Menstrual Phase Affects Coagulation and Hematological Parameters during Central Hypovolemia
Abstract
1. Introduction
2. Experimental Section
2.1. Subjects
2.2. Sample Size
2.3. Study and LBNP Protocol
2.4. Blood Sampling
2.5. Thrombin Generation Markers and Endothelial Activation Markers
2.6. Thrombin Generation Using Automated Fluorogenic Measurements
2.7. Tissue Factor Triggered TEM Assay (Thrombelastometer) Provide the Following
2.8. Statistics
3. Results
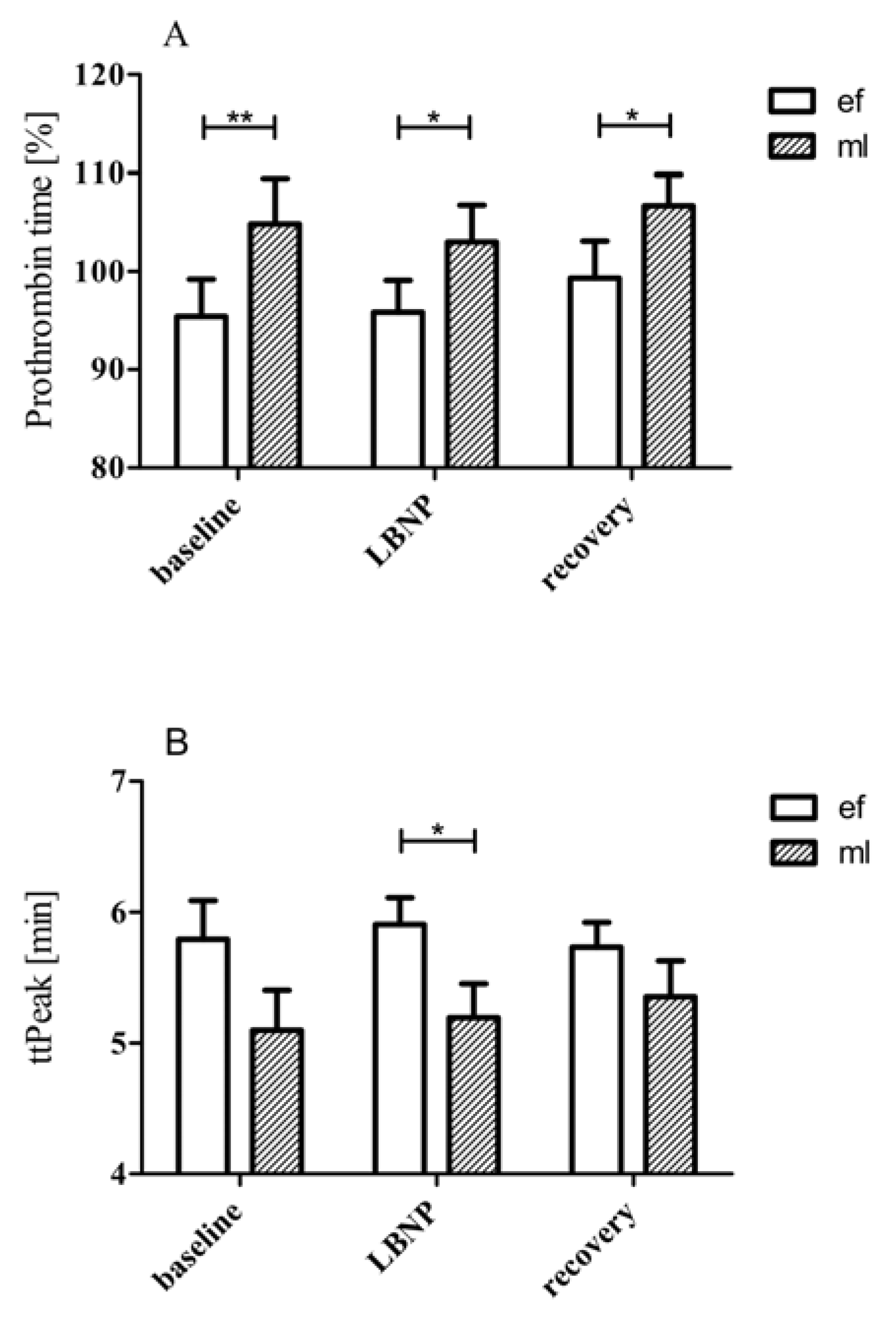
3.1. LBNP Effects on Standard Coagulation Parameters
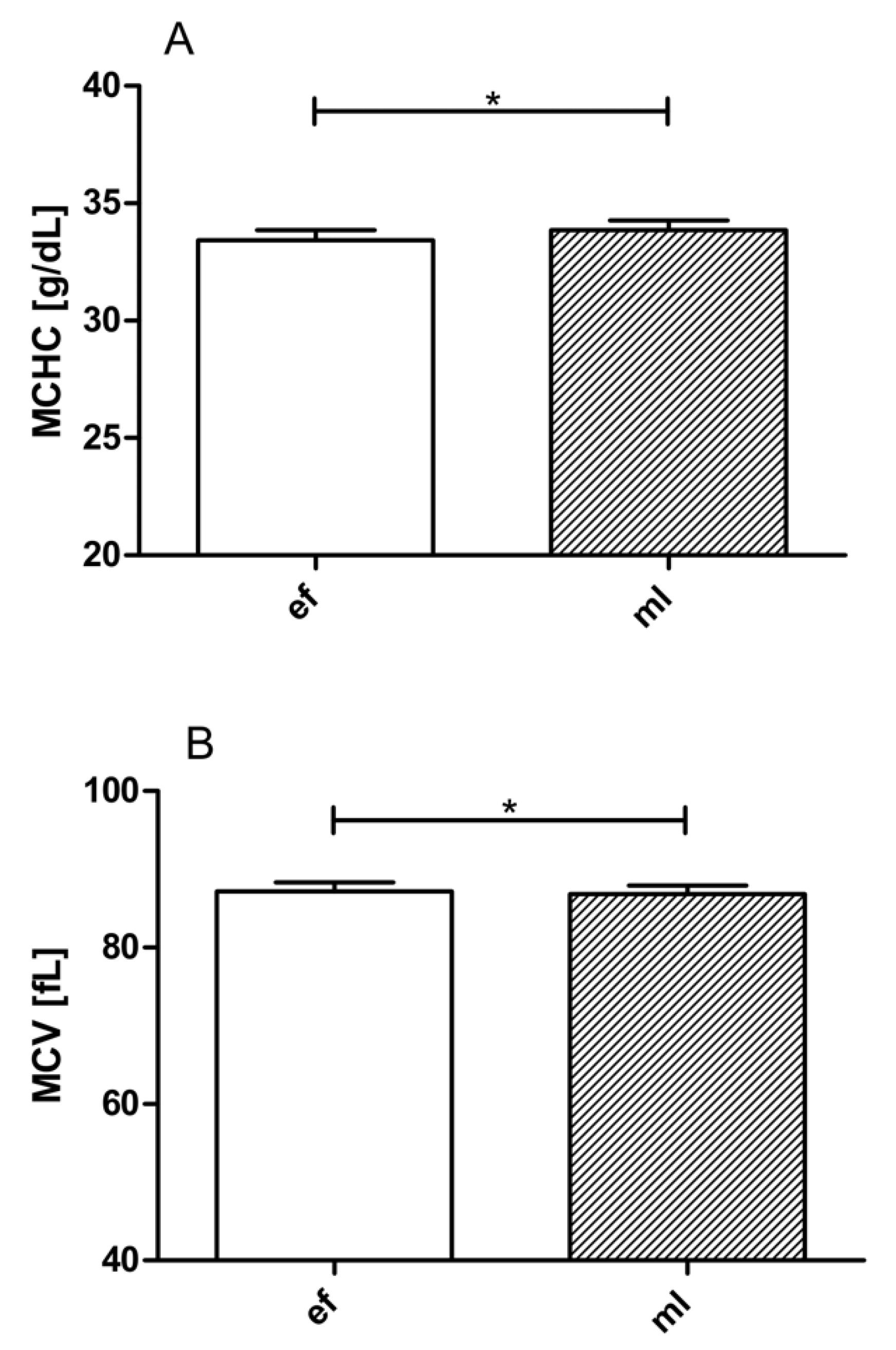
3.2. LBNP Effects on Blood Cell Count, Hematocrit, Hemoglobin, MCHC, MCH, and MCV
3.3. LBNP Effects on Thrombin Generation Markers
3.4. Effects of LBNP on Endothelial Activation Markers
3.5. Thrombin Generation Using CAT
3.6. Effects of LBNP on TEM Values
4. Discussion
5. Conclusions
- (1)
- During both phases, women are susceptible to increased coagulation during LBNP, as reflected in their decreased PTT and elevated FVIII, F1 + 2, and TAT levels;
- (2)
- During the mid-luteal phase, greater prothrombin time and shorter ttpeak values (implying faster maximum thrombin formation) suggest that women in the mid-luteal phase are relatively hypercoagulable compared with the early follicular phase;
- (3)
- LBNP represents a mild but efficient stimulus to expose individuals to a procoagulant challenge. It has been suggested recently that a simple sit-to-stand test might also have the potential to identify individuals with an increased risk of thrombosis [31]. However, a stand test is accompanied by an activation of both the endothelium and the coagulation cascade and might therefore not be appropriate in patients with higher risk of thrombosis. The present study shows that LBNP is a very mild but efficient coagulation stimulus and, thus, might be a suitable tool to screen for thrombosis.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Goswami, N.; Blaber, A.P.; Hinghofer-Szalkay, H.; Convertino, V.A. Lower Body Negative Pressure: Physiological Effects, Applications, and Implementation. Physiol. Rev. 2019, 99, 807–851. [Google Scholar] [CrossRef]
- Van Helmond, N.; Johnson, B.D.; Curry, T.B.; Cap, A.P.; Convertino, V.A.; Joyner, M.J. White blood cell concentrations during lower body negative pressure and blood loss in humans. Exp. Physiol. 2016, 101, 1265–1275. [Google Scholar] [CrossRef]
- Boulanger, L.; Joshi, A.V.; Tortella, B.J.; Menzin, J.; Caloyeras, J.P.; Russell, M.W. Excess mortality, length of stay, and costs associated with serious hemorrhage among trauma patients: Findings from the National Trauma Data Bank. Am. Surg. 2007, 73, 1269–1274. [Google Scholar] [CrossRef] [PubMed]
- Cvirn, G.; Waha, J.E.; Brix, B.; Rossler, A.; Jantscher, A.; Schlagenhauf, A.; Koestenberger, M.; Wonisch, W.; Wagner, T.; Goswami, N. Coagulation changes induced by lower body negative pessure in men and woman. J. Appl. Physiol. 2019, 126, 1214–1222. [Google Scholar] [CrossRef] [PubMed]
- Zaar, M.; Johansson, P.I.; Nielsen, L.B.; Crandall, C.G.; Shibasaki, M.; Hilsted, L.; Secher, N.H. Early activation of the coagulation system during lower body negative pressure. Clin. Physiol. Funct. Imaging 2009, 29, 427–430. [Google Scholar] [CrossRef]
- Zaar, M.; Fedyk, C.G.; Pidcoke, H.F.; Scherer, M.R.; Ryan, K.L.; Rickards, C.A.; Hinojosa-Laborde, C.; Convertino, V.A.; Cap, A.P. Platelet activation after presyncope by lower body negative pressure in humans. PLoS ONE 2014, 9, e116174. [Google Scholar] [CrossRef] [PubMed]
- Kraemer, M.; Kuepper, M.; Nebe-vom Stein, A.; Sorgenfrei, U.; Diehl, R.R. The influence of vasovagal response on the coagulation system. Clin. Auton. Res. 2010, 20, 105–111. [Google Scholar] [CrossRef]
- Swieringa, F.; Spronk, H.M.H.; Heemskerk, J.W.M.; van der Meijden, P.E.J. Integrating platelet and coagulation activation in fibrin clot formation. Res. Pract. Thromb. Haemost. 2018, 2, 450–460. [Google Scholar] [CrossRef] [PubMed]
- Sorensen, B.; Johansen, P.; Christiansen, K.; Woelke, M.; Ingerslev, J. Whole blood coagulation thrombelastographic profiles employing minimal tissue factor activation. J. Thromb. Haemost. 2003, 1, 551–558. [Google Scholar] [CrossRef]
- Lowe, G.D.; Rumley, A.; Woodward, M.; Morrison, C.E.; Philippou, H.; Lane, D.A.; Tunstall-Pedoe, H. Epidemiology of coagulation factors, inhibitors and activation markers: The Third Glasgow MONICA Survey. I. Illustrative reference ranges by age, sex and hormone use. Br. J. Haematol. 1997, 97, 775–784. [Google Scholar] [CrossRef]
- Hamelin, B.A.; Methot, J.; Arsenault, M.; Pilote, S.; Poirier, P.; Plante, S.; Bogaty, P. Influence of the menstrual cycle on the timing of acute coronary events in premenopausal women. Am. J. Med. 2003, 114, 599–602. [Google Scholar] [CrossRef]
- Cvirn, G.; Waha, J.E.; Ledinski, G.; Schlagenhauf, A.; Leschnik, B.; Koestenberger, M.; Tafeit, E.; Hinghofer-Szalkay, H.; Goswami, N. Bed rest does not induce hypercoagulability. Eur. J. Clin. Investig. 2015, 45, 63–69. [Google Scholar] [CrossRef] [PubMed]
- Waha, J.E.; Goswami, N.; Schlagenhauf, A.; Leschnik, B.; Koestenberger, M.; Reibnegger, G.; Roller, R.E.; Hinghofer-Szalkay, H.; Cvirn, G. Effects of exercise and nutrition on the coagulation system during bedrest immobilization. Medicine 2015, 94, e1555. [Google Scholar] [CrossRef]
- Cvirn, G.; Schlagenhauf, A.; Leschnik, B.; Koestenberger, M.; Roessler, A.; Jantscher, A.; Vrecko, K.; Juergens, G.; Hinghofer-Szalkay, H.; Goswami, N. Coagulation changes during presyncope and recovery. PLoS ONE 2012, 7, 1–8. [Google Scholar] [CrossRef]
- Hinghofer-Szalkay, H.G.; Goswami, N.; Rossler, A.; Grasser, E.; Schneditz, D. Reactive hyperemia in the human liver. Am. J. Physiol. Gastroint. Liver Physiol. 2008, 295, 332–337. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Goswami, N.; Loeppky, J.; Hinghofer-Szalkay, H. LBNP: Past protocols and technical considerations for experimental design. Aviat. Space Environ. Med. 2008, 79, 459–471. [Google Scholar] [CrossRef]
- Blaber, A.P.; Goswami, N.; Bondar, R.L.; Kassam, M.S. Impairment of cerebral blood flow regulation in astronauts with post flight orthostatic intolerance. Stroke 2011, 42, 1844–1850. [Google Scholar] [CrossRef]
- Hemker, H.C.; Giesen, P.; Al Dieri, R.; Regnault, V.; de Smedt, E.; Wagenvoord, R.; Lecompte, T.; Beguin, S. Calibrated automated thrombin generation measurement in clotting plasma. Pathophysiol. Haemost. Thromb. 2003, 33, 4–15. [Google Scholar] [CrossRef]
- Knol, H.M.; Kemperman, R.F.; Kluin-Nelemans, H.C.; Mulder, A.B.; Meijer, K. Haemostatic variables during normal menstrual cycle. A systematic review. Thromb. Haemost. 2012, 107, 22–29. [Google Scholar] [CrossRef]
- Zaar, M.; Morkeberg, J.; Pott, F.C.; Johansson, P.I.; Secher, N.H. Coagulation competence and fluid recruitment after moderate blood loss in young men. Blood Coagul. Fibrinolysis 2014, 25, 592–596. [Google Scholar] [CrossRef]
- Cai, Y.; Holm, S.; Jenstrup, M.; Stromstad, M.; Eigtved, A.; Warberg, J.; Hojgaard, L.; Friberg, L.; Secher, N.H. Electrical admittance for filling of the heart during lower body negative pressure in humans. J. Appl. Physiol. 2000, 89, 1569–1576. [Google Scholar] [CrossRef] [PubMed]
- van Helmond, N.; Johnson, B.D.; Curry, T.B.; Cap, A.P.; Convertino, V.A.; Joyner, M.J. Coagulation changes during lower body negative pressure and blood loss in humans. Am. J. Physiol. Heart Circ. Physiol. 2015, 309, H1591–H1597. [Google Scholar] [CrossRef] [PubMed]
- Pecins-Thompson, M.; Keller-Wood, M. Effects of progesterone on blood pressure, plasma volume, and responses to hypotension. Am. J. Physiol. 1997, 272, R377–R385. [Google Scholar] [CrossRef] [PubMed]
- Van Beaumont, W.; Rochelle, R.H. Erythrocyte volume stability with plasma osmolarity changes in exercising man. Proc. Soc. Exp. Biol. Med. 1974, 145, 240–243. [Google Scholar] [CrossRef] [PubMed]
- Brochner-Mortensen, J.; Paaby, P.; Fjeldborg, P.; Raffn, K.; Larsen, C.E.; Moller-Petersen, J. Renal haemodynamics and extracellular homeostasis during the menstrual cycle. Scand. J. Clin. Lab. Investig. 1987, 47, 829–835. [Google Scholar] [CrossRef]
- Vellar, O.D. Changes in hemoglobin concentration and hematocrit during the menstrual cycle. I. A cross-sectional study. Acta Obstet. Gynecol. Scand. 1974, 53, 243–246. [Google Scholar] [CrossRef]
- Libre, E.P.; Cowan, D.H.; Watkins, S.P., Jr.; Shulman, N.R. Relationships between spleen, platelets and factor 8 levels. Blood 1968, 31, 358–368. [Google Scholar] [CrossRef]
- Schagatay, E.; Andersson, J.P.; Hallen, M.; Palsson, B. Selected contribution: Role of spleen emptying in prolonging apneas in humans. J. Appl. Physiol. 2001, 90, 1623–1629. [Google Scholar] [CrossRef]
- Park, S.H.; Park, C.J.; Lee, B.R.; Kim, M.J.; Han, M.Y.; Cho, Y.U.; Jang, S. Establishment of Age- and Gender-Specific Reference Ranges for 36 Routine and 57 Cell Population Data Items in a New Automated Blood Cell Analyzer, Sysmex XN-2000. Ann. Lab. Med. 2016, 36, 244–249. [Google Scholar] [CrossRef]
- Chidambaram, M.; Duncan, J.A.; Lai, V.S.; Cattran, D.C.; Floras, J.S.; Scholey, J.W.; Miller, J.A. Variation in the renin angiotensin system throughout the normal menstrual cycle. J. Am. Soc. Nephrol. 2002, 13, 446–452. [Google Scholar]
- Cvirn, G.; Kneihsl, M.; Rossmann, C.; Paar, M.; Gattringer, T.; Schlagenhauf, A.; Leschnik, B.; Koestenberger, M.; Tafeit, E.; Reibnegger, G.; et al. Orthostatic challenge shifts the hemostatic system of patients recovered from stroke toward hypercoagulability. Front. Physiol. 2017, 8, 12. [Google Scholar] [CrossRef] [PubMed]
| Baseline | LBNP | Recovery | Ptime (ANOVA) | ||
|---|---|---|---|---|---|
| Early follicular phase | aPTT [s] | 36.14 ± 0.94 | 34.37 ± 0.96 | 33.13 ± 1.41 | 0.011 |
| PT [%] | 97.00 ± 4.08 | 95.83 v 3.28 | 99.33 ± 3.77 | 0.281 | |
| F II [%] | 87.00 ± 7.96 | 87.33 ± 8.09 | 91.83 ± 5.04 | 0.445 | |
| FVII [%] | 104.70 ± 7.67 | 106.00 ± 7.27 | 104.60 ± 6.92 | 0.861 | |
| F VIII [%] | 71.57 ± 7.53 | 79.71 ± 8.09 | 90.29 ± 10.16 | 0.003 | |
| PC [%] | 96.67 ± 5.73 | 101.50 ± 8.57 | 101.00 ± 5.84 | 0.605 | |
| PS [%] | 91.83 ± 15.99 | 84.83 ± 11.90 | 76.67 ± 4.43 | 0.403 | |
| WBC [103/µL] | 4.61 ± 0.50 | 4.11 ± 0.42 | 4.73 ± 0.61 | 0.009 | |
| Plt [103/mL] | 218.30 ± 19.88 | 218.40 ± 20.20 | 224.90 ± 22.41 | 0.257 | |
| RBC [106/mL] | 3.96 ± 0.11 | 3.98 ± 0.12 | 4.053 ± 0.13 | 0.170 | |
| Hct [%] | 34.49 ± 0.52 | 34.60 ± 0.68 | 35.23 ± 0.73 | 0.182 | |
| Hb [g/dL] | 11.54 ± 0.27 | 11.67 ± 0.35 | 11.90 ± 0.37 | 0.055 | |
| MCHC [g/dL] | 33.43 ± 0.43 | 33.70 ± 0.43 | 33.73 ± 0.43 | 0.004 | |
| MCH [pg] | 29.14 ± 0.39 | 29.36 ± 0.39 | 29.39 ± 0.42 | 0.038 | |
| MCV [fL] | 87.19 ± 1.12 | 87.20 ± 1.13 | 87.10 ± 1.12 | 0.733 | |
| F1 + 2 [pmol/L] | 225.3 ± 17.20 | 415.5 ± 71.37 | 731.3 ± 154.4 | 0.002 | |
| TAT [ng/mL] | 5.25 ± 1.66 | 29.81 ± 8.51 | 49.54 ± 8.71 | 0.001 | |
| TF [pg/mL] | 313.30 ± 27.39 | 362.10 ± 37.51 | 576.90 ± 170.30 | 0.121 | |
| tPA [ng/mL] | 1.58 ± 0.50 | 1.35 ± 0.41 | 1.39 ± 0.40 | 0.397 | |
| LT [min] | 2.43 ± 0.12 | 2.39 ± 0.06 | 2.30 ± 0.08 | 0.321 | |
| ETP [nM•min] | 1464 ± 203 | 1406 ± 186 | 1421 ± 214 | 0.137 | |
| Peak [nM] | 250.30 ± 36.66 | 232.80 ± 33.72 | 241.00 ± 35.11 | 0.081 | |
| ttPeak [min] | 5.79 ± 0.30 | 5.91 ± 0.21 | 5.73 ± 0.19 | 0.485 | |
| VelIndex [nm/min] | 81.33 ± 18.66 | 70.01 ± 13.86 | 73.86 ± 14.40 | 0.126 | |
| StartTail [min] | 21.87 ± 0.39 | 21.98 ± 0.27 | 22.00 ± 0.38 | 0.937 | |
| CT [s] | 167.00 ± 17.02 | 159.60 ± 15.17 | 163.40 ± 15.66 | 0.391 | |
| CFT [s] | 142.30 ± 20.35 | 115.90 ± 12.59 | 117.70 ± 12.47 | 0.052 | |
| MCF [mm] | 58.14 ± 2.22 | 59.43 ± 1.96 | 59.57 ± 1.88 | 0.205 | |
| Alpha [°] | 63.71 ± 3.19 | 67.57 ± 2.25 | 67.00 ± 2.23 | 0.044 | |
| Mid-luteal phase | aPTT [s] | 34.70 ± 1.35 | 33.17 ± 1.69 | 32.21 ± 1.54 | 0.009 |
| PT [%] | 104.9 ± 4.63 | 103.0 ± 3.77 | 106.07 ± 3.14 | 0.104 | |
| F II [%] | 92.33 ± 6.44 | 90.67 ± 6.66 | 92.00 ± 6.18 | 0.750 | |
| FVII [%] | 106.5 ± 13.6 | 107.7 ± 15.1 | 108.0 ± 13.7 | 0.849 | |
| F VIII [%] | 87.20 ± 13.32 | 94.00 ± 15.09 | 96.00 ± 12.59 | 0.024 | |
| PC [%] | 88.25 ± 16.06 | 96.67 ± 3.00 | 111.00 ± 7.27 | 0.312 | |
| PS [%] | 67.86 ± 10.41 | 72.86 ± 8.23 | 76.43 ± 7.24 | 0.328 | |
| WBC [103/µL] | 4.64 ± 0.43 | 4.07 ± 0.37 | 4.90 ± 0.47 | 0.001 | |
| Plt [103/mL] | 225.00 ± 18.63 | 218.40 ± 16.07 | 223.60 ± 18.40 | 0.430 | |
| RBC [106/mL] | 3.91 ± 0.13 | 3.89 ± 0.15 | 4.02 ± 0.15 | 0.012 | |
| Hct [%] | 33.84 ± 0.87 | 33.70 ± 1.05 | 34.81 ± 1.00 | 0.011 | |
| Hb [g/dL] | 11.47 ± 0.35 | 11.43 ± 0.42 | 11.81 ± 0.42 | 0.014 | |
| MCHC [g/dL] | 33.86 ± 0.41 | 33.89 ± 0.45 | 33.90 ± 0.44 | 0.933 | |
| MCH [pg] | 29.39 ± 0.47 | 29.43 ± 0.48 | 29.41 ± 0.48 | 0.946 | |
| MCV [fL] | 86.80 ± 1.11 | 86.86 ± 1.17 | 86.79 ± 1.14 | 0.902 | |
| F1 + 2 [pmol/L] | 212.3 ± 27.80 | 546.2 ± 99.37 | 584.3 ± 108.7 | 0.036 | |
| TAT [ng/mL] | 3.30 ± 0.32 | 41.77 ± 8.81 | 41.19 ± 9.96 | 0.004 | |
| TF [pg/mL] | 295.00 ± 64.93 | 345.50 ± 83.44 | 547.50 ± 198.60 | 0.330 | |
| tPA [ng/mL] | 1.96 ± 0.57 | 2.40 ± 0.73 | 3.24 ± 0.75 | 0.287 | |
| LT [min] | 2.13 ± 0.10 | 2.22 ± 0.11 | 2.27 ± 0.14 | 0.184 | |
| ETP [nM•min] | 1688 ± 214 | 1645 ± 210 | 1645 ± 197 | 0.347 | |
| Peak [nM] | 312.80 ± 43.15 | 304.50 ± 42.68 | 297.30 ± 38.44 | 0.315 | |
| ttPeak [min] | 5.10 ± 0.31 | 5.19 ± 0.26 | 5.35 ± 0.28 | 0.239 | |
| VelIndex [nm/min] | 116.60 ± 24.64 | 110.90 ± 21.30 | 103.20 ± 19.57 | 0.079 | |
| StartTail [min] | 21.46 ± 0.43 | 21.55 ± 0.39 | 21.44 ± 0.41 | 0.906 | |
| CT [s] | 155.60 ± 13.11 | 144.40 ± 13.00 | 148.40 ± 13.48 | 0.076 | |
| CFT [s] | 121.70 ± 15.53 | 107.30 ± 20.56 | 99.86 ± 11.04 | 0.122 | |
| MCF [mm] | 60.86 ± 2.06 | 62.00 ± 1.98 | 62.00 ± 2.01 | 0.500 | |
| Alpha [°] | 66.14 ± 2.49 | 69.57 ± 3.12 | 70.00 ± 2.05 | 0.035 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Goswami, N.; Brix, B.; Roessler, A.; Koestenberger, M.; Reibnegger, G.; Cvirn, G. Menstrual Phase Affects Coagulation and Hematological Parameters during Central Hypovolemia. J. Clin. Med. 2020, 9, 3118. https://doi.org/10.3390/jcm9103118
Goswami N, Brix B, Roessler A, Koestenberger M, Reibnegger G, Cvirn G. Menstrual Phase Affects Coagulation and Hematological Parameters during Central Hypovolemia. Journal of Clinical Medicine. 2020; 9(10):3118. https://doi.org/10.3390/jcm9103118
Chicago/Turabian StyleGoswami, Nandu, Bianca Brix, Andreas Roessler, Martin Koestenberger, Gilbert Reibnegger, and Gerhard Cvirn. 2020. "Menstrual Phase Affects Coagulation and Hematological Parameters during Central Hypovolemia" Journal of Clinical Medicine 9, no. 10: 3118. https://doi.org/10.3390/jcm9103118
APA StyleGoswami, N., Brix, B., Roessler, A., Koestenberger, M., Reibnegger, G., & Cvirn, G. (2020). Menstrual Phase Affects Coagulation and Hematological Parameters during Central Hypovolemia. Journal of Clinical Medicine, 9(10), 3118. https://doi.org/10.3390/jcm9103118





