018, 18, 11. [Google Scholar] [CrossRef] [PubMed]
- Ao, J.; Goldblatt, F.; Casson, R.J. Review of the ophthalmic manifestations of gout and uric acid crystal deposition. Clin. Exp. Ophthalmol. 2017, 45, 73–80. [Google Scholar] [CrossRef]
- Mcwilliams, J.R. Ocular findings in gout*: Report of a case of conjunctival tophi. Am. J. Ophthalmol. 1952, 35, 1778–1783. [Google Scholar] [CrossRef]
- Sarma, P.; Das, D.; Deka, P.; Deka, A.C. Subconjunctival urate crystals: A case report. Cornea 2010, 29, 830–832. [Google Scholar] [CrossRef]
- Ferry, A.P.; Safir, A.; Melikian, H.E. Ocular abnormalities in patients with gout. Ann. Ophthalmol. 1985, 17, 632–635. [Google Scholar]
- Bakhritdinova, F.A. Morphometric parameters of the bulbar conjunctiva vessels in patients with gout. Vestn. Oftalmol. 1996, 112, 30–32. [Google Scholar]
- Ministrini, S.; Baronio, G.; Zorzi, F.; Bercich, L.; Grazioli, L.; Molfino, S.; Portolani, N. Unusual presentation of gouty tophus in the liver with subsequent appearance in the same site of HCC: A correlate diagnosis? Case Report. World J. Surg. Oncol. 2019, 17. [Google Scholar] [CrossRef]
- Khanna, D.; Tang, S.-J.; Wallace, W.D.; Roth, B.E.; Hahn, B.H. Gouty tophi in a pancreatic pseudocyst. Arthritis Rheum. 2002, 46, 565–566. [Google Scholar] [CrossRef]
- Sarungbam, J.; Jain, S.; Yusuf, Y. Urate crystals in pancreatic pseudocyst: A rare cause of intestinal perforation. Am. J. Clin. Pathol. 2012, 138, A235. [Google Scholar] [CrossRef][Green Version]
- Hawkins, C.F.; Ellis, H.A.; Rawson, A. Malignant gout with tophaceous small intestine and megaloblastic anaemia. Ann. Rheum. Dis. 1965, 24, 224–233. [Google Scholar] [CrossRef]
- Moiseev, S.V.; Shavarov, A.A.; Varshavsky, V.A.; Reshetin, V.V. Multiple pseudotumorous crystalline deposits in small intestine, mesentery and lungs in terminal heart failure patent without gouty arthritis. Eur. J. Heart Fail. Abstr. Supp. 2016, 18 (Suppl. 1), 317. [Google Scholar]
- Härle, P.; Schlottmann, K.; Ehrenstein, B.P.; Fleck, M.; Glück, T.; Herold, T.; Schubert, T.E.; Straub, R.H.; Schölmerich, J. A Patient with arthritis, severe back pain, impaired wound healing, and perforated sigmoid colon. Lancet 2006, 367, 2032. [Google Scholar] [CrossRef]
- Wu, H.; Klein, M.J.; Stahl, R.E.; Sanchez, M.A. Intestinal pseudotumorous gouty nodulosis: A colonic tophus without manifestation of gouty arthritis. Hum. Pathol. 2004, 35, 897–899. [Google Scholar] [CrossRef] [PubMed]
- Katoch, P.; Trier-Mørch, S.; Vyberg, M. Small intestinal tophus mimicking tumor. Hum. Pathol. Case Rep. 2014, 1, 2–5. [Google Scholar] [CrossRef][Green Version]
- Chen, C.-H.; Chen, C.K.-H.; Yeh, L.-R.; Pan, H.-B.; Yang, C.-F. Intra-abdominal gout mimicking pelvic abscess. Skeletal Radiol. 2005, 34, 229–233. [Google Scholar] [CrossRef]
- Pattanaprichakul, P.; Bunyaratavej, S.; McLain, P.M.; Varothai, S. Disseminated gouty panniculitis: An unusual presentation of extensive cutaneous tophi. Dermatol. Pract. Concept. 2014, 4, 33–35. [Google Scholar] [CrossRef]
- Antonio, I.G.; Londono, J.C.; Saaibi, D.L.; Peña, M.; Lizarazo, H.; Gonzalez, E.B. Gout nodulosis: Widespread subcutaneous deposits without gout. Arthritis Rheum. 1996, 9, 74–77. [Google Scholar] [CrossRef]
- Towiwat, P.; Chhana, A.; Dalbeth, N. The anatomical pathology of gout: A systematic literature review. BMC Musculoskelet. Disord. 2019, 20, 140. [Google Scholar] [CrossRef]
- Weberschock, T.; Gholam, P.; Hartschuh, W.; Hartmann, M. Gouty panniculitis in a 68-year-old man: Case report and review of the literature. Int. J. Dermatol. 2010, 49, 410–413. [Google Scholar] [CrossRef] [PubMed]
- Dahiya, A.; Leach, J.; Levy, H. Gouty panniculitis in a healthy male. J. Am. Acad. Dermatol. 2007, 57, S52–S54. [Google Scholar] [CrossRef] [PubMed]
- Mutlu, A.; Dündar, E.; İşeri, M.; Erçin, C.; Cefle, A. An unusual presentation of gout: Tophi in the middle ear. J. Int. Adv. Otol. 2016, 12, 216–218. [Google Scholar] [CrossRef] [PubMed]
- Faas, I. A gouty tophus in the temporomandibular joint and on the eustachian tube. Laryngol. Rhinol. Otol. 1983, 62, 574–577. [Google Scholar] [CrossRef]
- Saliba, J.; Sakano, H.; Friedman, R.A.; Harris, J.P. Tophaceous gout of the middle ear: Case reports and review of the literature. Audiol. Neurootol. 2019, 24, 51–55. [Google Scholar] [CrossRef] [PubMed]
- Lefkovits, A.M. Gouty involvement of the larynx report of a case and review of the literature. Arthritis Rheum. 1965, 8, 1019–1026. [Google Scholar] [CrossRef] [PubMed]
- Okada, T. Hoarseness due to gouty tophus in vocal cords. Arch. Otolaryngol. 1964, 79, 407–411. [Google Scholar] [CrossRef]
- Gacek, R.R.; Gacek, M.R.; Montgomery, W.W. Evidence for laryngeal paralysis in cricoarytenoid joint arthritis. Laryngoscope 1999, 109, 279–283. [Google Scholar] [CrossRef]
- Tsikoudas, A.; Coatesworth, A.P.; Martin-Hirsch, D.P. Laryngeal gout. J. Laryngol. Otol. 2002, 116, 140–142. [Google Scholar] [CrossRef]
- Wu, J.C.-H.; Chou, P.-Y.; Chen, C.-H. Nasal gouty tophus: Report a rare case presenting as a nasal hump with nasal obstruction. Biomed. J. 2016, 39, 295–297. [Google Scholar] [CrossRef][Green Version]
- Motrich, R.D.; Olmedo, J.J.; Molina, R.; Tissera, A.; Minuzzi, G.; Rivero, V.E. Uric acid crystals in the semen of a patient with symptoms of chronic prostatitis. Fertil. Steril. 2006, 85, 751–754. [Google Scholar] [CrossRef] [PubMed]
- Persson, B.E.; Ronquist, G.; Ekblom, M. Ameliorative effect of allopurinol on nonbacterial prostatitis: A parallel double-blind controlled study. J. Urol. 1996, 155, 961–964. [Google Scholar] [CrossRef]
- Sharifabad, M.A.; Tzeng, J.; Gharibshahi, S. Mammary gouty tophus: A case report and review of the literature. Breast J. 2006, 12, 263–265. [Google Scholar] [CrossRef]
- Adamson, R.; Lacy, J.M.; Cheng, A.M.; Park, D.R. Tophus causing bronchial obstruction. Am. J. Respir. Crit. Care Med. 2013, 188, e72–e73. [Google Scholar] [CrossRef] [PubMed]
- Zang, Y.-S.; Fang, Z.; Li, B. Gout-associated lung disease. Rheumatology 2012, 51, 756. [Google Scholar] [CrossRef] [PubMed]
- Flores Martín, J.F.; Vázquez Alonso, F.; Puche Sanz, I.; Berrio Campos, R.; Campaña Gutierrez, M.A.; Cózar Olmo, J.M. Gouty tophi in the penis: A case report and review of the literature. Case Rep. Urol. 2012, 2012, 594905. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Vela, P.; Pascual, E. Images in clinical medicine. An unusual tophus. N. Engl. J. Med. 2015, 372, e6. [Google Scholar] [CrossRef]
- Kleber, M.E.; Delgado, G.; Grammer, T.B.; Silbernagel, G.; Huang, J.; Krämer, B.K.; Ritz, E.; März, W. Uric acid and cardiovascular events: A mendelian randomization study. J. Am. Soc. Nephrol. 2015, 26, 2831–2838. [Google Scholar] [CrossRef]


| Organ System | Number of Articles | Diagnostics |
|---|---|---|
| Total | 290 | Clinical exam, surgery, autopsy, histopathology and/or imaging |
| Spine: Lumbar, thoracic, cervical: facet joint, lamina, pedicle, intervertebral disc, ligaments, ligamentum flavum, epidural space, paraspinal soft tissue | 113 | Surgery, autopsy, histopathology, imaging |
| Integumentary: Dermis, hypodermis | 48 | Clinical exam, autopsy, histopathology |
| Ocular: Periocular soft tissues, canthi, conjunctiva, sclera, cornea, lens, orbital fossa, retina, iris | 36 | Clinical exam, histopathology, imaging |
| Renal: Renal parenchyma (Cases of nephrolithiasis were excluded) | 25 | Autopsy, histopathology, imaging |
| Cardiovascular: Valves (mitral, pulmonic, aortic, tricuspid), myocardium, conduction pathway, coronary artery wall, arterial atherosclerotic plaque | 21 | Surgery, autopsy, histopathology, imaging |
| Larynx: Vocal cords, subglottis, cricoarytenoid joint | 11 | Clinical exam, histopathology |
| Bowel: Small intestine, colon, mesentery | 6 | Autopsy, histopathology, imaging |
| Middle Ear/ Eustachian tube | 6 | Clinical exam, histopathology, imaging |
| Breast | 5 | Histopathology, imaging |
| Pancreas | 4 | Histopathology, imaging |
| Nasal | 4 | Clinical exam, histopathology, imaging |
| Pulmonary: Bronchus, pleural effusion | 4 | Bronchoscopy, histopathology, imaging |
| Prostate Gland | 2 | Histopathology |
| Liver | 2 | Histopathology, imaging |
| Penis | 1 | Clinical exam, surgery, histopathology |
| Nailbed | 1 | Clinical exam, histopathology |
| Pelvis | 1 | Histopathology, imaging |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khanna, P.; Johnson, R.J.; Marder, B.; LaMoreaux, B.; Kumar, A. Systemic Urate Deposition: An Unrecognized Complication of Gout? J. Clin. Med. 2020, 9, 3204. https://doi.org/10.3390/jcm9103204
Khanna P, Johnson RJ, Marder B, LaMoreaux B, Kumar A. Systemic Urate Deposition: An Unrecognized Complication of Gout? Journal of Clinical Medicine. 2020; 9(10):3204. https://doi.org/10.3390/jcm9103204
Chicago/Turabian StyleKhanna, Puja, Richard J. Johnson, Bradley Marder, Brian LaMoreaux, and Ada Kumar. 2020. "Systemic Urate Deposition: An Unrecognized Complication of Gout?" Journal of Clinical Medicine 9, no. 10: 3204. https://doi.org/10.3390/jcm9103204
APA StyleKhanna, P., Johnson, R. J., Marder, B., LaMoreaux, B., & Kumar, A. (2020). Systemic Urate Deposition: An Unrecognized Complication of Gout? Journal of Clinical Medicine, 9(10), 3204. https://doi.org/10.3390/jcm9103204





