Application of A Convolutional Neural Network in The Diagnosis of Gastric Mesenchymal Tumors on Endoscopic Ultrasonography Images
Abstract
1. Introduction
2. Methods
2.1. Training and Test Image Datasets
2.2. Histopathology
2.3. Mesenchymal Tumor Classification Algorithm
2.3.1. Tumor Dataset Generation
2.3.2. Dataset Configuration
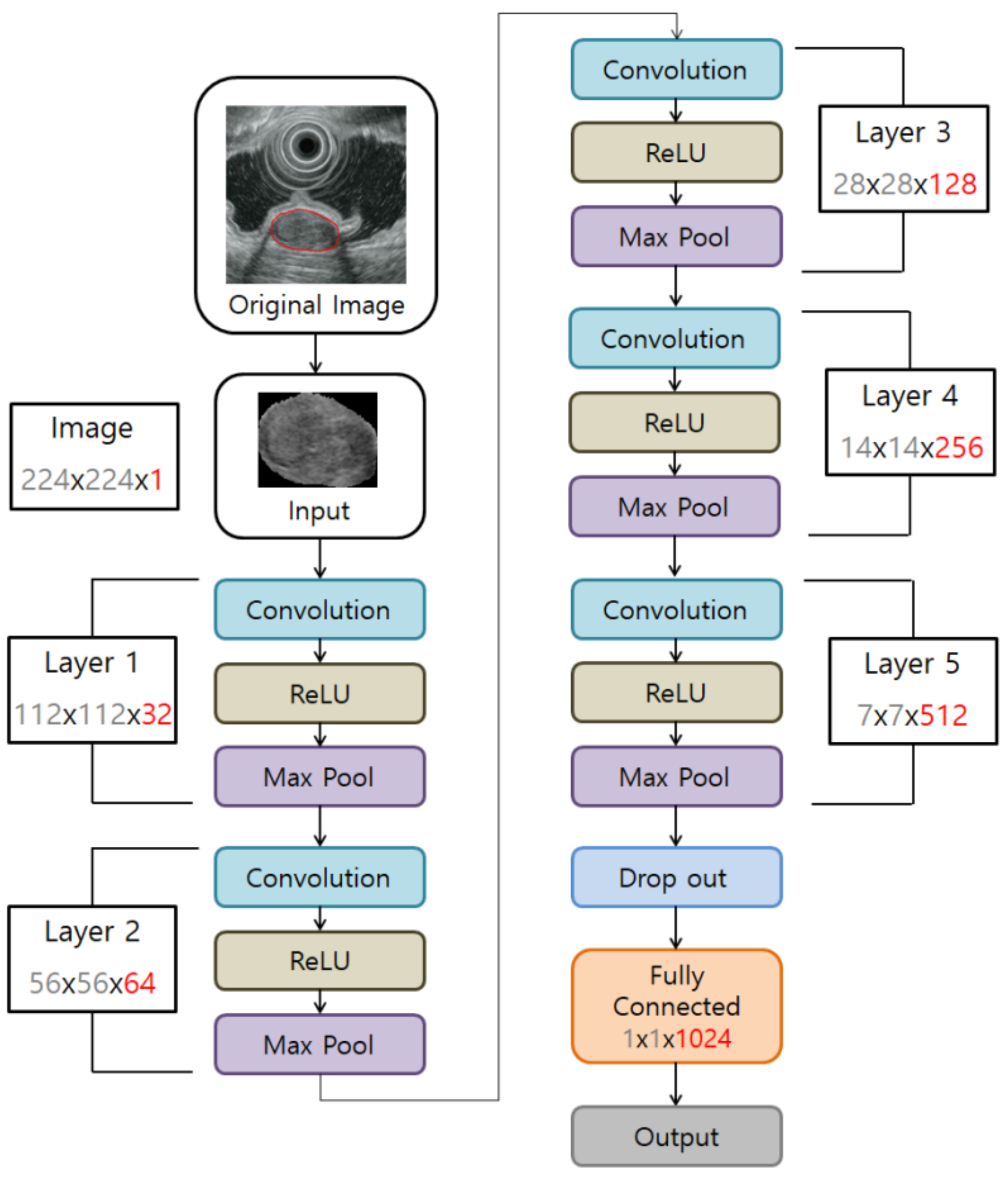
2.3.3. CNN Algorithm Construction to Develop a Tumor Classification Model
2.4. Outcome Measures
2.5. Statistical Analysis
3. Results
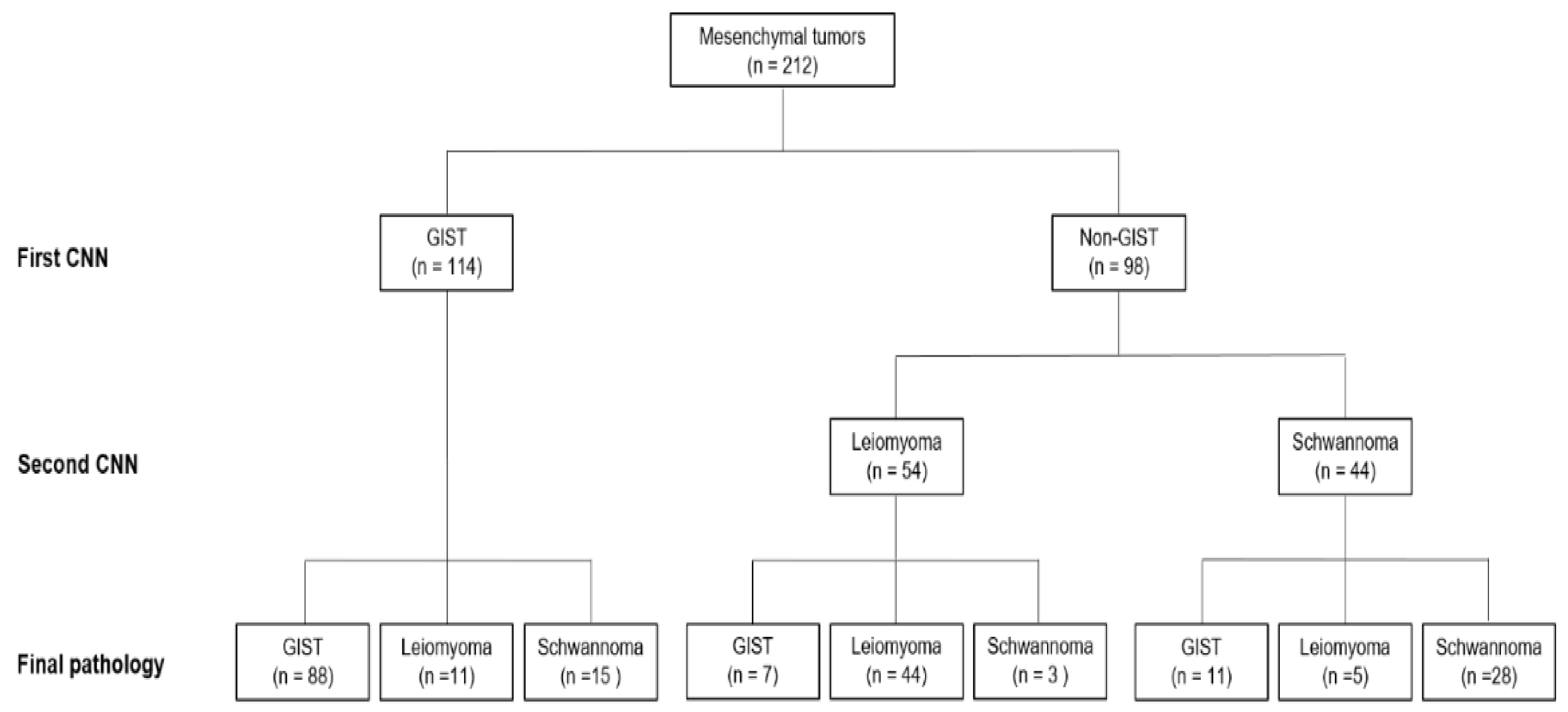
3.1. Performance of the CNN-CAD System in the Training Dataset
3.2. Performance of the CNN-CAD System in the Test Dataset
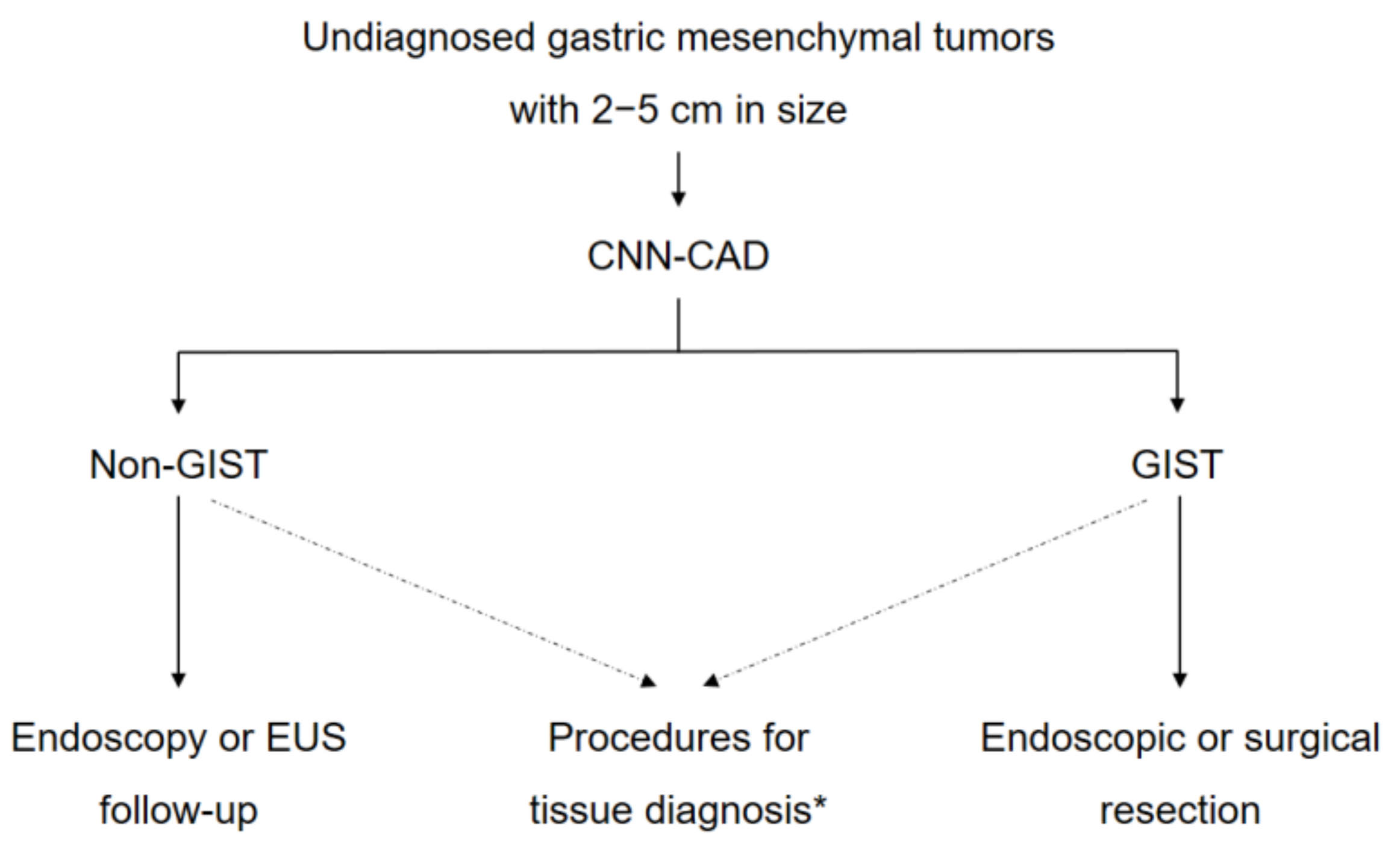
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lee, M.W.; Kim, G.H.; Kim, K.B.; Kim, Y.H.; Park, D.Y.; Choi, C.I.; Kim, D.H.; Jeon, T.Y. Digital image analysis-based scoring system for endoscopic ultrasonography is useful in predicting gastrointestinal stromal tumors. Gastric Cancer 2019, 22, 980–987. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Lee, H.L.; Ahn, Y.W.; Lee, K.N.; Jun, D.W.; Lee, O.Y.; Han, D.S.; Yoon, B.C.; Choi, H.S. Prevalence of gastric subepithelial tumors in Korea: A single center experience. Korean J. Gastroenterol. 2015, 66, 274–276. [Google Scholar] [CrossRef] [PubMed]
- Sarlomo-Rikala, M.; Kovatich, A.J.; Barusevicius, A.; Miettinen, M. CD117: A sensitive marker for gastrointestinal stromal tumors that is more specific than CD34. Mod. Pathol. 1998, 11, 728–734. [Google Scholar]
- Pidhorecky, I.; Cheney, R.T.; Kraybill, W.G.; Gibbs, J.F. Gastrointestinal stromal tumors: Current diagnosis, biologic behavior, and management. Ann. Surg. Oncol. 2000, 7, 705–712. [Google Scholar] [CrossRef] [PubMed]
- Miettinen, M.; Sobin, L.H.; Sarlomo-Rikala, M. Immunohistochemical spectrum of GISTs at different sites and their differential diagnosis with a reference to CD117 (KIT). Mod. Pathol. 2000, 13, 1134–1142. [Google Scholar] [CrossRef] [PubMed]
- Blay, J.Y.; Bonvalot, S.; Casali, P.; Choi, H.; Debiec-Richter, M.; Dei Tos, A.P.; Emile, J.F.; Gronchi, A.; Hogendoorn, P.C.; Joensuu, H.; et al. Consensus meeting for the management of gastrointestinal stromal tumors. Report of the GIST Consensus Conference of 20–21 March 2004, under the auspices of ESMO. Ann. Oncol. 2005, 16, 566–578. [Google Scholar] [CrossRef]
- Miettinen, M.; Sobin, L.H.; Lasota, J. Gastrointestinal stromal tumors of the stomach: A clinicopathologic, immunohistochemical, and molecular genetic study of 1765 cases with long-term follow-up. Am. J. Surg. Pathol. 2005, 29, 52–68. [Google Scholar] [CrossRef]
- Fletcher, C.D.; Berman, J.J.; Corless, C.; Gorstein, F.; Lasota, J.; Longley, B.J.; Miettinen, M.; O’Leary, T.J.; Remotti, H.; Rubin, B.P.; et al. Diagnosis of gastrointestinal stromal tumors: A consensus approach. Hum. Pathol. 2002, 33, 459–465. [Google Scholar] [CrossRef]
- Demetri, G.D.; von Mehren, M.; Antonescu, C.R.; DeMatteo, R.P.; Ganjoo, K.N.; Maki, R.G.; Pisters, P.W.; Raut, C.P.; Riedel, R.F.; Schuetze, S.; et al. NCCN Task Force report: Update on the management of patients with gastrointestinal stromal tumors. J. Natl. Compr. Canc. Netw. 2010, 8 (Suppl. 2), S1–S41, quiz S42–44. [Google Scholar] [CrossRef]
- National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology: Soft Tissue Sarcoma. Version 2.2020. Available online: https://www.nccn.org/professionals/physician_gls/pdf/sarcoma.pdf (accessed on 16 September 2010).
- Chak, A.; Canto, M.I.; Rosch, T.; Dittler, H.J.; Hawes, R.H.; Tio, T.L.; Lightdale, C.J.; Boyce, H.W.; Scheiman, J.; Carpenter, S.L.; et al. Endosonographic differentiation of benign and malignant stromal cell tumors. Gastrointest. Endosc. 1997, 45, 468–473. [Google Scholar] [CrossRef]
- Palazzo, L.; Landi, B.; Cellier, C.; Cuillerier, E.; Roseau, G.; Barbier, J.P. Endosonographic features predictive of benign and malignant gastrointestinal stromal cell tumours. Gut 2000, 46, 88–92. [Google Scholar] [CrossRef] [PubMed]
- Kim, G.H.; Park do, Y.; Kim, S.; Kim, D.H.; Choi, C.W.; Heo, J.; Song, G.A. Is it possible to differentiate gastric GISTs from gastric leiomyomas by EUS? World J. Gastroenterol. 2009, 15, 3376–3381. [Google Scholar] [CrossRef] [PubMed]
- Okai, T.; Minamoto, T.; Ohtsubo, K.; Minato, H.; Kurumaya, H.; Oda, Y.; Mai, M.; Sawabu, N. Endosonographic evaluation of c-kit-positive gastrointestinal stromal tumor. Abdom. Imaging 2003, 28, 301–307. [Google Scholar] [CrossRef] [PubMed]
- Catalano, M.F.; Sivak, M.V., Jr.; Bedford, R.A.; Falk, G.W.; van Stolk, R.; Presa, F.; Van Dam, J. Observer variation and reproducibility of endoscopic ultrasonography. Gastrointest. Endosc. 1995, 41, 115–120. [Google Scholar] [CrossRef]
- Gress, F.; Schmitt, C.; Savides, T.; Faigel, D.O.; Catalano, M.; Wassef, W.; Roubein, L.; Nickl, N.; Ciaccia, D.; Bhutani, M.; et al. Interobserver agreement for EUS in the evaluation and diagnosis of submucosal masses. Gastrointest. Endosc. 2001, 53, 71–76. [Google Scholar] [CrossRef]
- Kim, G.H.; Kim, K.B.; Lee, S.H.; Jeon, H.K.; Park, D.Y.; Jeon, T.Y.; Kim, D.H.; Song, G.A. Digital image analysis of endoscopic ultrasonography is helpful in diagnosing gastric mesenchymal tumors. BMC Gastroenterol. 2014, 14, 7. [Google Scholar] [CrossRef]
- Mori, Y.; Kudo, S.E.; Mohmed, H.E.N.; Misawa, M.; Ogata, N.; Itoh, H.; Oda, M.; Mori, K. Artificial intelligence and upper gastrointestinal endoscopy: Current status and future perspective. Dig. Endosc. 2019, 31, 378–388. [Google Scholar] [CrossRef]
- Ruffle, J.K.; Farmer, A.D.; Aziz, Q. Artificial intelligence-assisted gastroenterology-promises and pitfalls. Am. J. Gastroenterol. 2019, 114, 422–428. [Google Scholar] [CrossRef]
- Krizhevsky, A.; Sutskever, I.; Hinton, G.E. ImageNet classification with deep convolutional neural networks. In NIPS’12 Proceedings of the 25th International Conference on Neural Information Processing Systems, Lake Tahoe, NV, USA, 3–6 December 2012; Volume 1, pp. 1097–1105. [Google Scholar]
- Szegedy, C.; Liu, W.; Jia, Y.; Sermanet, P.; Reed, S.; Anguelov, D.; Erhan, D.; Vanhoucke, V.; Rabinovich, A. Going deeper with convolutions. In Proceedings of the IEEE Conference on Computer Vision and Pattern Recognition, Boston, MA, USA, 7–12 June 2015; pp. 1–9. [Google Scholar]
- Park, C.H.; Kim, G.H.; Lee, B.E.; Song, G.A.; Park, D.Y.; Choi, K.U.; Kim, D.H.; Jeon, T.Y. Two staging systems for gastrointestinal stromal tumors in the stomach: Which is better? BMC Gastroenterol. 2017, 17, 141. [Google Scholar] [CrossRef]
- Wong, S.C.; Gatt, A.; Stamatescu, V.; McDonnell, M.D. Understanding data augmentation for classification: When to warp? In Proceedings of the 2016 International Conference on Digital Image Computing: Techniques and Applications (DICTA), Gold Coast, QLD, Australia, 30 November–2 December 2016; pp. 1–6. [Google Scholar]
- Ribeiro, E.; Uhl, A.; Wimmer, G.; Hafner, M. Exploring deep learning and transfer learning for colonic polyp classification. Comput. Math. Methods Med. 2016, 2016, 6584725. [Google Scholar] [CrossRef]
- Zhu, Y.; Wang, Q.C.; Xu, M.D.; Zhang, Z.; Cheng, J.; Zhong, Y.S.; Zhang, Y.Q.; Chen, W.F.; Yao, L.Q.; Zhou, P.H.; et al. Application of convolutional neural network in the diagnosis of the invasion depth of gastric cancer based on conventional endoscopy. Gastrointest. Endosc. 2019, 89, 806–815. [Google Scholar] [CrossRef] [PubMed]
- Yoon, J.M.; Kim, G.H.; Park, D.Y.; Shin, N.R.; Ahn, S.; Park, C.H.; Lee, J.S.; Lee, K.J.; Lee, B.E.; Song, G.A. Endosonographic features of gastric schwannoma: A single center experience. Clin. Endosc. 2016, 49, 548–554. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.H.; Rulyak, S.D.; Kimmey, M.B.; American Gastroenterological Association Institute. American Gastroenterological Association Institute technical review on the management of gastric subepithelial masses. Gastroenterology 2006, 130, 2217–2228. [Google Scholar] [CrossRef] [PubMed]
- Nishida, T.; Blay, J.Y.; Hirota, S.; Kitagawa, Y.; Kang, Y.K. The standard diagnosis, treatment, and follow-up of gastrointestinal stromal tumors based on guidelines. Gastric Cancer 2016, 19, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Hirasawa, T.; Aoyama, K.; Tanimoto, T.; Ishihara, S.; Shichijo, S.; Ozawa, T.; Ohnishi, T.; Fujishiro, M.; Matsuo, K.; Fujisaki, J.; et al. Application of artificial intelligence using a convolutional neural network for detecting gastric cancer in endoscopic images. Gastric Cancer 2018, 21, 653–660. [Google Scholar] [CrossRef] [PubMed]

| Characteristics | Training Dataset (n = 179) | Test Dataset (n = 69) |
|---|---|---|
| Age, Years (mean ± SD) | 57.7 ± 11.9 | 56.9 ± 13.7 |
| Sex | ||
| Male | 80 (44.7) | 31 (44.9) |
| Female | 99 (55.3) | 38 (55.1) |
| Tumor Location | ||
| Upper | 105 (58.7) | 43 (62.3) |
| Middle | 57 (31.8) | 21 (30.4) |
| Lower | 17 (9.5) | 5 (7.2) |
| Tumor Size, cm (mean ± SD) | 3.6 ± 2.1 | 3.2 ± 1.6 |
| Final Histopathology | ||
| Leiomyoma | 33 (18.4) | 23 (33.3) |
| Schwannoma | 21 (11.7) | 14 (20.3) |
| Gastrointestinal Stromal Tumor | 125 (69.8) | 32 (46.4) |
| Very Low Risk | 27 | 7 |
| Low Risk | 50 | 14 |
| Intermediate Risk | 29 | 7 |
| High Risk | 19 | 4 |
| CNN-CAD System | Endoscopists | ||||||
|---|---|---|---|---|---|---|---|
| Experienced 1 | Experienced 2 | Experienced 3 | Junior 1 | Junior 2 | Junior 3 | ||
| Sensitivity, % | 83.0 (77.4–87.5) | 83.0 (77.4–87.5) | 74.5 (68.3–79.9) | 71.7 (65.3–77.3) | 84.0 (78.4–88.3) | 73.6 (67.3–79.1) | 84.9 (79.5–89.1) |
| Specificity, % | 75.5 (69.3–80.8) | 68.9 (62.3–74.7) | 61.3 * (54.6–67.6) | 56.6 * (49.9–63.1) | 63.2 (56.5–69.4) | 77.4 (71.3–82.5) | 53.8 * (47.1–60.4) |
| Positive predictive value, % | 77.2 (71.1–82.3) | 72.7 (66.4–78.3) | 65.8 (59.2–71.9) | 62.3 * (55.6–68.5) | 69.5 (63.0–75.3) | 76.5 (70.3–81.7) | 64.7 * (58.1–70.9) |
| Negative predictive value, % | 81.6 (75.9–86.3) | 80.2 (74.3–85.0) | 70.7 (64.2–76.4) | 66.7 * (60.1–72.7) | 79.8 (73.8–84.6) | 74.5 (68.3–79.9) | 78.1 (72.0–83.1) |
| Accuracy, % | 79.2 (73.3–84.2) | 75.9 (69.8–81.2) | 67.9 * (61.4–73.8) | 64.2 * (57.5–70.3) | 73.6 (67.3–79.1) | 75.5 (69.3–80.8) | 69.3 * (62.8–75.2) |
| CNN-CAD System | Endoscopists | ||||||
|---|---|---|---|---|---|---|---|
| Experienced 1 | Experienced 2 | Experienced 3 | Junior 1 | Junior 2 | Junior 3 | ||
| Accuracy, % | 75.5 (69.3–80.8) | 72.6 (66.3–78.2) | 61.8 * (55.1–68.1) | 59.0 * (52.2–65.4) | 67.0 (60.4–73.0) | 68.4 (61.9–74.3) | 66.0 * (59.4–72.1) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, Y.H.; Kim, G.H.; Kim, K.B.; Lee, M.W.; Lee, B.E.; Baek, D.H.; Kim, D.H.; Park, J.C. Application of A Convolutional Neural Network in The Diagnosis of Gastric Mesenchymal Tumors on Endoscopic Ultrasonography Images. J. Clin. Med. 2020, 9, 3162. https://doi.org/10.3390/jcm9103162
Kim YH, Kim GH, Kim KB, Lee MW, Lee BE, Baek DH, Kim DH, Park JC. Application of A Convolutional Neural Network in The Diagnosis of Gastric Mesenchymal Tumors on Endoscopic Ultrasonography Images. Journal of Clinical Medicine. 2020; 9(10):3162. https://doi.org/10.3390/jcm9103162
Chicago/Turabian StyleKim, Yoon Ho, Gwang Ha Kim, Kwang Baek Kim, Moon Won Lee, Bong Eun Lee, Dong Hoon Baek, Do Hoon Kim, and Jun Chul Park. 2020. "Application of A Convolutional Neural Network in The Diagnosis of Gastric Mesenchymal Tumors on Endoscopic Ultrasonography Images" Journal of Clinical Medicine 9, no. 10: 3162. https://doi.org/10.3390/jcm9103162
APA StyleKim, Y. H., Kim, G. H., Kim, K. B., Lee, M. W., Lee, B. E., Baek, D. H., Kim, D. H., & Park, J. C. (2020). Application of A Convolutional Neural Network in The Diagnosis of Gastric Mesenchymal Tumors on Endoscopic Ultrasonography Images. Journal of Clinical Medicine, 9(10), 3162. https://doi.org/10.3390/jcm9103162







