Atrial Substrate Underlies the Recurrence after Catheter Ablation in Patients with Atrial Fibrillation
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Electrophysiological Studies and Catheter Ablation
2.3. Post-Ablation Management and Follow-Up
2.4. Analysis of MR LGE Images
2.5. Statistical Analysis
3. Results
3.1. Patient Characteristics
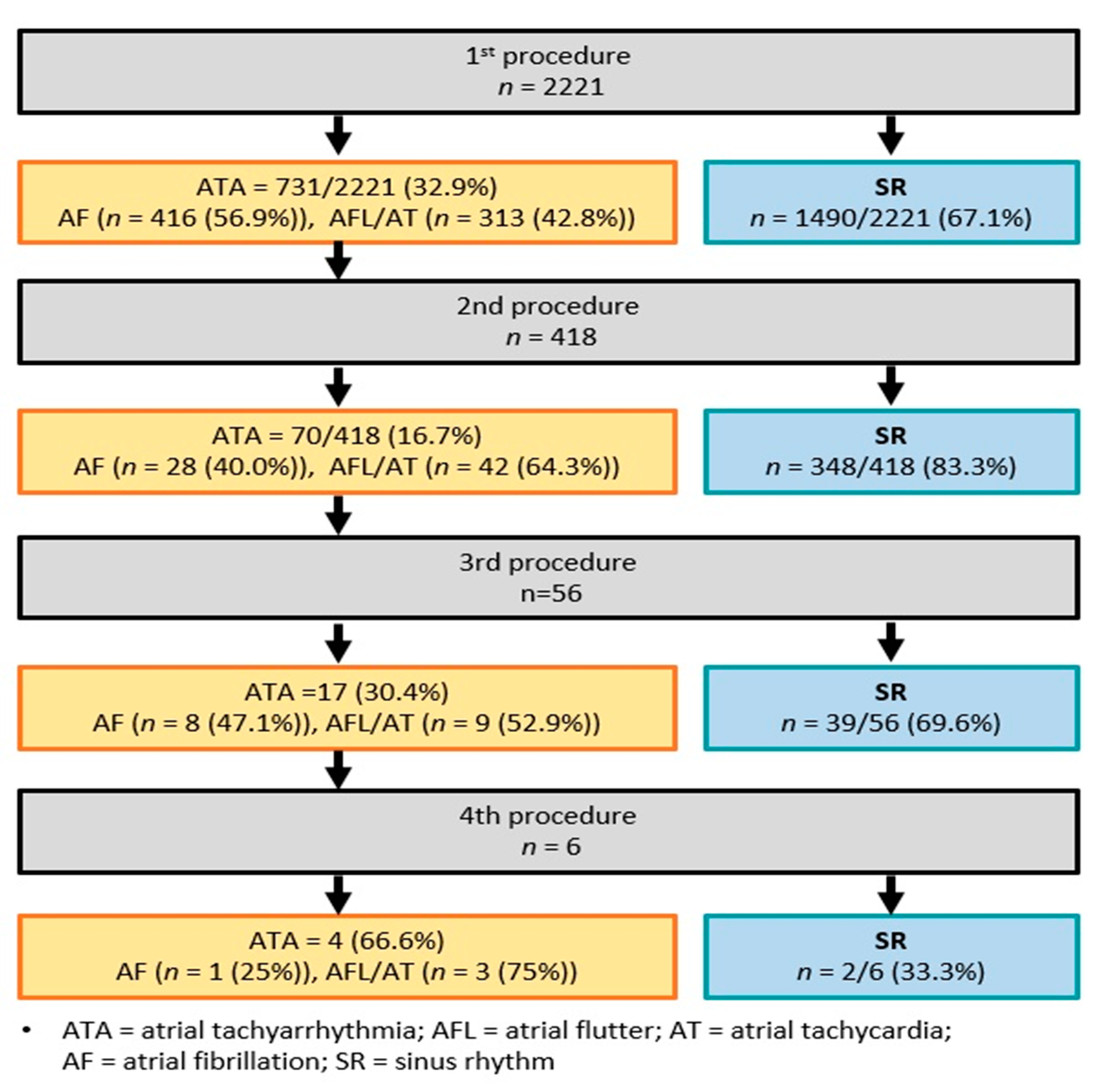
3.2. Radiofrequency Catheter Ablation: Index and Repeat Procedures
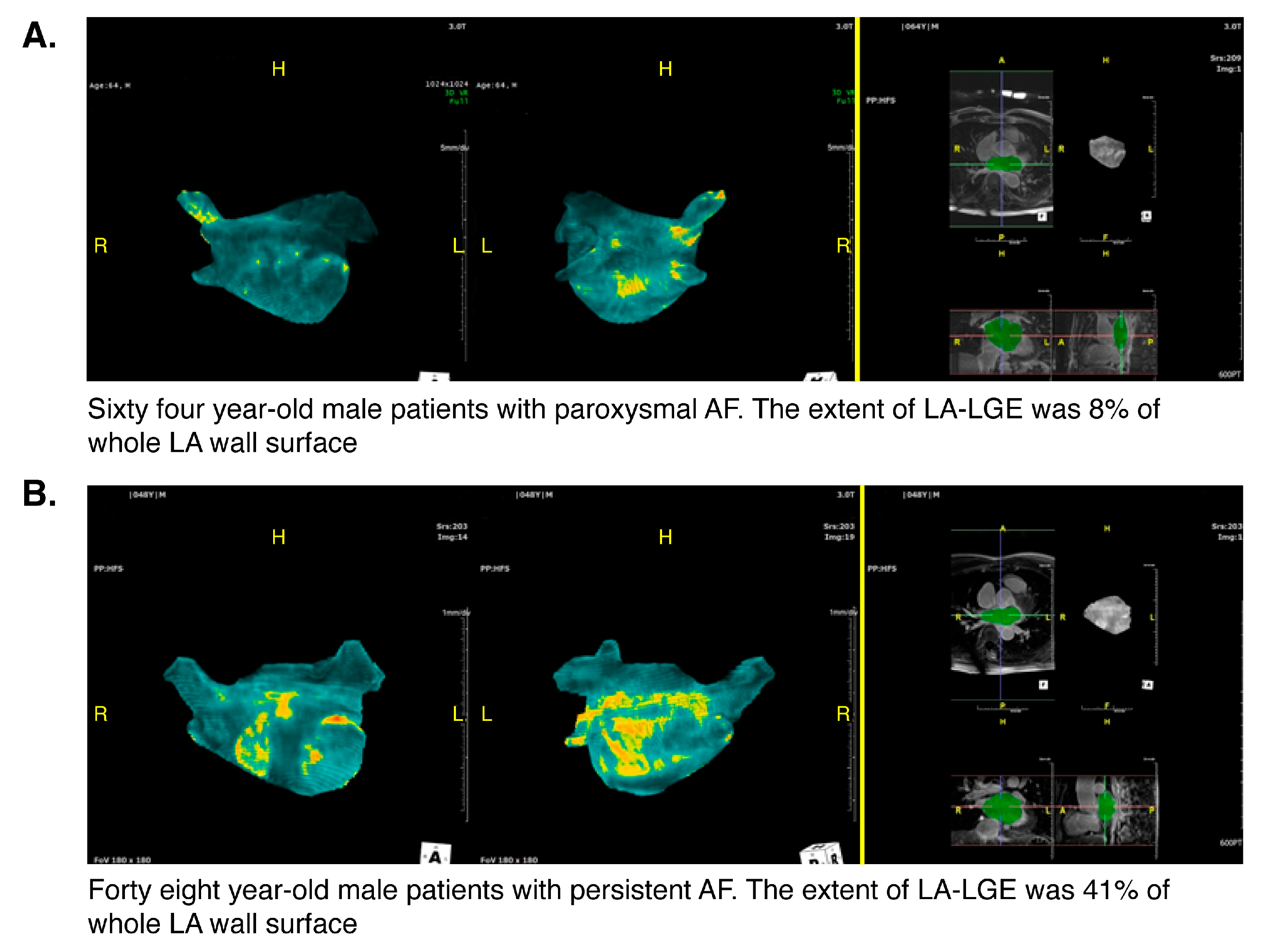
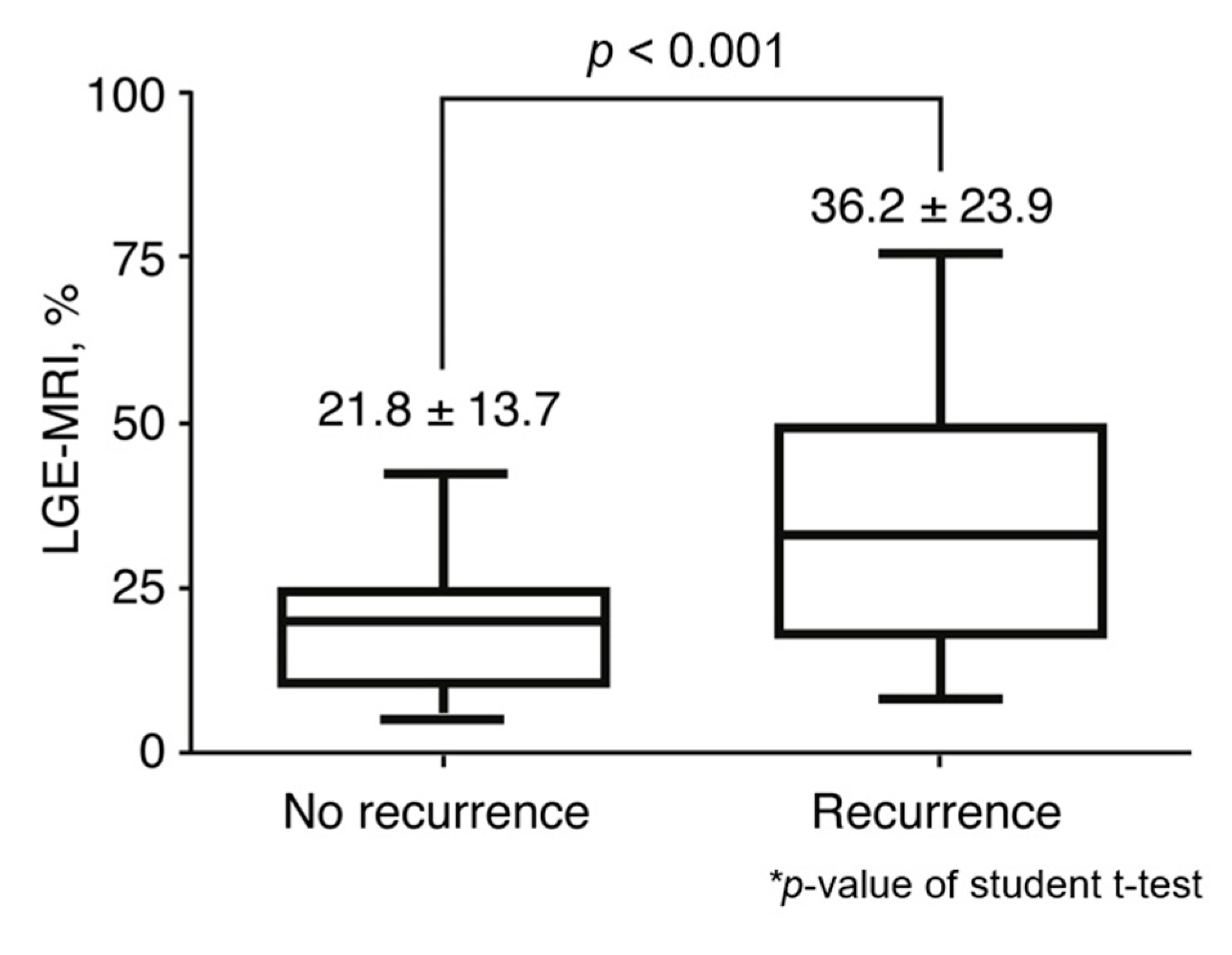
3.3. Relationship between MR LGE and Recurrence
3.4. Predictors of Clinical Recurrence
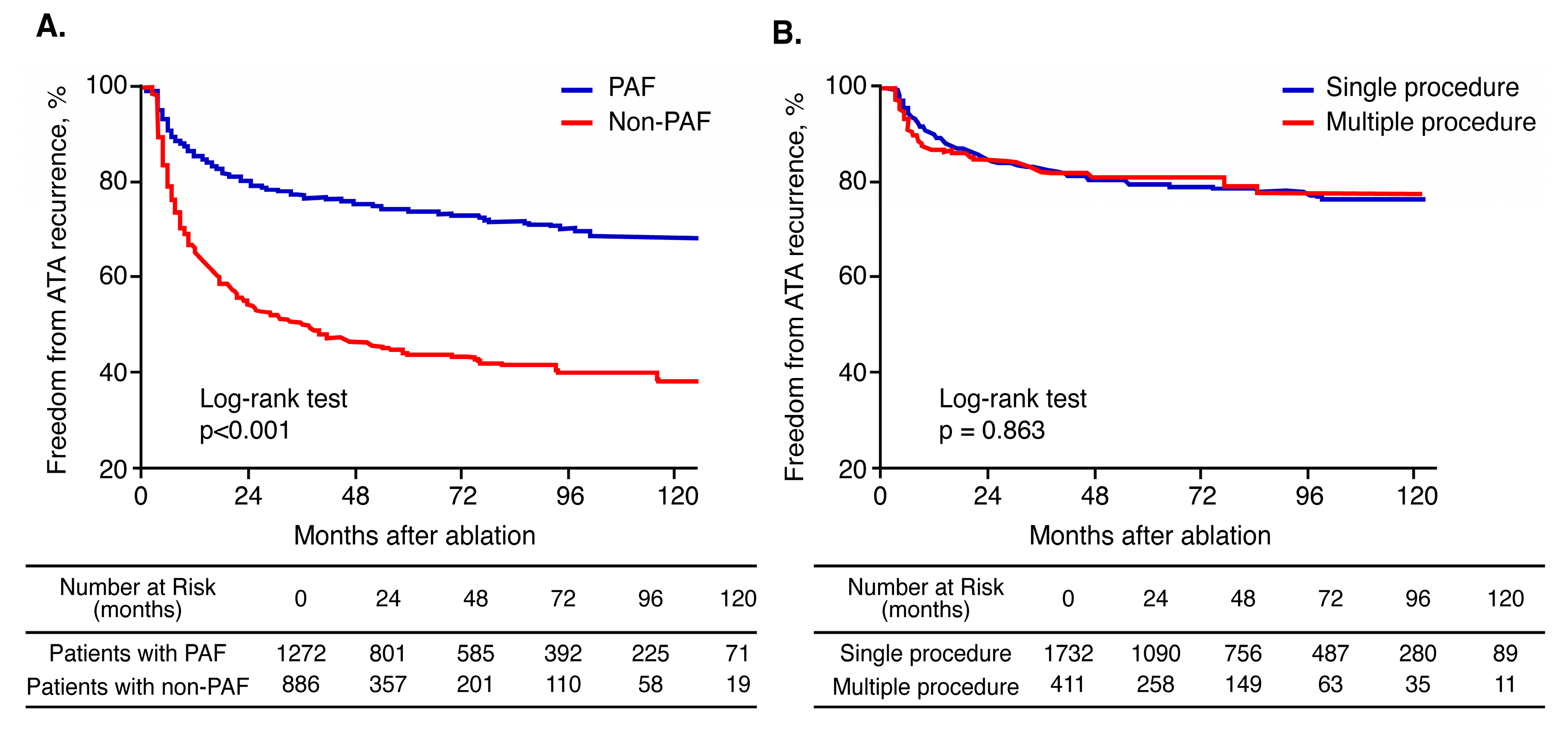
3.5. Effect of Multiple Procedures According to AF Type
4. Discussion
4.1. Main Findings
4.2. Long-Term Clinical Outcomes According to AF Type
4.3. Clinical Predictors of SR Maintenance After Catheter Ablation
4.4. Effect of Atrial Substrate on Clinical Recurrence
4.5. Study Limitations
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Kirchhof, P.; Benussi, S.; Kotecha, D.; Ahlsson, A.; Atar, D.; Casadei, B.; Castellà, M.; Diener, H.-C.; Heidbuchel, H.; Hendriks, J.; et al. 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur. Hear. J. 2016, 37, 2893–2962. [Google Scholar] [CrossRef]
- January, C.T.; Wann, L.S.; Alpert, J.S.; Calkins, H.; Cigarroa, J.E.; Cleveland, J.C.; Conti, J.B.; Ellinor, P.T.; Ezekowitz, M.D.; Field, M.E.; et al. 2014 AHA/ACC/HRS Guideline for the Management of Patients with Atrial Fibrillation. J. Am. Coll. Cardiol. 2014, 64, e1–e76. [Google Scholar] [CrossRef]
- Tilz, R.R.; Heeger, C.-H.; Wick, A.; Saguner, A.M.; Metzner, A.; Rillig, A.; Wohlmuth, P.; Reissmann, B.; Lemes, C.; Maurer, T.; et al. Ten-Year Clinical Outcome After Circumferential Pulmonary Vein Isolation Utilizing the Hamburg Approach in Patients with Symptomatic Drug-Refractory Paroxysmal Atrial Fibrillation. Circ. Arrhythm. Electrophysiol. 2018, 11, e005250. [Google Scholar] [CrossRef]
- Ganesan, A.N.; Shipp, N.J.; Brooks, A.G.; Kuklik, P.; Lau, D.H.; Lim, H.S.; Sullivan, T.; Roberts-Thomson, K.C.; Sanders, P. Long-term Outcomes of Catheter Ablation of Atrial Fibrillation: A Systematic Review and Meta-analysis. J. Am. Hear. Assoc. 2013, 2. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.G.; Newton-Cheh, C.; Almgren, P.; Struck, J.; Morgenthaler, N.G.; Bergmann, A.; Platonov, P.G.; Hedblad, B.; Engström, G.; Wang, T.J.; et al. Assessment of Conventional Cardiovascular Risk Factors and Multiple Biomarkers for the Prediction of Incident Heart Failure and Atrial Fibrillation. J. Am. Coll. Cardiol. 2010, 56, 1712–1719. [Google Scholar] [CrossRef]
- Marrouche, N.F.; Wilber, D.; Hindricks, G.; Jais, P.; Akoum, N.; Marchlinski, F.; Kholmovski, E.; Burgon, N.; Hu, N.; Mont, L.; et al. Association of Atrial Tissue Fibrosis Identified by Delayed Enhancement MRI and Atrial Fibrillation Catheter Ablation. JAMA 2014, 311, 498–506. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.-N.; Roh, S.-Y.; Baek, Y.-S.; Park, H.-S.; Ahn, J.; Kim, D.-H.; Lee, D.I.; Shim, J.; Choi, J.-I.; Park, S.-W.; et al. Long-Term Clinical Comparison of Procedural End Points After Pulmonary Vein Isolation in Paroxysmal Atrial Fibrillation. Circ. Arrhythm. Electrophysiol. 2018, 11. [Google Scholar] [CrossRef] [PubMed]
- Hwang, S.H.; Oh, Y.-W.; Lee, D.I.; Shim, J.; Park, S.-W.; Kim, Y.-H. Relation between left atrial wall composition by late gadolinium enhancement and complex fractionated atrial electrograms in patients with persistent atrial fibrillation: Influence of non-fibrotic substrate in the left atrium. Int. J. Cardiovasc. Imaging 2015, 31, 1191–1199. [Google Scholar] [CrossRef]
- Kim, Y.G.; Shim, J.; Oh, S.-K.; Park, H.-S.; Lee, K.-N.; Hwang, S.H.; Choi, J.-I.; Kim, Y.-H. Different Responses of Left Atrium and Left Atrial Appendage to Radiofrequency Catheter Ablation of Atrial Fibrillation: A Follow Up MRI study. Sci. Rep. 2018, 8, 7871. [Google Scholar] [CrossRef]
- Ouyang, F.; Tilz, R.; Chun, J.; Schmidt, B.; Wissner, E.; Zerm, T.; Neven, K.; Köktürk, B.; Konstantinidou, M.; Metzner, A.; et al. Long-Term Results of Catheter Ablation in Paroxysmal Atrial Fibrillation: Lessons from a 5-Year Follow-Up. Circulation 2010, 122, 2368–2377. [Google Scholar] [CrossRef]
- Münkler, P.; Kröger, S.; Liosis, S.; Abdin, A.; Lyan, E.; Eitel, C.; Eitel, I.; Meyer, C.; Willems, S.; Heeger, C.-H.; et al. Ablation Index for Catheter Ablation of Atrial Fibrillation—Clinical Applicability and Comparison with Force-Time Integral. Circ. J. 2018, 82, 2722–2727. [Google Scholar] [CrossRef] [PubMed]
- Verma, A.; Jiang, C.; Betts, T.R.; Chen, J.; Deisenhofer, I.; Mantovan, R.; Macle, L.; Morillo, C.; Haverkamp, W.; Weerasooriya, R.; et al. Approaches to Catheter Ablation for Persistent Atrial Fibrillation. N. Engl. J. Med. 2015, 372, 1812–1822. [Google Scholar] [CrossRef] [PubMed]
- Scherr, D.; Khairy, P.; Miyazaki, S.; Aurillac-Lavignolle, V.; Pascale, P.; Wilton, S.B.; Ramoul, K.; Komatsu, Y.; Roten, L.; Jadidi, A.; et al. Five-Year Outcome of Catheter Ablation of Persistent Atrial Fibrillation Using Termination of Atrial Fibrillation as a Procedural Endpoint. Circ. Arrhythm. Electrophysiol. 2015, 8, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Garvanski, I.; Simova, I.; Angelkov, L.; Matveev, M. Predictors of Recurrence of AF in Patients After Radiofrequency Ablation. Eur. Cardiol. Rev. 2019, 14, 165–168. [Google Scholar] [CrossRef]
- Sultan, A.; Lüker, J.; Andresen, D.; Kuck, K.H.; Hoffmann, E.; Brachmann, J.; Hochadel, M.; Willems, S.; Eckardt, L.; Lewalter, T.; et al. Predictors of Atrial Fibrillation Recurrence after Catheter Ablation: Data from the German Ablation Registry. Sci. Rep. 2017, 7, 16678. [Google Scholar] [CrossRef]
- Montefusco, A.; Biasco, L.; Blandino, A.; Cristoforetti, Y.; Scaglione, M.; Caponi, D.; Di Donna, P.; Boffano, C.; Cesarani, F.; Coin, D.; et al. Left atrial volume at MRI is the main determinant of outcome after pulmonary vein isolation plus linear lesion ablation for paroxysmal-persistent atrial fibrillation. J. Cardiovasc. Med. 2010, 11, 593–598. [Google Scholar] [CrossRef]
- Tedrow, U.B.; Conen, D.; Ridker, P.M.; Cook, N.R.; Koplan, B.A.; Manson, J.E.; Buring, J.E.; Albert, C.M. The Long- and Short-Term Impact of Elevated Body Mass Index on the Risk of New Atrial Fibrillation. J. Am. Coll. Cardiol. 2010, 55, 2319–2327. [Google Scholar] [CrossRef]
- Cha, Y.-M.; Friedman, P.A.; Asirvatham, S.J.; Shen, W.-K.; Munger, T.M.; Rea, R.F.; Brady, P.A.; Jahangir, A.; Monahan, K.H.; Hodge, D.O.; et al. Catheter Ablation for Atrial Fibrillation in Patients with Obesity. Circulation 2008, 117, 2583–2590. [Google Scholar] [CrossRef]
- Baek, Y.-S.; Yang, P.; Kim, T.-H.; Uhm, J.; Kim, J.; Joung, B.; Lee, M.; Pak, H.-N. Delayed recurrence of atrial fibrillation 2 years after catheter ablation is associated with metabolic syndrome. Int. J. Cardiol. 2016, 223, 276–281. [Google Scholar] [CrossRef]
- Wazni, O.M.; Marrouche, N.F.; Martin, D.O.; Verma, A.; Bhargava, M.; Saliba, W.; Bash, D.; Schweikert, R.; Brachmann, J.; Günther, J.; et al. Radiofrequency Ablation vs. Antiarrhythmic Drugs as First-line Treatment of Symptomatic Atrial Fibrillation. JAMA 2005, 293, 2634–2640. [Google Scholar] [CrossRef]
- Akoum, N.; Daccarett, M.; McGann, C.; Segerson, N.; Vergara, G.; Kuppahally, S.; Badger, T.; Burgon, N.; Haslam, T.; Kholmovski, E.; et al. Atrial Fibrosis Helps Select the Appropriate Patient and Strategy in Catheter Ablation of Atrial Fibrillation: A DE-MRI Guided Approach. J. Cardiovasc. Electrophysiol. 2010, 22, 16–22. [Google Scholar] [CrossRef] [PubMed]
- Kottkamp, H.; Schreiber, D.; Moser, F.; Rieger, A. Therapeutic Approaches to Atrial Fibrillation Ablation Targeting Atrial Fibrosis. JACC Clin. Electrophysiol. 2017, 3, 643–653. [Google Scholar] [CrossRef] [PubMed]
- Ali, R.L.; Hakim, J.B.; Boyle, P.M.; Zahid, S.; Sivasambu, B.; Marine, J.E.; Calkins, H.; Trayanova, N.A.; Spragg, D.D. Arrhythmogenic propensity of the fibrotic substrate after atrial fibrillation ablation: A longitudinal study using magnetic resonance imaging-based atrial models. Cardiovasc. Res. 2019, 115, 1757–1765. [Google Scholar] [CrossRef]
- Fochler, F.; Yamaguchi, T.; Kheirkahan, M.; Kholmovski, E.G.; Morris, A.K.; Marrouche, N.F. Late Gadolinium Enhancement Magnetic Resonance Imaging Guided Treatment of Post-Atrial Fibrillation Ablation Recurrent Arrhythmia. Circ. Arrhythm. Electrophysiol. 2019, 12, e007174. [Google Scholar] [CrossRef] [PubMed]

| Overall | No Recurrence Group | Recurrence Group | * p Value | |
|---|---|---|---|---|
| (n = 2221) | (n = 1490) | (n = 731) | ||
| Age (years) | 55.1 ± 10.8 | 55.0 ± 10.8 | 56.5 ± 10.7 | 0.004 |
| Female, n (%) | 451 (20.3) | 303 (20.3) | 148 (20.2) | 0.961 |
| Non-paroxysmal AF, n (%) | 911 (41.0) | 487 (32.7) | 424 (58.0) | <0.001 |
| Height, cm | 168.2 ± 8.2 | 168.0 ± 8.0 | 168.5 ± 8.1 | 0.269 |
| Weight, kg | 70.8 ± 11.4 | 70.4 ± 11.6 | 71.7 ± 11.1 | 0.040 |
| BSA, m2 | 1.8 ± 0.4 | 1.8 ± 0.5 | 1.8 ± 0.2 | 0.927 |
| BMI, kg/m2 | 25.1 ± 3.4 | 24.9 ± 3.5 | 25.3 ± 3.2 | 0.078 |
| HF, n (%) | 320 (14.4) | 193 (13.0) | 127 (17.4) | 0.005 |
| Hypertension, n (%) | 732 (33.0) | 467 (31.3) | 265 (36.3) | 0.021 |
| DM, n (%) | 171 (7.7) | 103 (6.9) | 68 (9.3) | 0.047 |
| Stroke/TIA, n (%) | 116 (5.2) | 72 (4.8) | 44 (6.0) | 0.237 |
| Vascular disease, n (%) | 104 (4.7) | 76 (5.1) | 28 (3.8) | 0.200 |
| CHA2DS2-VASc score | 1.15 ± 1.22 | 1.09 ± 1.20 | 1.28 ± 1.24 | <0.001 |
| Post-ABL medication | ||||
| Beta-blocker, n (%) | 403 (18.4) | 268 (18.2) | 248 (34.3) | <0.001 |
| Statin, n (%) | 516 (23.5) | 224 (15.2) | 179 (24.8) | <0.001 |
| AAD, n (%) | 342 (15.6) | 192 (13.0) | 150 (20.7) | <0.001 |
| Laboratory findings | ||||
| BUN (mg/dl) | 15.7 ± 4.8 | 15.7 ± 4.9 | 15.6 ± 4.5 | 0.733 |
| Creatinine (mg/dl) | 1.0 ± 0.4 | 1.0 ± 0.4 | 1.0 ± 0.2 | 0.381 |
| Total cholesterol (mg/dl) | 179.7 ± 37.1 | 180.3 ± 35.9 | 177.8 ± 40.4 | 0.419 |
| HDL cholesterol (mg/dl) | 48.2 ± 11.8 | 49.3 ± 12.3 | 49.2 ± 12.2 | 0.843 |
| LDL cholesterol (mg/dl) | 108.0 ± 30.5 | 109.0 ± 30.4 | 105.9 ± 30.4 | 0.053 |
| Triglyceride (mg/dl) | 136.3 ± 83.9 | 136.3 ± 86.1 | 136.3 ± 79.1 | 0.996 |
| HbA1c | 5.9 ± 0.8 | 5.9 ± 0.8 | 5.9 ± 0.7 | 0.465 |
| AF duration, years | 4.6 ± 4.7 | 4.2 ± 4.4 | 5.3 ± 5.2 | <0.001 |
| Overall | No Recurrence Group | Recurrence Group | * p Value | |
|---|---|---|---|---|
| (n = 2221) | (n = 1490) | (n = 731) | ||
| Echocardiographic findings | ||||
| LA dimension (mm) | 41.2 ± 6.1 | 40.4 ± 5.9 | 42.8 ± 6.1 | <0.001 |
| LV ejection fraction (%) | 55.1 ± 5.8 | 55.4 ± 5.6 | 54.4 ± 6.3 | 0.001 |
| LA dimension ≥45 mm (%) | 519 (23.6) | 277 (18.8) | 242 (33.3) | <0.001 |
| Ablation findings | ||||
| Total ablation time (min) | 114 ± 54 | 102 ± 47 | 129 ± 59 | <0.001 |
| Total fluoroscopy time (min) | 68 ± 34 | 63 ± 32 | 76 ± 36 | <0.001 |
| Total procedure time (min) | 275 ± 108 | 252 ± 98 | 309 ± 113 | <0.001 |
| Ablation lesion set | ||||
| CPVI, n (%) | 2221 (100) | 1490 (100) | 731 (100) | NA |
| CTI, n (%) | 1425 (64.0) | 907 (61.0) | 518 (71.0) | <0.001 |
| CFAE, n (%) | 507 (22.8) | 288 (19.3) | 219 (30.0) | <0.001 |
| Linear ablation, n (%) | 268 (12.1) | 131 (8.8) | 137 (18.7) | <0.001 |
| Biatrial ablation, n (%) | 520 (23.0) | 236 (16.0) | 284 (39.0) | <0.001 |
| Univariate | Multivariate Model 1 * | Multivariate Model 2 † | |||||||
|---|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | p Value | HR | 95% CI | p Value | HR | 95% CI | p Value | |
| Age | 1.013 | 1.006–1.020 | <0.001 | 1.007 | 1.000–1.015 | 0.051 | 1.008 | 1.000–1.015 | 0.043 |
| Female | 0.978 | 0.817–1.172 | 0.810 | 1.013 | 0.840–1.222 | 0.890 | 1.013 | 0.840–1.222 | 0.893 |
| Non-paroxysmal AF | 2.587 | 2.231–2.999 | <0.001 | 2.254 | 1.919–2.648 | <0.001 | 2.238 | 1.905–2.629 | <0.001 |
| Overweight or obese | 1.216 | 1.006–1.470 | 0.043 | 1.391 | 1.176–1.937 | 0.018 | 1.314 | 1.107–1.559 | 0.020 |
| HF | 1.355 | 1.119–1.641 | 0.002 | 1.070 | 0.879–1.302 | 0.499 | 1.076 | 0.884–1.310 | 0.464 |
| HTN | 1.212 | 1.042–1.410 | 0.013 | 1.076 | 0.917–1.264 | 0.777 | 1.068 | 0.910–1.254 | 0.421 |
| DM | 1.325 | 1.032–1.700 | 0.027 | 1.231 | 0.955–1.588 | 0.109 | 1.240 | 0.962–1.599 | 0.097 |
| LA dimension ≥45 mm | 1.918 | 1.646–2.234 | <0.001 | 1.279 | 1.005–1.033 | 0.004 | 1.284 | 1.085–1.518 | 0.004 |
| MR LGE ≥25% | 1.022 | 1.014–1.031 | <0.001 | 1.726 | 1.330–2.239 | <0.001 | |||
| AF duration, per year | 1.017 | 1.003–1.031 | 0.017 | 1.019 | 1.005–1.033 | 0.008 | 1.020 | 1.006–1.034 | 0.004 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baek, Y.-S.; Choi, J.-I.; Kim, Y.G.; Lee, K.-N.; Roh, S.-Y.; Ahn, J.; Kim, D.-H.; Lee, D.I.; Hwang, S.H.; Shim, J.; et al. Atrial Substrate Underlies the Recurrence after Catheter Ablation in Patients with Atrial Fibrillation. J. Clin. Med. 2020, 9, 3164. https://doi.org/10.3390/jcm9103164
Baek Y-S, Choi J-I, Kim YG, Lee K-N, Roh S-Y, Ahn J, Kim D-H, Lee DI, Hwang SH, Shim J, et al. Atrial Substrate Underlies the Recurrence after Catheter Ablation in Patients with Atrial Fibrillation. Journal of Clinical Medicine. 2020; 9(10):3164. https://doi.org/10.3390/jcm9103164
Chicago/Turabian StyleBaek, Yong-Soo, Jong-Il Choi, Yun Gi Kim, Kwang-No Lee, Seung-Young Roh, Jinhee Ahn, Dong-Hyeok Kim, Dae In Lee, Sung Ho Hwang, Jaemin Shim, and et al. 2020. "Atrial Substrate Underlies the Recurrence after Catheter Ablation in Patients with Atrial Fibrillation" Journal of Clinical Medicine 9, no. 10: 3164. https://doi.org/10.3390/jcm9103164
APA StyleBaek, Y.-S., Choi, J.-I., Kim, Y. G., Lee, K.-N., Roh, S.-Y., Ahn, J., Kim, D.-H., Lee, D. I., Hwang, S. H., Shim, J., Kim, J. S., Kim, D.-H., Park, S.-W., & Kim, Y.-H. (2020). Atrial Substrate Underlies the Recurrence after Catheter Ablation in Patients with Atrial Fibrillation. Journal of Clinical Medicine, 9(10), 3164. https://doi.org/10.3390/jcm9103164





