Behavioral Factors Related to the Incidence of Frailty in Older Adults
Abstract
1. Introduction
2. Methods
2.1. Participants
2.2. Operational Definition of Physical Frailty
2.3. Measurements of Lifestyle Activity
2.4. Potential Confounding Factors
2.5. Statistical Analysis
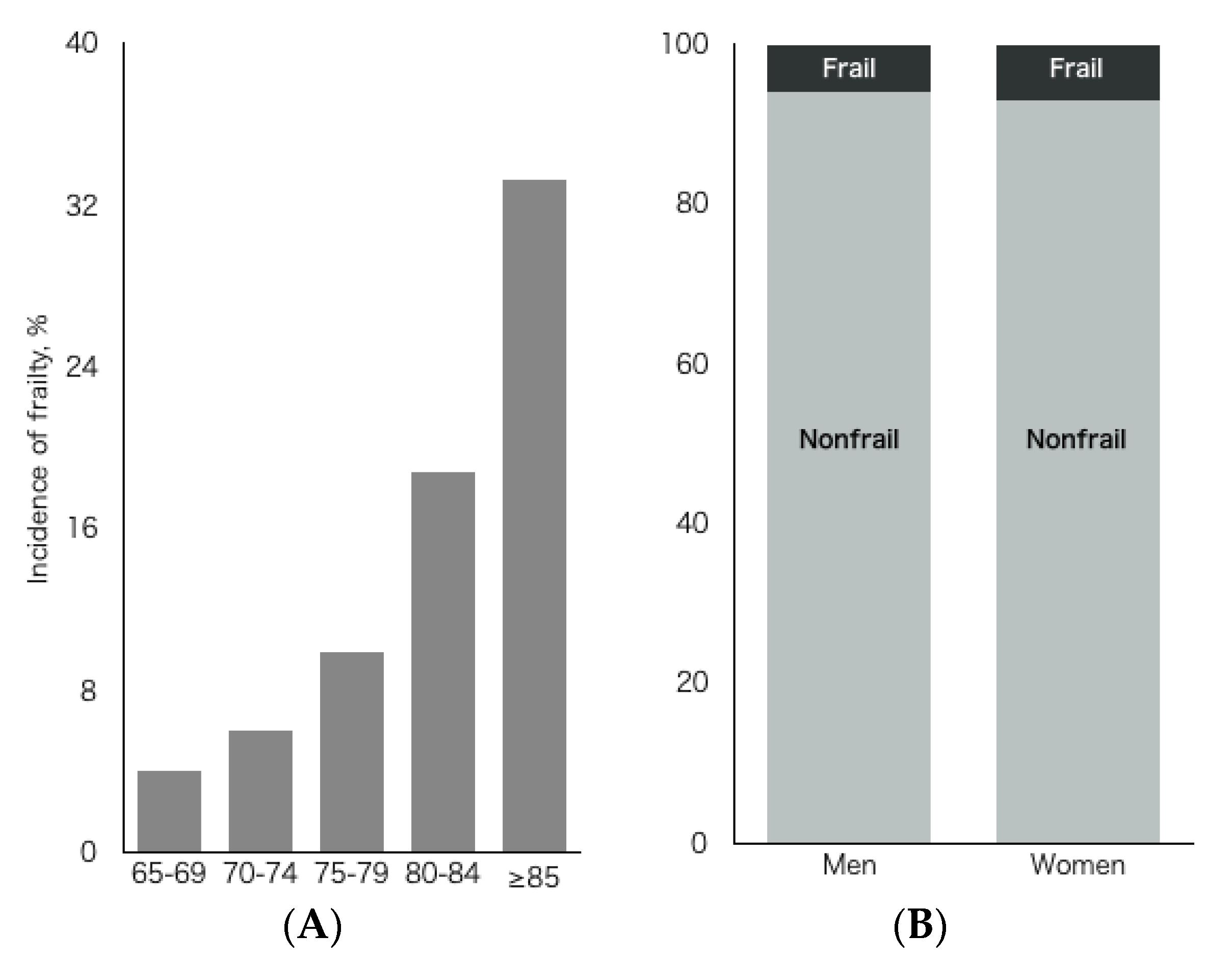
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Clegg, A.; Young, J.; Iliffe, S.; Rikkert, M.O.; Rockwood, K. Frailty in elderly people. Lancet 2013, 381, 752–762. [Google Scholar] [CrossRef]
- Rodriguez-Manas, L.; Feart, C.; Mann, G.; Vina, J.; Chatterji, S.; Chodzko-Zajko, W.; Gonzalez-Colaco Harmand, M.; Bergman, H.; Carcaillon, L.; Nicholson, C.; et al. Searching for an operational definition of frailty: A Delphi method based consensus statement. The frailty operative definition-consensus conference project. J. Gerontol. A Biol. Sci. Med. Sci. 2013, 68, 62–67. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Mitnitski, A.; Rockwood, K. Prevalence and 10-year outcomes of frailty in older adults in relation to deficit accumulation. J. Am. Geriatr. Soc. 2010, 58, 681–687. [Google Scholar] [CrossRef] [PubMed]
- Fried, L.P.; Tangen, C.M.; Walston, J.; Newman, A.B.; Hirsch, C.; Gottdiener, J.; Seeman, T.; Tracy, R.; Kop, W.J.; Burke, G.; et al. Frailty in older adults: Evidence for a phenotype. J. Gerontol. A Biol. Sci. Med. Sci. 2001, 56, M146–M156. [Google Scholar] [CrossRef] [PubMed]
- Paulo, T.R.; Tribess, S.; Sasaki, J.E.; Meneguci, J.; Martins, C.A.; Freitas, I.F., Jr.; Romo-Perez, V.; Virtuoso, J.S., Jr. A Cross-Sectional Study of the Relationship of Physical Activity with Depression and Cognitive Deficit in Older Adults. J. Aging Phys. Act. 2016, 24, 311–321. [Google Scholar] [CrossRef]
- Shimada, H.; Makizako, H.; Lee, S.; Doi, T.; Lee, S. Lifestyle activities and the risk of dementia in older Japanese adults. Geriatr. Gerontol. Int. 2018, 18, 1491–1496. [Google Scholar] [CrossRef]
- Shimada, H.; Doi, T.; Lee, S.; Makizako, H. Reversible predictors of reversion from mild cognitive impairment to normal cognition: A 4-year longitudinal study. Alzheimers Res. Ther. 2019, 11, 24. [Google Scholar] [CrossRef]
- Statistics Bureau of Japan. Population Estimates Monthly Report. Available online: http://www.stat.go.jp/english/data/jinsui/tsuki/index.html (accessed on 31 March 2020).
- Fabrigoule, C.; Letenneur, L.; Dartigues, J.F.; Zarrouk, M.; Commenges, D.; Barberger-Gateau, P. Social and leisure activities and risk of dementia: A prospective longitudinal study. J. Am. Geriatr. Soc. 1995, 43, 485–490. [Google Scholar] [CrossRef]
- Wang, H.X.; Karp, A.; Winblad, B.; Fratiglioni, L. Late-life engagement in social and leisure activities is associated with a decreased risk of dementia: A longitudinal study from the Kungsholmen project. Am. J. Epidemiol. 2002, 155, 1081–1087. [Google Scholar] [CrossRef]
- Karp, A.; Paillard-Borg, S.; Wang, H.X.; Silverstein, M.; Winblad, B.; Fratiglioni, L. Mental, physical and social components in leisure activities equally contribute to decrease dementia risk. Dement. Geriatr. Cogn. Disord. 2006, 21, 65–73. [Google Scholar] [CrossRef]
- Laurin, D.; Verreault, R.; Lindsay, J.; MacPherson, K.; Rockwood, K. Physical activity and risk of cognitive impairment and dementia in elderly persons. Arch. Neurol. 2001, 58, 498–504. [Google Scholar] [CrossRef]
- Scarmeas, N.; Levy, G.; Tang, M.X.; Manly, J.; Stern, Y. Influence of leisure activity on the incidence of Alzheimer’s disease. Neurology 2001, 57, 2236–2242. [Google Scholar] [CrossRef] [PubMed]
- Wilson, R.S.; Mendes De Leon, C.F.; Barnes, L.L.; Schneider, J.A.; Bienias, J.L.; Evans, D.A.; Bennett, D.A. Participation in cognitively stimulating activities and risk of incident Alzheimer disease. JAMA 2002, 287, 742–748. [Google Scholar] [CrossRef] [PubMed]
- Wilson, R.S.; Bennett, D.A.; Bienias, J.L.; Aggarwal, N.T.; Mendes De Leon, C.F.; Morris, M.C.; Schneider, J.A.; Evans, D.A. Cognitive activity and incident AD in a population-based sample of older persons. Neurology 2002, 59, 1910–1914. [Google Scholar] [CrossRef] [PubMed]
- Sorman, D.E.; Sundstrom, A.; Ronnlund, M.; Adolfsson, R.; Nilsson, L.G. Leisure activity in old age and risk of dementia: A 15-year prospective study. J. Gerontol. B Psychol. Sci. Soc. Sci. 2014, 69, 493–501. [Google Scholar] [CrossRef]
- Akbaraly, T.N.; Portet, F.; Fustinoni, S.; Dartigues, J.F.; Artero, S.; Rouaud, O.; Touchon, J.; Ritchie, K.; Berr, C. Leisure activities and the risk of dementia in the elderly: Results from the Three-City Study. Neurology 2009, 73, 854–861. [Google Scholar] [CrossRef]
- Shimada, H.; Tsutsumimoto, K.; Lee, S.; Doi, T.; Makizako, H.; Lee, S.; Harada, K.; Hotta, R.; Bae, S.; Nakakubo, S.; et al. Driving continuity in cognitively impaired older drivers. Geriatr. Gerontol. Int. 2015, 16, 508–514. [Google Scholar] [CrossRef]
- Miller, E.A.; Weissert, W.G. Predicting elderly people’s risk for nursing home placement, hospitalization, functional impairment, and mortality: A synthesis. Med. Care Res. Rev. 2000, 57, 259–297. [Google Scholar] [CrossRef]
- Chen, L.K.; Liu, L.K.; Woo, J.; Assantachai, P.; Auyeung, T.W.; Bahyah, K.S.; Chou, M.Y.; Chen, L.Y.; Hsu, P.S.; Krairit, O.; et al. Sarcopenia in Asia: Consensus report of the Asian Working Group for Sarcopenia. J. Am. Med. Dir. Assoc. 2014, 15, 95–101. [Google Scholar] [CrossRef]
- Shimada, H.; Makizako, H.; Doi, T.; Yoshida, D.; Tsutsumimoto, K.; Anan, Y.; Uemura, K.; Ito, T.; Lee, S.; Park, H.; et al. Combined Prevalence of Frailty and Mild Cognitive Impairment in a Population of Elderly Japanese People. J. Am. Med. Dir. Assoc. 2013, 14, 518–524. [Google Scholar] [CrossRef]
- Shimada, H.; Suzuki, T.; Suzukawa, M.; Makizako, H.; Doi, T.; Yoshida, D.; Tsutsumimoto, K.; Anan, Y.; Uemura, K.; Ito, T.; et al. Performance-based assessments and demand for personal care in older Japanese people: A cross-sectional study. BMJ Open 2013, 3, e002424. [Google Scholar] [CrossRef] [PubMed]
- Fukutomi, E.; Okumiya, K.; Wada, T.; Sakamoto, R.; Ishimoto, Y.; Kimura, Y.; Chen, W.L.; Imai, H.; Kasahara, Y.; Fujisawa, M.; et al. Relationships between each category of 25-item frailty risk assessment (Kihon Checklist) and newly certified older adults under Long-Term Care Insurance: A 24-month follow-up study in a rural community in Japan. Geriatr. Gerontol. Int. 2014, 15, 864–871. [Google Scholar] [CrossRef]
- Folstein, M.F.; Folstein, S.E.; McHugh, P.R. “Mini-mental state.” A practical method for grading the cognitive state of patients for the clinician. J. Psychiatr. Res. 1975, 12, 189–198. [Google Scholar] [CrossRef]
- Sheikh, J.I.; Yesavage, J.A. Geriatric Depression Scale (GDS): Recent evidence and development of a shorter version. Clin. Gerontol. 1986, 5, 165–173. [Google Scholar] [CrossRef]
- Lesher, E.L.; Berryhill, J.S. Validation of the Geriatric Depression Scale--Short Form among inpatients. J. Clin. Psychol. 1994, 50, 256–260. [Google Scholar] [CrossRef]
- Stuck, A.E.; Walthert, J.M.; Nikolaus, T.; Bula, C.J.; Hohmann, C.; Beck, J.C. Risk factors for functional status decline in community-living elderly people: A systematic literature review. Soc. Sci. Med. 1999, 48, 445–469. [Google Scholar] [CrossRef]
- Ishizaki, T.; Watanabe, S.; Suzuki, T.; Shibata, H.; Haga, H. Predictors for functional decline among nondisabled older Japanese living in a community during a 3-year follow-up. J. Am. Geriatr. Soc. 2000, 48, 1424–1429. [Google Scholar] [CrossRef]
- WHO Expert Consultation. Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet 2004, 363, 157–163. [Google Scholar] [CrossRef]
- Ofori-Asenso, R.; Chin, K.L.; Mazidi, M.; Zomer, E.; Ilomaki, J.; Zullo, A.R.; Gasevic, D.; Ademi, Z.; Korhonen, M.J.; LoGiudice, D.; et al. Global Incidence of Frailty and Prefrailty Among Community-Dwelling Older Adults: A Systematic Review and Meta-analysis. JAMA Netw. Open 2019, 2, e198398. [Google Scholar] [CrossRef]
- Beauchet, O.; Annweiler, C.; Allali, G.; Berrut, G.; Herrmann, F.R.; Dubost, V. Recurrent falls and dual task-related decrease in walking speed: Is there a relationship? J. Am. Geriatr. Soc. 2008, 56, 1265–1269. [Google Scholar] [CrossRef]
- Kang, H.G.; Dingwell, J.B. Effects of walking speed, strength and range of motion on gait stability in healthy older adults. J. Biomech. 2008, 41, 2899–2905. [Google Scholar] [CrossRef]
- Guralnik, J.M.; Ferrucci, L.; Pieper, C.F.; Leveille, S.G.; Markides, K.S.; Ostir, G.V.; Studenski, S.; Berkman, L.F.; Wallace, R.B. Lower extremity function and subsequent disability: Consistency across studies, predictive models, and value of gait speed alone compared with the short physical performance battery. J. Gerontol. A Biol. Sci. Med. Sci. 2000, 55, M221–M231. [Google Scholar] [CrossRef] [PubMed]
- Cesari, M.; Kritchevsky, S.B.; Penninx, B.W.; Nicklas, B.J.; Simonsick, E.M.; Newman, A.B.; Tylavsky, F.A.; Brach, J.S.; Satterfield, S.; Bauer, D.C.; et al. Prognostic value of usual gait speed in well-functioning older people—Results from the Health, Aging and Body Composition Study. J. Am. Geriatr. Soc. 2005, 53, 1675–1680. [Google Scholar] [CrossRef]
- Cesari, M.; Kritchevsky, S.B.; Newman, A.B.; Simonsick, E.M.; Harris, T.B.; Penninx, B.W.; Brach, J.S.; Tylavsky, F.A.; Satterfield, S.; Bauer, D.C.; et al. Added value of physical performance measures in predicting adverse health-related events: Results from the Health, Aging And Body Composition Study. J. Am. Geriatr. Soc. 2009, 57, 251–259. [Google Scholar] [CrossRef]
- Onder, G.; Penninx, B.W.; Ferrucci, L.; Fried, L.P.; Guralnik, J.M.; Pahor, M. Measures of physical performance and risk for progressive and catastrophic disability: Results from the Women’s Health and Aging Study. J. Gerontol. A Biol. Sci. Med. Sci. 2005, 60, 74–79. [Google Scholar] [CrossRef] [PubMed]
- Studenski, S.; Perera, S.; Wallace, D.; Chandler, J.M.; Duncan, P.W.; Rooney, E.; Fox, M.; Guralnik, J.M. Physical performance measures in the clinical setting. J. Am. Geriatr. Soc. 2003, 51, 314–322. [Google Scholar] [CrossRef] [PubMed]
- Simonsick, E.M.; Newman, A.B.; Visser, M.; Goodpaster, B.; Kritchevsky, S.B.; Rubin, S.; Nevitt, M.C.; Harris, T.B. Mobility limitation in self-described well-functioning older adults: Importance of endurance walk testing. J. Gerontol. A Biol. Sci. Med. Sci. 2008, 63, 841–847. [Google Scholar] [CrossRef]
- Shinkai, S.; Watanabe, S.; Kumagai, S.; Fujiwara, Y.; Amano, H.; Yoshida, H.; Ishizaki, T.; Yukawa, H.; Suzuki, T.; Shibata, H. Walking speed as a good predictor for the onset of functional dependence in a Japanese rural community population. Age Ageing 2000, 29, 441–446. [Google Scholar] [CrossRef]
- Nofuji, Y.; Shinkai, S.; Taniguchi, Y.; Amano, H.; Nishi, M.; Murayama, H.; Fujiwara, Y.; Suzuki, T. Associations of Walking Speed, Grip Strength, and Standing Balance With Total and Cause-Specific Mortality in a General Population of Japanese Elders. J. Am. Med. Dir. Assoc. 2016, 17, 184 e181–187. [Google Scholar] [CrossRef]
- Liu, B.; Hu, X.; Zhang, Q.; Fan, Y.; Li, J.; Zou, R.; Zhang, M.; Wang, X.; Wang, J. Usual walking speed and all-cause mortality risk in older people: A systematic review and meta-analysis. Gait Posture 2016, 44, 172–177. [Google Scholar] [CrossRef]
- Montero-Odasso, M.; Schapira, M.; Soriano, E.R.; Varela, M.; Kaplan, R.; Camera, L.A.; Mayorga, L.M. Gait velocity as a single predictor of adverse events in healthy seniors aged 75 years and older. J. Gerontol. A Biol. Sci. Med. Sci. 2005, 60, 1304–1309. [Google Scholar] [CrossRef]
- Volpato, S.; Guralnik, J.M.; Ferrucci, L.; Balfour, J.; Chaves, P.; Fried, L.P.; Harris, T.B. Cardiovascular disease, interleukin-6, and risk of mortality in older women: The women’s health and aging study. Circulation 2001, 103, 947–953. [Google Scholar] [CrossRef] [PubMed]
- Shimada, H.; Makizako, H.; Doi, T.; Tsutsumimoto, K.; Suzuki, T. Incidence of Disability in Frail Older Persons with or Without Slow Walking Speed. J. Am. Med. Dir. Assoc. 2015, 16, 690–696. [Google Scholar] [CrossRef] [PubMed]
- Beaudart, C.; Dawson, A.; Shaw, S.C.; Harvey, N.C.; Kanis, J.A.; Binkley, N.; Reginster, J.Y.; Chapurlat, R.; Chan, D.C.; Bruyere, O.; et al. Nutrition and physical activity in the prevention and treatment of sarcopenia: Systematic review. Osteoporos. Int. 2017, 28, 1817–1833. [Google Scholar] [CrossRef] [PubMed]
- Ng, T.P.; Feng, L.; Nyunt, M.S.; Feng, L.; Niti, M.; Tan, B.Y.; Chan, G.; Khoo, S.A.; Chan, S.M.; Yap, P.; et al. Nutritional, Physical, Cognitive, and Combination Interventions and Frailty Reversal Among Older Adults: A Randomized Controlled Trial. Am. J. Med. 2015, 128, 1225.e1–1236.e1. [Google Scholar] [CrossRef] [PubMed]
- Luger, E.; Dorner, T.E.; Haider, S.; Kapan, A.; Lackinger, C.; Schindler, K. Effects of a Home-Based and Volunteer-Administered Physical Training, Nutritional, and Social Support Program on Malnutrition and Frailty in Older Persons: A Randomized Controlled Trial. J. Am. Med. Dir. Assoc. 2016, 17, 671.e9–671.e16. [Google Scholar] [CrossRef]
- Glass, T.A.; de Leon, C.M.; Marottoli, R.A.; Berkman, L.F. Population based study of social and productive activities as predictors of survival among elderly Americans. BMJ 1999, 319, 478–483. [Google Scholar] [CrossRef]
- Jung, Y.; Gruenewald, T.L.; Seeman, T.E.; Sarkisian, C.A. Productive activities and development of frailty in older adults. J. Gerontol. B Psychol. Sci. Soc. Sci. 2010, 65, 256–261. [Google Scholar] [CrossRef]
- Feng, Z.; Lugtenberg, M.; Franse, C.; Fang, X.; Hu, S.; Jin, C.; Raat, H. Risk factors and protective factors associated with incident or increase of frailty among community-dwelling older adults: A systematic review of longitudinal studies. PLoS ONE 2017, 12, e0178383. [Google Scholar] [CrossRef]
- Kristman, V.; Manno, M.; Cote, P. Loss to follow-up in cohort studies: How much is too much? Eur. J. Epidemiol. 2004, 19, 751–760. [Google Scholar] [CrossRef]
| Included Participants (n = 2631) | Excluded Participants (n = 2473) | p | |
|---|---|---|---|
| Age (years) † | 71.0 (4.7) | 73.6 (6.4) | <0.01 |
| Sex (% male) | 49.5 | 48.2 | 0.34 |
| Education (years) † | 11.7 (2.5) | 10.9 (2.6) *14 | <0.01 |
| BMI > 27.5 (% yes) | 7.6 | 10.7 | <0.01 |
| BMI < 18.5 (% yes) | 3.3 | 5.8 | <0.01 |
| Stroke (% yes) | 4.0 | 7.1 | <0.01 |
| Heart disease (% yes) | 15.6 | 18.4 | <0.01 |
| Pulmonary disease (% yes) | 10.9 | 12.1 | 0.20 |
| Osteoarthritis (% yes) | 13.8 | 15.4 | 0.11 |
| Diabetes mellitus (% yes) | 11.4 | 15.6 | <0.01 |
| History of falls (% yes) | 13.1 | 17.9 | <0.01 |
| Medication (number) † | 1.9 (1.9) | 2.4 (2.4) | <0.01 |
| Minimental state examination (points) † | 26.6 (2.5) | 25.6 (3.0) *13 | <0.01 |
| Geriatric depression scale (points) † | 2.4 (2.4) | 3.5 (2.9) *18 | <0.01 |
| Serum albumin (g/dL) † | 4.3 (0.2) | 4.3 (0.3) *39 | <0.01 |
| Instrumental activities of daily living (% yes) | |||
| Going outdoors using bus and train | 93.1 | 84.5 | <0.01 |
| Cash handling and banking | 90.7 | 86.6 | <0.01 |
| Driving a car | 76.7 | 60.2 | <0.01 |
| Using map to go unfamiliar places | 67.6 | 53.9 | <0.01 |
| Cognitive activity (% yes) | |||
| Reading of book or newspaper | 97.1 | 93.3 | <0.01 |
| Cognitive stimulation such as board game and learning | 53.7 | 42.2 | <0.01 |
| Culture lesson | 46.8 | 32.6 | <0.01 |
| Using personal computer | 39.6 | 25.2 | <0.01 |
| Social activity (% yes) | |||
| Daily conversation | 97.1 | 93.9 | <0.01 |
| Giving someone a helping hand | 93.7 | 87.3 | <0.01 |
| Attending a meeting in the community | 55.5 | 44.3 | <0.01 |
| Hobby or sports activity | 79.9 | 61.5 | <0.01 |
| Productive activity (% yes) | |||
| Housecleaning | 87.7 | 84.2 | <0.01 |
| Field work or gardening | 75.5 | 66 | <0.01 |
| Taking care of grandchild or pet | 57.8 | 50.1 | <0.01 |
| Working | 32.2 | 25.5 | <0.01 |
| Activity score (points) † | |||
| Instrumental activities of daily living | 3.3 (0.8) | 2.9 (1.1) *40 | <0.01 |
| Cognitive activity | 2.4 (1.1) | 1.9 (1.0) *31 | <0.01 |
| Social activity | 3.3 (0.8) | 2.9 (1.0) *71 | <0.01 |
| Productive activity | 2.5 (0.9) | 2.3 (1.0) *38 | <0.01 |
| Total | 11.4 (2.4) | 9.9 (2.9) *151 | <0.01 |
| Participants without Frailty (n = 2459) | Participants with Frailty (n = 172) | p Value | |
|---|---|---|---|
| Age (years) * | 70.8 (4.5) | 73.9 (5.9) | <0.01 |
| Sex (% male) | 49.8 | 44.8 | 0.20 |
| Education (years) * | 11.8 (2.5) | 10.7 (2.4) | <0.01 |
| BMI > 27.5 (% yes) | 7.1 | 15.7 | <0.01 |
| BMI < 18.5 (% yes) | 3.3 | 3.5 | 0.89 |
| Stroke (% yes) | 3.6 | 9.9 | <0.01 |
| Heart disease (% yes) | 15.5 | 16.9 | 0.63 |
| Pulmonary disease (% yes) | 10.7 | 15.1 | 0.07 |
| Osteoarthritis (% yes) | 13.8 | 13.4 | 0.87 |
| Diabetes mellitus (% yes) | 11.0 | 16.9 | 0.02 |
| History of falls (% yes) | 12.3 | 25.0 | <0.01 |
| Medication (number) * | 1.8 (1.9) | 2.5 (2.1) | <0.01 |
| Mini-mental state examination (points) * | 26.7 (2.4) | 25.3 (3.2) | <0.01 |
| Geriatric depression scale (points) * | 2.3 (2.3) | 3.6 (2.7) | <0.01 |
| Serum albumin * | 4.3 (0.2) | 4.3 (0.2) | <0.01 |
| Activity score (points) * | |||
| Instrumental activities of daily living | 3.3 (0.8) | 2.9 (1.0) | <0.01 |
| Cognitive activity | 2.4 (1.0) | 1.9 (1.0) | <0.01 |
| Social activity | 3.3 (0.8) | 2.7 (1.0) | <0.01 |
| Productive activity | 2.5 (0.9) | 2.3 (0.9) | <0.01 |
| Total | 11.6 (2.3) | 9.7 (2.7) | <0.01 |
| Model 1 | Model 2 | |||
|---|---|---|---|---|
| Odds Ratio (95% CI) | p Value | Odds Ratio (95% CI) | p Value | |
| Instrumental activities of daily living (% yes) | ||||
| Going outdoors using bus and train | 0.47 (0.28–0.81) | <0.01 | 0.66 (0.37–1.18) | 0.16 |
| Cash handling and banking | 0.72 (0.42–1.21) | 0.21 | 0.86 (0.49–1.51) | 0.60 |
| Driving a car | 1.09 (0.72–1.65) | 0.68 | 1.33 (0.86–2.05) | 0.20 |
| Using map to go unfamiliar place | 0.66 (0.46–0.95) | 0.02 | 0.81 (0.55–1.18) | 0.27 |
| Cognitive activity (% yes) | ||||
| Reading of book or newspaper | 0.99 (0.42–2.32) | 0.98 | 1.40 (0.57–3.44) | 0.47 |
| Cognitive stimulation such as board game and learning | 0.69 (0.48–0.98) | 0.04 | 1.04 (0.7–1.55) | 0.86 |
| Culture lessons | 0.64 (0.45–0.93) | 0.02 | 1.12 (0.73–1.72) | 0.61 |
| Using personal computer | 0.57 (0.36–0.90) | 0.02 | 0.68 (0.42–1.09) | 0.11 |
| Social activity (% yes) | ||||
| Daily conversation | 0.48 (0.25–0.95) | 0.03 | 0.66 (0.32–1.37) | 0.27 |
| Giving someone advice | 0.52 (0.31–0.86) | 0.01 | 0.71 (0.41–1.22) | 0.22 |
| Attending a meeting in the community | 0.49 (0.35–0.69) | <0.01 | 0.61 (0.42–0.88) | 0.01 |
| Hobby or sports activity | 0.32 (0.22–0.46) | <0.01 | 0.35 (0.23–0.52) | <0.01 |
| Productive activity (% yes) | ||||
| Housecleaning | 0.82 (0.49–1.38) | 0.45 | 1.04 (0.60–1.80) | 0.90 |
| Field work or gardening | 0.82 (0.57–1.18) | 0.53 | 0.99 (0.67–1.46) | 0.97 |
| Taking care of grandchild or pet | 0.83 (0.60–1.16) | 0.28 | 0.94 (0.66–1.33) | 0.73 |
| Working | 0.93 (0.63–1.39) | 0.74 | 0.88 (0.58–1.33) | 0.54 |
| Model 1 | Model 2 | |||
|---|---|---|---|---|
| Odds Ratio (95% CI) | p Value | Odds Ratio (95% CI) | p Value | |
| Total score of IADL (point) | 0.78 (0.64–0.96) | 0.02 | 0.92 (0.74–1.14) | 0.44 |
| Total score of cognitive activity (point) | 0.74 (0.62–0.89) | <0.01 | 0.92 (0.75–1.11) | 0.38 |
| Total score of social activity (point) | 0.52 (0.43–0.63) | <0.01 | 0.55 (0.44–0.67) | <0.01 |
| Total score of productive activity (point) | 0.86 (0.72–1.04) | 0.12 | 0.98 (0.81–1.19) | 0.87 |
| Total score of all activity (point) | 0.81 (0.75–0.87) | <0.01 | ||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shimada, H.; Doi, T.; Tsutsumimoto, K.; Lee, S.; Bae, S.; Arai, H. Behavioral Factors Related to the Incidence of Frailty in Older Adults. J. Clin. Med. 2020, 9, 3074. https://doi.org/10.3390/jcm9103074
Shimada H, Doi T, Tsutsumimoto K, Lee S, Bae S, Arai H. Behavioral Factors Related to the Incidence of Frailty in Older Adults. Journal of Clinical Medicine. 2020; 9(10):3074. https://doi.org/10.3390/jcm9103074
Chicago/Turabian StyleShimada, Hiroyuki, Takehiko Doi, Kota Tsutsumimoto, Sangyoon Lee, Seongryu Bae, and Hidenori Arai. 2020. "Behavioral Factors Related to the Incidence of Frailty in Older Adults" Journal of Clinical Medicine 9, no. 10: 3074. https://doi.org/10.3390/jcm9103074
APA StyleShimada, H., Doi, T., Tsutsumimoto, K., Lee, S., Bae, S., & Arai, H. (2020). Behavioral Factors Related to the Incidence of Frailty in Older Adults. Journal of Clinical Medicine, 9(10), 3074. https://doi.org/10.3390/jcm9103074






