Abstract
Despite the availability of new therapies that have led to improved outcomes for patients with multiple myeloma, most patients will eventually relapse. With triplet and even quadruplet combination therapies becoming standard in the first and second line, many patients will have few treatment options after second-line treatment. Melflufen (melphalan flufenamide) is a first-in-class peptide–drug conjugate (PDC) that targets aminopeptidases and rapidly releases alkylating agents into tumor cells. Once inside the tumor cells, melflufen is hydrolyzed by peptidases to release alkylator molecules, which become entrapped. Melflufen showed anti-myeloma activity in myeloma cells that were resistant to bortezomib and the alkylator melphalan. In early phase studies (O-12-M1 and HORIZON [OP-106]), melflufen plus dexamethasone has demonstrated encouraging clinical activity and a manageable safety profile in heavily pretreated patients with relapsed/refractory multiple myeloma, including those with triple-class refractory disease and extramedullary disease. The Phase III OCEAN study (OP-104) is further evaluating melflufen plus dexamethasone in patients with relapsed/refractory multiple myeloma. The safety profile of melflufen is characterized primarily by clinically manageable hematologic adverse events. Melflufen, with its novel mechanism of action, has the potential to provide clinically meaningful benefits to patients with relapsed/refractory multiple myeloma, including those with high unmet needs.
1. Introduction
Despite improved outcomes in patients with multiple myeloma following the advent of proteasome inhibitors, immunomodulatory agents (IMiDs), and anti-CD38 monoclonal antibodies, the majority of patients with multiple myeloma will eventually relapse [1,2]. For younger, fit patients with multiple myeloma, the current frontline therapy includes a proteasome inhibitor plus dexamethasone in triplet combination, often with an IMiD such as thalidomide or lenalidomide, followed by autologous stem cell transplant and lenalidomide maintenance therapy [3,4,5]. Quadruplet regimens consisting of anti-CD38 monoclonal antibodies in combination with an IMiD, a proteasome inhibitor, and a steroid in frontline therapy are also used in some patients [6,7,8]. For patients with newly diagnosed multiple myeloma who are not eligible for stem cell transplant, several multi-agent regimens are recommended. Most of these regimens are based on bortezomib plus dexamethasone or lenalidomide plus dexamethasone as a backbone [9,10,11,12,13]. Several new combinations that have been approved in first-line therapy for patients who are not eligible to receive a stem cell transplant include triplet combination regimens with daratumumab plus lenalidomide and dexamethasone as well as quadruplet regimens with daratumumab plus bortezomib in combination with melphalan and prednisone or thalidomide and dexamethasone [8,14].
Treatment choice following relapse is largely dependent on prior received therapy and prior response to therapy, with class switching often prioritized [15]. Because multiple myeloma is a heterogeneous disease [16] and sequential therapeutic intervention is required to maintain disease control, additional mutations develop throughout the course of the disease, many of which drive resistance to therapy [17,18]. Furthermore, patients are receiving several drug classes during upfront therapy and the use of newer drugs has moved to earlier lines of therapy, resulting in many patients being faced with disease that is refractory to multiple drug classes and multiple drugs within each class after second-line therapy [2,3,15]. Importantly, treatment duration and time to disease progression get progressively shorter with subsequent lines of therapy, while the frequency of toxicities and comorbidities become higher [19].
Thus, new therapies with novel mechanisms of action that are also tolerable are needed for third-line treatment and beyond, particularly for patients with relapsed and/or refractory multiple myeloma who have disease that is refractory to standard-of-care agents including IMiDs, proteasome inhibitors, and anti-CD38 antibodies. Several new agents with novel mechanisms of action are currently under investigation.
The US Food and Drug Administration granted accelerated approval to selinexor in July 2019, a selective inhibitor of exportin 1, in combination with dexamethasone for the treatment of adult patients with relapsed/refractory multiple myeloma who have received ≥4 prior lines of therapy and whose disease is penta-refractory (i.e., ≥2 proteasome inhibitors, ≥2 IMiDs, and ≥1 anti-CD38 monoclonal antibody) [20]. Approval was based on the results from the STORM study, a Phase II study of selinexor plus dexamethasone in heavily pretreated patients with triple-class refractory multiple myeloma (refractory to an IMiD, a proteasome inhibitor, and an anti-CD38 monoclonal antibody) [21].
Venetoclax, a Bcl-2 inhibitor, has shown promising efficacy and an acceptable safety profile when given in combination with bortezomib and dexamethasone in patients with relapsed/refractory multiple myeloma in a Phase Ib study (n = 66) [22]. Additional studies are ongoing [23]. Another mechanism of action being explored is the targeting of B cell maturation antigen (BCMA), which is an antigen with expression that is primarily restricted to late stages of B cell differentiation (e.g., late memory B cells and plasma cells) and that is also expressed at high levels in malignant multiple myeloma cells [24]. Immunotherapies targeting BCMAs include anti-BCMA antibody–drug conjugates, bispecific antibodies, bispecific T cell engagers (BiTEs), and chimeric antigen receptor (CAR) T cells [24,25,26]. In the Phase II DREAMM-2 study, belantamab mafodotin, an anti-BCMA antibody–drug conjugate, has demonstrated single-agent activity and a manageable safety profile in patients with relapsed/refractory multiple myeloma who had received ≥3 lines of therapy and were refractory to an IMiD, a proteasome inhibitor, and an anti-CD38 antibody (n = 196) [26]. The overall response rate (ORR) was 33% across two different doses of belantamab mafodotin (2.5 and 3.4 mg/kg) and the median progression-free survival (PFS) was 3 months with a 2.5 kg/mg dose and 5 months with a 3.4 kg/mg dose with a short duration of follow-up (median: 6.3 and 6.9 months, respectively). Hematologic events (thrombocytopenia, 27%; anemia, 23%) and keratopathy (24%) were the most commonly reported Grade 3/4 adverse events (AEs) [26]. Several types of CAR T cells have been evaluated in a total of >300 patients with relapsed/refractory multiple myeloma in early phase studies [25]. CAR T cells have shown promising clinical activity with high response rates (ranging from 20–100%; most were ≥80%) in these Phase I studies [25]. For example, in a Phase I study, idecabtagene vicleucel (bb2121) showed an 85% ORR among 33 evaluable patients with a median duration of response of 10.9 months and a median PFS of 11.8 months. Hematologic events were the most common Grade 3/4 AEs (neutropenia, 85%; leukopenia, 58%; anemia, 45%; thrombocytopenia, 45%) [27].
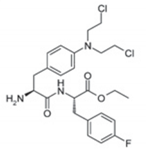
Melphalan flufenamide (melflufen) is a first-in-class peptide–drug conjugate (PDC) that targets aminopeptidases and rapidly releases alkylating agents into tumor cells [28,29,30,31,32]. Because of its distinct mechanism of action, melflufen is well suited for use in third-line therapy both in patients who have not received prior alkylator therapy, such as older patients and those who are not eligible for transplant, as well as in patients who have prior exposure to melphalan [29]. This review describes the historical development of melflufen, its mechanism of action, and the ongoing clinical development program for melflufen in multiple myeloma.
2. Preclinical Development
2.1. Mechanism of Action
Melflufen is a PDC that is being investigated in multiple myeloma and other hematologic malignancies, immunoglobulin light chain (AL) amyloidosis, and solid tumors [29,33,34].
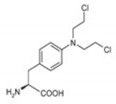
Melflufen is highly lipophilic, which promotes its rapid uptake by cells [29,30,31,35] (Figure 1). Once within the cell, melflufen releases its hydrophilic alkylator payloads via the hydrolytic activity of intracellular peptidases (e.g., aminopeptidases) [30]. Aminopeptidases are Zn2+-dependent metalloproteinases that remove amino acids at the N-terminal position from oligopeptides and have been associated with multiple tumorigenic processes such as proliferation, apoptosis, differentiation, angiogenesis, and motility [36,37].
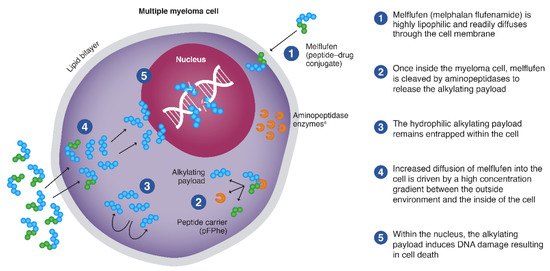
Figure 1.
Mechanism of action of melflufen. Melflufen readily diffuses through the cell membrane into the myeloma cell, where it is cleaved by aminopeptidases to release the alkylating payload that are entrapped. a Aminopeptidases are proteolytic enzymes expressed in cancer cells, including multiple myeloma cells. pFPhe, p-L fluoro-phenylalanine ethyl ester. From Oriol A, et al. Expert Opin Invest Drugs. 2020 Sep 14 [online ahead of print] doi: 10.1080/13543784.2020.1808884. Copyright © 2020 Taylor & Francis. Reprinted with permission from Taylor & Francis Ltd.
The dependence of melflufen on aminopeptidases was initially demonstrated by the reduced cytotoxic activity of melflufen—but not the alkylator melphalan—when cells were pretreated with bestatin, an antibiotic that is a potent aminopeptidase inhibitor [31]. In addition, structure analogs designed to resist peptide hydrolysis (N-methyl derivative and derivative with d-amino acid) were shown to be almost 100-fold less potent than melflufen [31]. Subsequent in vitro studies demonstrated that hydrolytic cleavage of melflufen by aminopeptidases releases alkylator payloads, including melphalan [30]. In vitro, the activity of melflufen is multi-pronged, including induction of DNA damage, induction of apoptosis, inhibition of VEGF-dependent cell migration, and inhibition of tumor-associated angiogenesis, which have been further reviewed elsewhere [29]. Downregulation of aminopeptidases resulted in reduced melflufen-mediated cytotoxic activity and apoptotic signaling in cultured cells [30].
2.2. Preclinical Anti-Tumor Activity
The anti-tumor activity of melflufen in multiple primary cultures of human cancer and leukemia cells, as well as established cell lines, was first reported in 2003 [31,33]. Compared with the known alkylator melphalan, melflufen had a higher cytotoxic activity in this broad range of malignant human cells, with a mean IC50 value that was 35-fold lower with melflufen than melphalan [29] (Table 1). In cells from hematological malignancies, the higher potency of melflufen compared with melphalan was even more pronounced (mean IC50 values were ≈50-fold lower with melflufen) [28,29,38,39]. Primary cultures of patient-derived acute myeloid leukemia cells were 7-fold more sensitive than normal peripheral blood mononuclear cells, indicating a 7-fold in vitro therapeutic index [39].

Table 1.
Comparison between melflufen and the known alkylator melphalan [30,33,40].
Melflufen also has a higher lipophilicity than melphalan, and peak intracellular concentration of melphalan can be achieved much faster with melflufen than melphalan [30,33,40]. More recently, the anti-tumor activity of melflufen has been reported in ovarian cancer, breast cancer, osteosarcoma, acute myeloid leukemia, neuroblastoma, and multiple myeloma cell lines [28,39,41,42,43,44]. In addition, melflufen demonstrated anti-tumor activity in a multiple myeloma xenograft model [28] and a genetically engineered myeloma model in transgenic immunocompetent Vk*MYK mice, postulated to be predictive of clinical activity [45].
Melflufen is rapidly taken up into cells, with the maximum concentration of intracellular melphalan reached within 15 min and full therapeutic activity obtained after 30 min of exposure in vitro, which is faster than that of melphalan [30,31]. In multiple myeloma cells, a high concentration of intracellular melphalan can be reached with a lower dose of drug (5 μM melflufen vs. 100 μM melphalan) [28]. In patient-derived myeloma tumor samples, melflufen demonstrated ≈50-fold higher cytotoxicity than melphalan, and a 50-fold higher melphalan exposure than direct administration of melphalan [42]. Melflufen has demonstrated anti-tumor activity against multiple myeloma cells that show resistance to melphalan, bortezomib, and dexamethasone. This activity likely stems from the multiple downstream effects elicited by melflufen, including induction of apoptosis and triggering rapid, robust, and irreversible DNA damage [28,32]. Unlike with melphalan, the DNA damage induced by melflufen is not dependent on activation of p53 [28], which likely contributes to the activity of melflufen in melphalan-resistant cells. In patients with multiple myeloma who have the 17p13 [del(17p)] adverse risk genotype, mutations/deletions in TP53 are found in approximately one-third of newly diagnosed patients and at least 50% of those with relapsed and refractory disease. Mutations in TP53 confer a poor prognosis and are associated with resistance to therapy [46].
Melflufen has also demonstrated synergistic activity when combined with standard-of-care agents in myeloma such as dexamethasone in dexamethasone-sensitive multiple myeloma cell lines [28] and bortezomib and lenalidomide in cell lines that were resistant to standard-of-care drugs [28,42]. Melflufen has anti-angiogenic activity and inhibits cell migration in multiple myeloma cells [28]. The potent anti-angiogenic effect of melflufen has been demonstrated in multiple in vitro and in vivo models [47]. There is some evidence that melflufen can also overcome the cytoprotective effects of the bone marrow microenvironment [28]. These lines of evidence show that (1) high intracellular concentrations of alkylator can be achieved with melflufen; (2) melflufen is highly cytotoxic and more potent than melphalan; (3) melflufen has broad anti-tumor activity in multiple myeloma with no apparent cross-resistance to other drugs; and (4) melflufen shows synergistic activity with standard- of-care agents, and together support the potential for melflufen as a myeloma therapy.
3. Pharmacokinetics
The pharmacokinetics of melflufen were first evaluated in an open-label, multicenter, dose-finding Phase I/II study in patients with solid tumors [48]. A total of 29 patients were evaluated for pharmacokinetics. Most patients received 50 mg melflufen, but doses ranged from 25 to 130 mg. Melflufen and melphalan (resulting from aminopeptidase cleavage of melflufen) concentrations were assessed before the start of infusion and then at several intervals up to 360 min after the start of the infusion, including at the end of infusion, which occurred at 30 min. Among patients who received 50 mg melflufen, the peak plasma concentration (Cmax) of melflufen was generally observed right before the end of infusion, whereas the Cmax of melphalan was observed 5 to 15 min after the end of infusion (Table 2). The release of melphalan following infusion with melflufen was rapid, as suggested by the fact that the Cmax and area under the curve over the time of infusion (AUC0–0.5) were higher for melphalan than melflufen. The elimination and clearance of melphalan was not affected by body weight or sex.

Table 2.
Pharmacokinetics of melflufen and melphalan following administration with 50 mg melflufen [48].
BRIDGE (OP-107) is a Phase II study evaluating the pharmacokinetics of melphalan during treatment with melflufen and dexamethasone in patients with relapsed/refractory multiple myeloma and moderate to severely impaired renal function (NCT03639610). Given that renal impairment is a common complication in patients with multiple myeloma, occurring in ≈50% of patients with multiple myeloma [49], the results from BRIDGE will be of relevance for patients with multiple myeloma and renal insufficiency. Because melflufen is rapidly and completely metabolized by aminopeptidases and melphalan is primarily eliminated from the plasma by spontaneous hydrolysis [31,50]—a process independent of renal function and hepatic metabolism—the hypothesis is that renal impairment will have no effect on melflufen pharmacokinetics and only a minor effect on melphalan pharmacokinetics.
4. Clinical Development
4.1. Early Development in Multiple Myeloma
Melflufen was first evaluated clinically in multiple myeloma in O-12-M1 (NCT01897714), a Phase I/II, multicenter, dose-escalation and dose-expansion study of melflufen with or without dexamethasone in patients with relapsed/refractory multiple myeloma who had received ≥2 prior lines of therapy, including lenalidomide and bortezomib, and were refractory to the last line of therapy [51]. A total of 75 heavily pretreated patients were enrolled in the study. In the Phase I dose-finding portion of the study, melflufen was administered intravenously over 30 min on Day 1 of each 21-day cycle. A total of 4 four dose levels of melflufen (15, 25, 40, and 55 mg) were assessed. At the 55 mg dose, 4 of 6 patients experienced Grade 4 dose-limiting hematologic toxicities. Therefore, the recommended dose for expansion was 40 mg melflufen. Among 58 patients treated at the recommended dose in the Phase II portion of the study, 13 received single-agent melflufen and 45 received melflufen in combination with 40 mg dexamethasone weekly. Of the 45 patients who received the combination therapy, 28 initiated treatment with 21-day cycles, but the Data Safety Monitoring Committee recommended increasing the cycle length to 28 days to prolong the hematologic recovery time between cycles. An additional 17 patients started treatment with a 28-day cycles. Among the 45 patients who received the combination therapy, the median number of prior therapies was 4 (range, 2–14) and 67% of patients were refractory to a proteasome inhibitor and an IMiD. The ORR (≥partial response (PR)) was 31%, with 5 patients achieving a very good PR (VGPR) and 9 patients achieving a PR (Table 3) with a median duration of response of 8.4 months [51]. The ORR was 41% among patients who received ≥2 doses of study treatment and had a post-baseline response assessment (n = 34). Responses (≥minimal response) were also observed in 4 of 9 patients with melphalan-refractory disease. At a median follow-up of 27.9 months, the median PFS was 5.7 months and the median overall survival (OS) was 20.7 months. In a subsequent analysis, with a median follow-up of 46 months, the median OS was also 20.7 months [52].

Table 3.
Efficacy of melflufen combination therapies in patients with multi-refractory multiple myeloma [51,52,56,57].
Melflufen plus dexamethasone was generally manageable in this heavily pretreated patient population [51]. All patients experienced ≥1 AE, most commonly hematologic AEs including thrombocytopenia (73%), neutropenia (69%), and anemia (64%). The most common non-hematologic AEs included pyrexia (40%), asthenia (31%), fatigue (29%), nausea (27%), and diarrhea (24%). Melflufen-related Grade ≥3 AEs occurred in 82% of patients, most commonly reversible thrombocytopenia and neutropenia. The incidence of Grade 4 thrombocytopenia was reduced from 32% to 0%, and the median duration of study treatment increased (from 105 to 182 days) after the study cycle was lengthened from 21 to 28 days. The most common Grade 3/4 non-hematologic AEs were asthenia, pneumonia, and hyperglycemia, and C-reactive protein increase (7% each). Serious AEs (SAEs) occurred in 38% of patients and were considered by the investigator to be related to melflufen in 27% of patients. The most common SAE was pneumonia. Overall, the safety profile of melflufen plus dexamethasone is generally comparable to that of other doublet combinations in patients with heavily pretreated relapsed/refractory multiple myeloma [53,54], with hematologic AEs being the most frequently reported AEs. In addition, gastrointestinal AEs (e.g., nausea, vomiting, diarrhea) and pyrexia are among the most frequently observed non-hematologic AEs in these patients. Pneumonia, which was also reported with melflufen plus dexamethasone (16% overall; 7% Grade 3/4), has also been frequently reported with other regimens, including pomalidomide plus dexamethasone (16%; Grade 3/4, 11%) and bortezomib plus dexamethasone (13%; Grade 3/4, 11%). Peripheral neuropathy, an AE frequently observed with bortezomib plus dexamethasone (67%; Grade 3/4, 15%), was not commonly observed with melflufen [53,54]. Among patients who received melflufen plus dexamethasone (n = 45), 10 received at least 8 cycles of therapy and 35 discontinued treatment before 8 cycles of therapy, most commonly due to AEs (n = 18) and disease progression (n = 13). Of the 18 patients who discontinued the combination therapy due to AEs, 16 (89%) had received the 21-day regimen and 2 (11%) the 28-day regimen [51].
Among the 13 patients who received single-agent melflufen, the median number of prior therapies was 5 (range, 4–8), the ORR was 8% (1 PR), median PFS was 4.4 months, and median OS was 15.5 months. Of note, patients treated with single-agent melflufen appeared to have a more advanced disease than those treated with melflufen plus dexamethasone (e.g., median years since diagnosis: 8 vs. 5; median prior lines of therapy: 5 vs. 4; and prior daratumumab exposure: 46% vs. 13%, respectively). Overall, results from the single-agent group are comparable to those of other studies being conducted at the time in similar patient populations, including studies of pomalidomide plus dexamethasone and daratumumab alone [53,55]. However, due to a better efficacy signal in the combination cohort, the single-agent arm of the study was terminated early. Results from the melflufen plus dexamethasone combination arm of the O-12-M1 study supported the further development of melflufen in combination with dexamethasone, including evaluation in potential triplet combination regimens.
4.2. Efficacy and Safety of Melflufen Combination Therapies
HORIZON (OP-106; NCT02963493), a pivotal, single-arm, multicenter Phase II study evaluating the efficacy and safety of melflufen in combination with dexamethasone, demonstrated efficacy and a manageable safety profile for the doublet in patients with heavily pretreated and poor-risk relapsed/refractory multiple myeloma refractory to pomalidomide and/or an anti-CD38 monoclonal antibody in an interim analysis (data cutoff date 1 October 2019) (Table 3 and Table 4) [56]. Of 154 patients who had received study treatment at the time of the data cutoff, all patients had prior exposure to IMiDs and proteasome inhibitors, 79% had prior exposure to anti-CD38 monoclonal antibodies, 71% were triple-class refractory, and 97% were refractory to treatment in the last line. The median treatment duration was 14.3 weeks.

Table 4.
Ongoing studies of melflufen in multiple myeloma.
Among 125 patients evaluable for response, the ORR (≥PR) was 29%, with 1 patient achieving a stringent complete response (CR) and 10 patients achieving a VGPR. The median duration of response was 4.4 months. The ORR was 21% among 47 patients with high-risk cytogenetics, 24% among 93 patients with triple-class refractory disease, and 24% among 42 patients with extramedullary disease. The median PFS and OS were 4.2 and 11.6 months for all patients, 4.0 and 11.3 months for patients with triple-class refractory disease, and 3.0 and 8.1 months for patients with extramedullary disease, respectively. Overall, 97% of patients experienced any-grade AEs and 85% of patients experienced Grades 3/4 AEs, most commonly hematologic AEs (thrombocytopenia [69%], neutropenia [66%], and anemia [37%]). The most common (occurring in ≥5% of patients) SAEs and treatment-related SAEs were infections (19% and 5%), febrile neutropenia (5% and 5%), and thrombocytopenia (5% and 5%). A total of 108 patients (70%) had discontinued treatment as of the data cutoff, 73 (47%) due to disease progression and 21 (14%) due to AEs. The rate of treatment discontinuation due to AEs in HORIZON was similar or lower than those reported for other doublet combination therapies, including pomalidomide plus dexamethasone (6%), bortezomib plus dexamethasone (20%), and selinexor plus dexamethasone (33%) [21,53,54]. Overall, 5 deaths were reported in the study, with none deemed to be related to melflufen. In general, the safety profile of melflufen plus dexamethasone in HORIZON was consistent with that reported in O-12-M1.
ANCHOR (OP-104; NCT03481556) is a Phase I/II study evaluating the safety and efficacy of melflufen and dexamethasone in triplet combinations with daratumumab or bortezomib in patients with relapsed/refractory multiple myeloma [57]. Eligible patients had to have received 1–4 prior lines of therapy and be refractory to an IMiD and/or a proteasome inhibitor (only applies to patients in the daratumumab cohort). In an interim analysis of the ANCHOR study (data cutoff date 8 October 2019), the triplet combinations of melflufen, dexamethasone, and daratumumab or bortezomib showed encouraging clinical activity and no new safety signals (Table 3 and Table 4). In the dose-escalation portion of the study, patients received 1 of 2 doses of melflufen (30 or 40 mg) on Day 1 of each 28-day cycle.
In the daratumumab cohort (combined Phase I/II), patients received melflufen plus dexamethasone (40 mg) plus 16 mg/kg daratumumab [57]. Of 33 patients treated up to the data cutoff date, 6 received 30 mg melflufen and 27 received 40 mg melflufen. Most patients (88%) had prior exposure to alkylator therapy and 4 patients (12%) were refractory to alkylator therapy. At a median follow-up of 6.6 months, the median duration of treatment was 6.2 months, and 67% of patients remained on study treatment. No dose-limiting toxicities (DLTs) were reported. The ORR (≥PR) was 76%, with 1 patient achieving a stringent CR and 11 patients achieving a VGPR, and the median PFS was 14.3 months. With 30 and 40 mg melflufen, Grade 3/4 treatment-related AEs, most commonly hematologic AEs, were reported in 83% and 81% of patients, respectively. Overall, SAEs were reported in 36% of patients and 3 patients died due to progressive disease, including 1 patient who had Grade 5 sepsis and pneumonia while in progression.
In the bortezomib cohort (Phase I only), patients received melflufen plus dexamethasone (20–40 mg) plus 1.3 mg/m2 bortezomib. Of 6 patients treated up to the data cutoff date, 3 received 30 mg melflufen and 3 received 40 mg melflufen. All patients had received prior proteasome inhibitor therapy and 5 had received prior alkylator therapy. At a median follow-up of 13.4 months, the median duration of treatment was 9.3 months, and 50% of patients remained on the study treatment. No DLTs were reported. The ORR (≥PR) was 67%, with 2 patients achieving VGPR, and the median PFS was not reached. The most common AEs and Grade 3/4 treatment-related AEs were hematologic events that were clinically manageable. In total, SAEs were reported in 5 patients and 2 patients died due to disease progression after discontinuation of study treatment.
5. Additional Clinical Development
The preliminary data from the HORIZON study, demonstrating encouraging clinical efficacy and a manageable safety profile for melflufen plus dexamethasone [56], support the further development of this combination. Based on clinical data to date, OCEAN (OP-103; NCT03151811)—a randomized, head-to-head, superiority, open-label, global Phase III study of melflufen plus dexamethasone versus pomalidomide plus dexamethasone in patients with multiple myeloma who have received 2 to 4 prior therapies, including lenalidomide within 18 months and are refractory to last line of therapy—was initiated (Table 4) [58,59].
In addition, the combination of melflufen and dexamethasone is also being evaluated in a Phase I/II study (OP201; NCT04115956) in patients with AL amyloidosis [34], which is a rare neoplastic disease of the plasma cells that results in the accumulation of aggregates of misfolded immunoglobulin free light chains within vital organs, leading to organ damage [60]. Despite treatments commonly used in multiple myeloma also being used in patients with AL amyloidosis, there are currently no approved therapies for this patient population with a high unmet medical need [60,61,62].
6. Management of Melflufen
Melflufen (40 mg) is given as a 30-min central intravenous infusion (Day 1 of a 28-day cycle) in combination with 40 mg oral dexamethasone (Days 1, 8, 15, and 22) [51]. Peripheral administration of melflufen will be investigated to enable treatment of patients without central venous access.
Given that neutropenia and thrombocytopenia are the most common toxicities with melflufen, monitoring for these cytopenias and providing appropriate management and supportive care are recommended [63]. During the O-12-M1 study, the cycle length was modified from 21 to 28 days to allow for hematologic recovery [51]. In addition, dose modifications of melflufen due to hematologic toxicities were permitted. If patients did not meet the hematologic criteria for beginning a new cycle (absolute neutrophil count ≥1.0 × 109/L; platelet count ≥50.0 × 109/L) by Day 1 of the next cycle, patients were re-evaluated weekly. If criteria for initiation were met on Day 29 or 36, no dose adjustments were needed; if they were met on Day 43, a one-level dose reduction (from 40 mg to 25 mg) could occur at the investigator’s discretion. If the criteria for initiation were met on Day 50 or 57, a one-level dose level reduction was required. An additional week could be added to the cycle length of subsequent cycles, at the investigator’s discretion, but treatment had to be initiated by Day 42 [51]. Additional management strategies include growth factor support, platelet transfusions, and the use of romiplostim (US only) [63,64].
7. Conclusions
Given that current standard-of-care regimens utilize triplet and quadruplet combination regimens in the first and second lines, there will likely be a large unmet need for patients with multiple myeloma that is refractory to multiple agents in the second line and beyond [2,3,15]. Importantly, with more treatments, patients will have progressively shorter duration of response to each subsequent therapy [19]. The novel agent melflufen, a PDC, has the potential to fill an unmet clinical need in the multiple myeloma treatment landscape. Melflufen has a novel mechanism of action and has demonstrated preclinical and clinically meaningful activity in multiple myeloma, which is refractory to prior standard-of-care therapies. In the early Phase O-12-M1 and HORIZON studies, melflufen plus dexamethasone showed efficacy and a manageable safety profile in patients with heavily pretreated relapsed/refractory multiple myeloma, including patients with triple-class refractory multiple myeloma and those with extramedullary disease [51,52,56]. Importantly, 83% and 57% of patients in HORIZON had been exposed to and were refractory to alkylators, respectively [56], which suggests that melflufen is efficacious in patients who are refractory to alkylators, and this is supported by the preclinical data indicating that melflufen has activity in multiple myeloma cells that are resistant to melphalan [28]. This would not be surprising given that melflufen has three factors that distinguish it from melphalan: (1) melflufen is lipophilic and can be rapidly taken up by myeloma cells; (2) melflufen can achieve higher intracellular concentrations of drug more rapidly than melphalan; and (3) melflufen has ≈50-fold higher cytotoxicity than melphalan in patient-derived myeloma tumor samples [28,29,32,42].
To date, the safety profile of melflufen has been consistent across studies, and no new safety concerns have been identified when melflufen is administered in doublet and triplet combinations [51,56,57]. In general, hematologic events that are manageable have been identified as the most common toxicity, which is not surprising because hematologic toxicities are commonly reported with other investigational agents in heavily pretreated patients with relapsed/refractory multiple myeloma [21,65,66].
Taken together, these data suggest that melflufen plus dexamethasone, as a doublet and in combination with other drugs (daratumumab, bortezomib), has the potential to be beneficial for a broad range of patients with relapsed/refractory multiple myeloma in third- or even second-line therapy. Additionally, melflufen could also have a potential role as a conditioning regimen in patients with relapsed/refractory multiple myeloma who are eligible for stem cell transplant [67].
Author Contributions
M.-V.M., S.B., E.M.O., and P.G.R. contributed to the conception and design of the manuscript. All authors contributed to the interpretation of the literature, critically reviewed all versions of the manuscript, and approved the final version. All authors have read and agreed to the published version of the manuscript.
Funding
The authors received medical editorial support for the development of this manuscript, which was funded by Oncopeptides AB.
Acknowledgments
We would like to thank Katherine Mills-Lujan, CMPP, of Team9 Science for medical editorial assistance with this manuscript, which was funded by Oncopeptides AB.
Conflicts of Interest
M.-V.M. reports having been an advisor/consultant for and having received honoraria from AbbVie, Adaptive, Amgen, Celgene, EDO Mundipharma, GlaxoSmithKline, Janssen, Seattle Genetics, and Takeda and having received honoraria from Oncopeptides. J.B. reports having been an advisor for and having received honoraria from Amgen, Celgene, Janssen, and Takeda. S.B. reports having received honoraria from Amgen, Bristol Myers Squibb, Celgene, and Janssen and having been an advisor/consultant for Amgen, Celgene, Janssen, Karyopharma, and Takeda. E.M.O. reports having received grants/research support from Amgen, Array, Celgene, and EDO Mundipharma and honoraria or consulting fees from Amgen, Asofarma, Bristol Myers Squibb, Celgene, EDO Mundipharma, Janssen, MDS, Oncopeptides, Sanofi, Secura-Bio, and Takeda. Y.E. reports having received honoraria from Takeda; participating in an advisory board or speakers bureau for Akcea, Janssen, and Takeda; and receiving research funding from Celgene. L.P. has nothing to disclose. F.G. reports having been an advisor for AbbVie, Adaptive, Amgen, Bristol-Myers Squibb, Celgene, Janssen, Oncpeptides, Roche, and Takeda and having received honoraria from Bristol-Myers Squibb, Celgene, Janssen, Takeda, and Sanofi. P.S. reports having received research support from and being a consultant for Amgen, Celgene, Janssen, SkylineDx, and Takeda and being a consultant for Oncopeptides. J.G. reports being a co-founder and shareholder in addition to having received honoraria from Oncopeptides. P.G.R. reports having been an advisor for Celgene, Janssen, Karyopharm, Oncopeptides, Sanofi, SecuraBio, and Takeda and having received grants from Bristol-Myers Squibb, Celgene, Karyopharma, Oncopeptides, and Takeda.
References
- Kumar, S.K.; Dimopoulos, M.A.; Kastritis, E.; Terpos, E.; Nahi, H.; Goldschmidt, H.; Hillengass, J.; Leleu, X.; Beksac, M.; Alsina, M.; et al. Natural history of relapsed myeloma, refractory to immunomodulatory drugs and proteasome inhibitors: A multicenter IMWG study. Leukemia 2017, 31, 2443–2448. [Google Scholar] [CrossRef] [PubMed]
- Gandhi, U.H.; Cornell, R.F.; Lakshman, A.; Gahvari, Z.J.; McGehee, E.; Jagosky, M.H.; Gupta, R.; Vamado, W.; Fiala, M.A.; Chhabra, S.; et al. Outcomes of patients with multiple myeloma refractory to CD38-targeted monoclonal antibody therapy. Leukemia 2019, 33, 2266–2275. [Google Scholar] [CrossRef] [PubMed]
- Moreau, P.; San Miguel, J.; Sonneveld, P.; Mateos, M.V.; Zamagni, E.; Avet-Loiseau, H.; Hajek, R.; Dimopoulos, M.A.; Ludwig, H.; Einsele, H.; et al. Multiple myeloma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2017, 28, iv52–iv61. [Google Scholar] [CrossRef] [PubMed]
- National Comprehensive Cancer Network. NCCN Guidelines: Multiple Myeloma; Version 2. 2020. Available online: https://www.nccn.org/professionals/physician_gls/pdf/myeloma.pdf (accessed on 23 September 2020).
- Cejalvo, M.J.; de la Rubia, J. Which therapies will move to the front line for multiple myeloma? Expert Rev. Hematol. 2017, 10, 383–392. [Google Scholar] [CrossRef]
- Moreau, P.; Attal, M.; Hulin, C.; Arnulf, B.; Belhadj, K.; Benboubker, L.; Bene, M.C.; Broijl, A.; Caillon, H.; Caillot, D.; et al. Bortezomib, thalidomide, and dexamethasone with or without daratumumab before and after autologous stem-cell transplantation for newly diagnosed multiple myeloma (CASSIOPEIA): A randomised, open-label, phase 3 study. Lancet 2019, 394, 29–38. [Google Scholar] [CrossRef]
- Facon, T.; Kumar, S.; Plesner, T.; Orlowski, R.Z.; Moreau, P.; Bahlis, N.; Basu, S.; Nahi, H.; Hulin, C.; Quach, H.; et al. Daratumumab plus lenalidomide and dexamethasone for untreated myeloma. N. Engl. J. Med. 2019, 380, 2104–2115. [Google Scholar] [CrossRef]
- Mateos, M.V.; Dimopoulos, M.A.; Cavo, M.; Suzuki, K.; Jakubowiak, A.; Knop, S.; Doyen, C.; Lucio, P.; Nagy, Z.; Kaplan, P.; et al. Daratumumab plus bortezomib, melphalan, and prednisone for untreated myeloma. N. Engl. J. Med. 2018, 378, 518–528. [Google Scholar] [CrossRef]
- Palumbo, A.; Bringhen, S.; Rossi, D.; Cavalli, M.; Larocca, A.; Ria, R.; Offidani, M.; Patriarca, F.; Nozzoli, C.; Guglielmelli, T.; et al. Bortezomib-melphalan-prednisone-thalidomide followed by maintenance with bortezomib-thalidomide compared with bortezomib-melphalan-prednisone for initial treatment of multiple myeloma: A randomized controlled trial. J. Clin. Oncol. 2010, 28, 5101–5109. [Google Scholar] [CrossRef]
- San Miguel, J.F.; Schlag, R.; Khuageva, N.K.; Dimopoulos, M.A.; Shpilberg, O.; Kropff, M.; Spicka, I.; Petrucci, M.T.; Palumbo, A.; Samoilova, O.S.; et al. Bortezomib plus melphalan and prednisone for initial treatment of multiple myeloma. N. Engl. J. Med. 2008, 359, 906–917. [Google Scholar] [CrossRef]
- Benboubker, L.; Dimopoulos, M.A.; Dispenzieri, A.; Catalano, J.; Belch, A.R.; Cavo, M.; Pinto, A.; Weisel, K.; Ludwig, H.; Bahlis, N.; et al. Lenalidomide and dexamethasone in transplant-ineligible patients with myeloma. N. Engl. J. Med. 2014, 371, 906–917. [Google Scholar] [CrossRef]
- Durie, B.G.M.; Hoering, A.; Abidi, M.H.; Rajkumar, S.V.; Epstein, J.; Kahanic, S.P.; Thakuri, M.; Reu, F.; Reynolds, C.M.; Sexton, R.; et al. Bortezomib with lenalidomide and dexamethasone versus lenalidomide and dexamethasone alone in patients with newly diagnosed myeloma without intent for immediate autologous stem-cell transplant (SWOG S0777): A randomised, open-label, phase 3 trial. Lancet 2017, 389, 519–527. [Google Scholar] [CrossRef]
- Rajkumar, S.V.; Jacobus, S.; Callander, N.S.; Fonseca, R.; Vesole, D.H.; Williams, M.E.; Abonour, R.; Siegel, D.S.; Katz, M.; Greipp, P.R.; et al. Lenalidomide plus high-dose dexamethasone versus lenalidomide plus low-dose dexamethasone as initial therapy for newly diagnosed multiple myeloma: An open-label randomised controlled trial. Lancet Oncol. 2010, 11, 29–37. [Google Scholar] [CrossRef]
- Takamatsu, H.; Iida, S.; Shibayama, H.; Shibayama, K.; Yamazaki, H.; Suzuki, K. Daratumumab, lenalidomide, and dexamethasone in Japanese patients with transplant-ineligible newly diagnosed multiple myeloma: A phase 1b study. Int. J. Hematol. 2020, 111, 692–701. [Google Scholar] [CrossRef]
- Moreau, P.; Zamagni, E.; Mateos, M.V. Treatment of patients with multiple myeloma progressing on frontline-therapy with lenalidomide. Blood Cancer J. 2019, 9, 38. [Google Scholar] [CrossRef] [PubMed]
- Chapman, M.A.; Lawrence, M.S.; Keats, J.J.; Cibulskis, K.; Sougnez, C.; Schinzel, A.C.; Harview, C.L.; Brunet, J.P.; Ahmann, G.J.; Adli, M.; et al. Initial genome sequencing and analysis of multiple myeloma. Nature 2011, 471, 467–472. [Google Scholar] [CrossRef]
- Egan, J.B.; Shi, C.X.; Tembe, W.; Christoforides, A.; Kurdoglu, A.; Sinari, S.; Middha, S.; Asmann, Y.; Schmidt, J.; Braggio, E.; et al. Whole-genome sequencing of multiple myeloma from diagnosis to plasma cell leukemia reveals genomic initiating events, evolution, and clonal tides. Blood 2012, 120, 1060–1066. [Google Scholar] [CrossRef]
- Robak, P.; Drozdz, I.; Szemraj, J.; Robak, T. Drug resistance in multiple myeloma. Cancer Treat. Rev. 2018, 70, 199–208. [Google Scholar] [CrossRef]
- Yong, K.; Delforge, M.; Driessen, C.; Fink, L.; Flinois, A.; Gonzalez-McQuire, S.; Safaei, R.; Karlin, L.; Mateos, M.V.; Raab, M.S.; et al. Multiple myeloma: Patient outcomes in real-world practice. Br. J. Haematol. 2016, 175, 252–264. [Google Scholar] [CrossRef]
- Xpovio (Selinexor) [Package Insert]; Karyopharm Therapeutics Inc.: Newton, MA, USA, 2019.
- Chari, A.; Vogl, D.T.; Gavriatopoulou, M.; Nooka, A.K.; Yee, A.J.; Huff, C.A.; Moreau, P.; Dingli, D.; Cole, C.; Lonial, S.; et al. Oral selinexor-dexamethasone for triple-class refractory multiple myeloma. N. Engl. J. Med. 2019, 381, 727–738. [Google Scholar] [CrossRef]
- Moreau, P.; Chanan-Khan, A.; Roberts, A.W.; Agarwal, A.B.; Facon, T.; Kumar, S.; Touzeau, C.; Punnoose, E.A.; Cordero, J.; Munasinghe, W.; et al. Promising efficacy and acceptable safety of venetoclax plus bortezomib and dexamethasone in relapsed/refractory MM. Blood 2017, 130, 2392–2400. [Google Scholar] [CrossRef]
- Kumar, S.; Harrison, S.; Cavo, M.; De la Rubia, J.; Popat, R.; Gasparetto, C.; Hungria, V.; Salwender, H.; Suzuki, K.; Kim, I.; et al. A phase 3 study of venetoclax or placebo in combination with bortezomib and dexamethasone in patients with relapsed/refractory multiple myeloma. Clin. Lymphoma Myeloma Leuk. 2019, 19, e31. [Google Scholar] [CrossRef]
- Cho, S.F.; Anderson, K.C.; Tai, Y.T. Targeting B Cell Maturation Antigen (BCMA) in Multiple Myeloma: Potential Uses of BCMA-Based Immunotherapy. Front. Immunol. 2018, 9, 1821. [Google Scholar] [CrossRef]
- D’Agostino, M.; Raje, N. Anti-BCMA CAR T-cell therapy in multiple myeloma: Can we do better? Leukemia 2019, 34, 21–34. [Google Scholar] [CrossRef] [PubMed]
- Lonial, S.; Lee, H.C.; Badros, A.; Trudel, S.; Nooka, A.K.; Chari, A.; Abdallah, A.O.; Callander, N.; Lendvai, N.; Sborov, D.; et al. Belantamab mafodotin for relapsed or refractory multiple myeloma (DREAMM-2): A two-arm, randomised, open-label, phase 2 study. Lancet Oncol. 2019, 21, 207–221. [Google Scholar] [CrossRef]
- Raje, N.; Berdeja, J.; Lin, Y.; Siegel, D.; Jagannath, S.; Madduri, D.; Liedtke, M.; Rosenblatt, J.; Maus, M.V.; Turka, A.; et al. Anti-BCMA CAR T-cell therapy bb2121 in relapsed or refractory multiple myeloma. N. Engl. J. Med. 2019, 380, 1726–1737. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, D.; Ray, A.; Viktorsson, K.; Spira, J.; Paba-Prada, C.; Munshi, N.; Richardson, P.; Lewensohn, R.; Anderson, K.C. In vitro and in vivo antitumor activity of a novel alkylating agent, melphalan-flufenamide, against multiple myeloma cells. Clin. Cancer Res. 2013, 19, 3019–3031. [Google Scholar] [CrossRef] [PubMed]
- Wickstrom, M.; Nygren, P.; Larsson, R.; Harmenberg, J.; Lindberg, J.; Sjoberg, P.; Jerling, M.; Lehmann, F.; Richardson, P.; Anderson, K.; et al. Melflufen—A peptidase-potentiated alkylating agent in clinical trials. Oncotarget 2017, 8, 66641–66655. [Google Scholar] [CrossRef]
- Wickström, M.; Viktorsson, K.; Lundholm, L.; Aesoy, R.; Nygren, H.; Sooman, L.; Fryknäs, M.; Vogel, L.K.; Lewensohn, R.; Larsson, R.; et al. The alkylating prodrug J1 can be activated by aminopeptidase N, leading to a possible target directed release of melphalan. Biochem. Pharm. 2010, 79, 1281–1290. [Google Scholar] [CrossRef]
- Gullbo, J.; Wickstrom, M.; Tullberg, M.; Ehrsson, H.; Lewensohn, R.; Nygren, P.; Luthman, K.; Larsson, R. Activity of hydrolytic enzymes in tumour cells is a determinant for anti-tumour efficacy of the melphalan containing prodrug J1. J. Drug Target. 2003, 11, 355–363. [Google Scholar] [CrossRef]
- Ray, A.; Ravillah, D.; Das, D.S.; Song, Y.; Nordstrom, E.; Gullbo, J.; Richardson, P.G.; Chauhan, D.; Anderson, K.C. A novel alkylating agent Melflufen induces irreversible DNA damage and cytotoxicity in multiple myeloma cells. Br. J. Haematol. 2016, 174, 397–409. [Google Scholar] [CrossRef]
- Gullbo, J.; Dhar, S.; Luthman, K.; Ehrsson, H.; Lewensohn, R.; Nygren, P.; Larsson, R. Antitumor activity of the alkylating oligopeptides J1 (L-melphalanyl-p-L-fluorophenylalanine ethyl ester) and P2 (L-prolyl-m-L-sarcolysyl-p-L-fluorophenylalanine ethyl ester): Comparison with melphalan. Anticancer Drugs 2003, 14, 617–624. [Google Scholar] [CrossRef] [PubMed]
- Palladini, G.; Schonland, S.; Lentzsch, S.; Cibeira, M.T.; Hajek, R.; Jaccard, A.; Jamroziak, K.; Kastritis, E.; Sanchorawala, V.; Schjesvold, F.H.; et al. OP201: A phase 1/2 study of melflufen and dexamethasone in patients with immunoglobulin light chain amyloidosis. In Proceedings of the 61st American Society of Hematology Annual Meeting, Orlando, FL, USA, 7–10 December 2019; p. 3163. [Google Scholar]
- Gullbo, J.; Tullberg, M.; Vabeno, J.; Ehrsson, H.; Lewensohn, R.; Nygren, P.; Larsson, R.; Luthman, K. Structure-activity relationship for alkylating dipeptide nitrogen mustard derivatives. Oncol. Res. 2003, 14, 113–132. [Google Scholar] [CrossRef] [PubMed]
- Wickstrom, M.; Larsson, R.; Nygren, P.; Gullbo, J. Aminopeptidase N (CD13) as a target for cancer chemotherapy. Cancer Sci. 2011, 102, 501–508. [Google Scholar] [CrossRef] [PubMed]
- Hitzerd, S.M.; Verbrugge, S.E.; Ossenkoppele, G.; Jansen, G.; Peters, G.J. Positioning of aminopeptidase inhibitors in next generation cancer therapy. Amino Acids 2014, 46, 793–808. [Google Scholar] [CrossRef] [PubMed]
- Delforoush, M.; Strese, S.; Wickstrom, M.; Larsson, R.; Enblad, G.; Gullbo, J. In vitro and in vivo activity of melflufen (J1) in lymphoma. BMC Cancer 2016, 16, 263. [Google Scholar] [CrossRef]
- Strese, S.; Hassan, S.B.; Velander, E.; Haglund, C.; Hoglund, M.; Larsson, R.; Gullbo, J. In vitro and in vivo anti-leukemic activity of the peptidase-potentiated alkylator melflufen in acute myeloid leukemia. Oncotarget 2017, 8, 6341–6352. [Google Scholar] [CrossRef][Green Version]
- Zhao, H.; Meng, X.; Yuan, H.; Lan, M. Novel melphalan and chlorambucil derivatives of 2,2,6,6-tetramethyl-1-piperidinyloxy radicals: Synthesis, characterization, and biological evaluation in vitro. Chem. Pharm. Bull. 2010, 58, 332–335. [Google Scholar] [CrossRef][Green Version]
- Carlier, C.; Strese, S.; Viktorsson, K.; Velander, E.; Nygren, P.; Uustalu, M.; Juntti, T.; Lewensohn, R.; Larsson, R.; Spira, J.; et al. Preclinical activity of melflufen (J1) in ovarian cancer. Oncotarget 2016, 7, 59322–59335. [Google Scholar] [CrossRef]
- Wickstrom, M.; Haglund, C.; Lindman, H.; Nygren, P.; Larsson, R.; Gullbo, J. The novel alkylating prodrug J1: Diagnosis directed activity profile ex vivo and combination analyses in vitro. Investig. New Drugs 2008, 26, 195–204. [Google Scholar] [CrossRef]
- Wickstrom, M.; Johnsen, J.I.; Ponthan, F.; Segerstrom, L.; Sveinbjornsson, B.; Lindskog, M.; Lovborg, H.; Viktorsson, K.; Lewensohn, R.; Kogner, P.; et al. The novel melphalan prodrug J1 inhibits neuroblastoma growth in vitro and in vivo. Mol. Cancer 2007, 6, 2409–2417. [Google Scholar] [CrossRef]
- Byrgazov, K.; Slipicevic, A.; Lehmann, F.; Lion, T.; Kager, L.; Taschner-Mandl, S. A peptidase-potentiated alkylating agent melflufen is an effective anti-neoplastic agent in osteosarcoma. In Proceedings of the European Society of Medical Oncology (ESMO) Annual Meeting, Barcelona, Spain, 27 September–19 October 2019; p. 1726. [Google Scholar]
- Chesi, M.; Matthews, G.M.; Garbitt, V.M.; Palmer, S.E.; Shortt, J.; Lefebure, M.; Stewart, A.K.; Johnstone, R.W.; Bergsagel, P.L. Drug response in a genetically engineered mouse model of multiple myeloma is predictive of clinical efficacy. Blood 2012, 120, 376–385. [Google Scholar] [CrossRef] [PubMed]
- Chin, M.; Sive, J.I.; Allen, C.; Roddie, C.; Chavda, S.J.; Smith, D.; Blombery, P.; Jones, K.; Ryland, G.L.; Popat, R.; et al. Prevalence and timing of TP53 mutations in del(17p) myeloma and effect on survival. Blood Cancer J. 2017, 7, e610. [Google Scholar] [CrossRef] [PubMed]
- Strese, S.; Wickstrom, M.; Fuchs, P.F.; Fryknas, M.; Gerwins, P.; Dale, T.; Larsson, R.; Gullbo, J. The novel alkylating prodrug melflufen (J1) inhibits angiogenesis in vitro and in vivo. Biochem. Pharm. 2013, 86, 888–895. [Google Scholar] [CrossRef] [PubMed]
- Berglund, A.; Ullen, A.; Lisyanskaya, A.; Orlov, S.; Hagberg, H.; Tholander, B.; Lewensohn, R.; Nygren, P.; Spira, J.; Harmenberg, J.; et al. First-in-human, phase I/IIa clinical study of the peptidase potentiated alkylator melflufen administered every three weeks to patients with advanced solid tumor malignancies. Investig. New Drugs 2015, 33, 1232–1241. [Google Scholar] [CrossRef] [PubMed]
- Dimopoulos, M.A.; Sonneveld, P.; Leung, N.; Merlini, G.; Ludwig, H.; Kastritis, E.; Goldschmidt, H.; Joshua, D.; Orlowski, R.Z.; Powles, R.; et al. International Myeloma Working Group Recommendations for the Diagnosis and Management of Myeloma-Related Renal Impairment. J. Clin. Oncol. 2016, 34, 1544–1557. [Google Scholar] [CrossRef] [PubMed]
- Nath, C.E.; Shaw, P.J.; Trotman, J.; Zeng, L.; Duffull, S.B.; Hegarty, G.; McLachlan, A.J.; Gurney, H.; Kerridge, I.; Kwan, Y.L.; et al. Population pharmacokinetics of melphalan in patients with multiple myeloma undergoing high dose therapy. Br. J. Clin. Pharm. 2010, 69, 484–497. [Google Scholar] [CrossRef]
- Richardson, P.; Bringhen, S.; Voorhees, P.; Plesner, T.; Mellqvist, U.H.; Reeves, B.; Paba-Prada, C.; Zubair, H.; Byrne, C.; Chauhan, D.; et al. Melflufen plus dexamethasone in relapsed and refractory multiple myeloma (O-12-M1): A multicentre, international, open-label, phase 1–2 study. Lancet Haematol. 2020, 7, e395–e407. [Google Scholar] [CrossRef]
- Bringhen, S.; Vorhees, P.M.; Plesner, T.; Mellqvist, U.H.; Reeves, B.; Sonneveld, P.; Byrne, C.; Nordstrom, E.; Harmenberg, J.; Obermuller, J.; et al. Updated progression-free survival and overall survival with melflufen and dexamethasone in patients with relapsed/refractory multiple myeloma: Results from the phase 2 study O-12-M1. In Proceedings of the 61st American Society of Hematology Annual Meeting, Orlando, FL, USA, 7–10 December 2019; p. 1839. [Google Scholar]
- Dimopoulos, M.A.; Palumbo, A.; Corradini, P.; Cavo, M.; Delforge, M.; Di Raimondo, F.; Weisel, K.C.; Oriol, A.; Hansson, M.; Vacca, A.; et al. Safety and efficacy of pomalidomide plus low-dose dexamethasone in STRATUS (MM-010): A phase 3b study in refractory multiple myeloma. Blood 2016, 128, 497–503. [Google Scholar] [CrossRef]
- San-Miguel, J.F.; Hungria, V.T.; Yoon, S.S.; Beksac, M.; Dimopoulos, M.A.; Elghandour, A.; Jedrzejczak, W.W.; Gunther, A.; Nakorn, T.N.; Siritanaratkul, N.; et al. Panobinostat plus bortezomib and dexamethasone versus placebo plus bortezomib and dexamethasone in patients with relapsed or relapsed and refractory multiple myeloma: A multicentre, randomised, double-blind phase 3 trial. Lancet Oncol. 2014, 15, 1195–1206. [Google Scholar] [CrossRef]
- Usmani, S.Z.; Weiss, B.M.; Plesner, T.; Bahlis, N.J.; Belch, A.; Lonial, S.; Lokhorst, H.M.; Voorhees, P.M.; Richardson, P.G.; Chari, A.; et al. Clinical efficacy of daratumumab monotherapy in patients with heavily pretreated relapsed or refractory multiple myeloma. Blood 2016, 128, 37–44. [Google Scholar] [CrossRef]
- Mateos, M.V.; Oriol, A.; Larocca, A.; Otero, P.R.; Bladé, J.; Cavo, M.; Hassoun, H.; Leleu, X.; Alegre, A.; Maisel, C.; et al. Clinical activity of melflufen in patients with triple-class refractory multiple myeloma and poor-risk features in an updated analysis of HORIZON (OP-106), a phase 2 study in patients with relapsed/refractory multiple myeloma refractory to pomalidomide and/or daratumumab. In Proceedings of the 61st American Society of Hematology Annual Meeting, Orlando, FL, USA, 7–10 December 2019; p. 1883. [Google Scholar]
- Ocio, E.M.; Efebera, Y.A.; Granell, M.; Hajek, R.; Maisnar, V.; Karlin, L.; Mateos, M.V.; Richardson, P.G.; Oriol, A.; Norin, S.; et al. ANCHOR (OP-104): Updated efficacy and safety from a phase 1/2 study of melflufen and dexamethasone plus bortezomib or daratumumab in patients with relapsed/refractory multiple myeloma (RRMM) refractory to an IMiD or a proteasome inhibitor (PI). Blood 2019, 133, 3124. [Google Scholar] [CrossRef]
- Schjesvold, F.; Robak, P.; Pour, L.; Aschan, J.; Sonneveld, P. OCEAN: A randomized phase III study of melphalan flufenamide + dexamethasone to treat relapsed refractory multiple myeloma. Future Oncol. 2020, 16, 631–641. [Google Scholar] [CrossRef] [PubMed]
- NIH US National Library of Medicine. ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ (accessed on 24 March 2020).
- Merlini, G.; Dispenzieri, A.; Sanchorawala, V.; Schonland, S.O.; Palladini, G.; Hawkins, P.N.; Gertz, M.A. Systemic immunoglobulin light chain amyloidosis. Nat. Rev. Dis. Primers 2018, 4, 38. [Google Scholar] [CrossRef] [PubMed]
- Varga, C.; Titus, S.E.; Toskic, D.; Comenzo, R.L. Use of novel therapies in the treatment of light chain amyloidosis. Blood Rev. 2019, 37, 100581. [Google Scholar] [CrossRef]
- Milani, P.; Palladini, G.; Merlini, G. New concepts in the treatment and diagnosis of amyloidosis. Expert Rev. Hematol. 2018, 11, 117–127. [Google Scholar] [CrossRef]
- Paner, A.; Okwuosa, T.M.; Richardson, K.J.; Libby, E.N. Triplet therapies—The new standard of care for multiple myeloma: How to manage common toxicities. Expert Rev. Hematol. 2018, 11, 957–973. [Google Scholar] [CrossRef]
- Parameswaran, R.; Lunning, M.; Mantha, S.; Devlin, S.; Hamilton, A.; Schwartz, G.; Soff, G. Romiplostim for management of chemotherapy-induced thrombocytopenia. Support. Care Cancer 2014, 22, 1217–1222. [Google Scholar] [CrossRef]
- Lakshman, A.; Abeykoon, J.P.; Kumar, S.K.; Rajkumar, S.V.; Dingli, D.; Buadi, F.K.; Gonsalves, W.I.; Leung, N.; Dispenzieri, A.; Kourelis, T.V.; et al. Efficacy of daratumumab-based therapies in patients with relapsed, refractory multiple myeloma treated outside of clinical trials. Am. J. Hematol. 2017, 92, 1146–1155. [Google Scholar] [CrossRef]
- Richardson, P.G.; San Miguel, J.F.; Moreau, P.; Hajek, R.; Dimopoulos, M.A.; Laubach, J.P.; Palumbo, A.; Luptakova, K.; Romanus, D.; Skacel, T.; et al. Interpreting clinical trial data in multiple myeloma: Translating findings to the real-world setting. Blood Cancer J. 2018, 8, 109. [Google Scholar] [CrossRef]
- Al Hamed, R.; Bazarbachi, A.H.; Malard, F.; Harousseau, J.L.; Mohty, M. Current status of autologous stem cell transplantation for multiple myeloma. Blood Cancer J. 2019, 9, 44. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).