Uninterrupted or Minimally Interrupted Direct Oral Anticoagulant Therapy is a Safe Alternative to Vitamin K Antagonists in Patients Undergoing Catheter Ablation for Atrial Fibrillation: An Updated Meta-Analysis
Abstract
1. Introduction
2. Methods
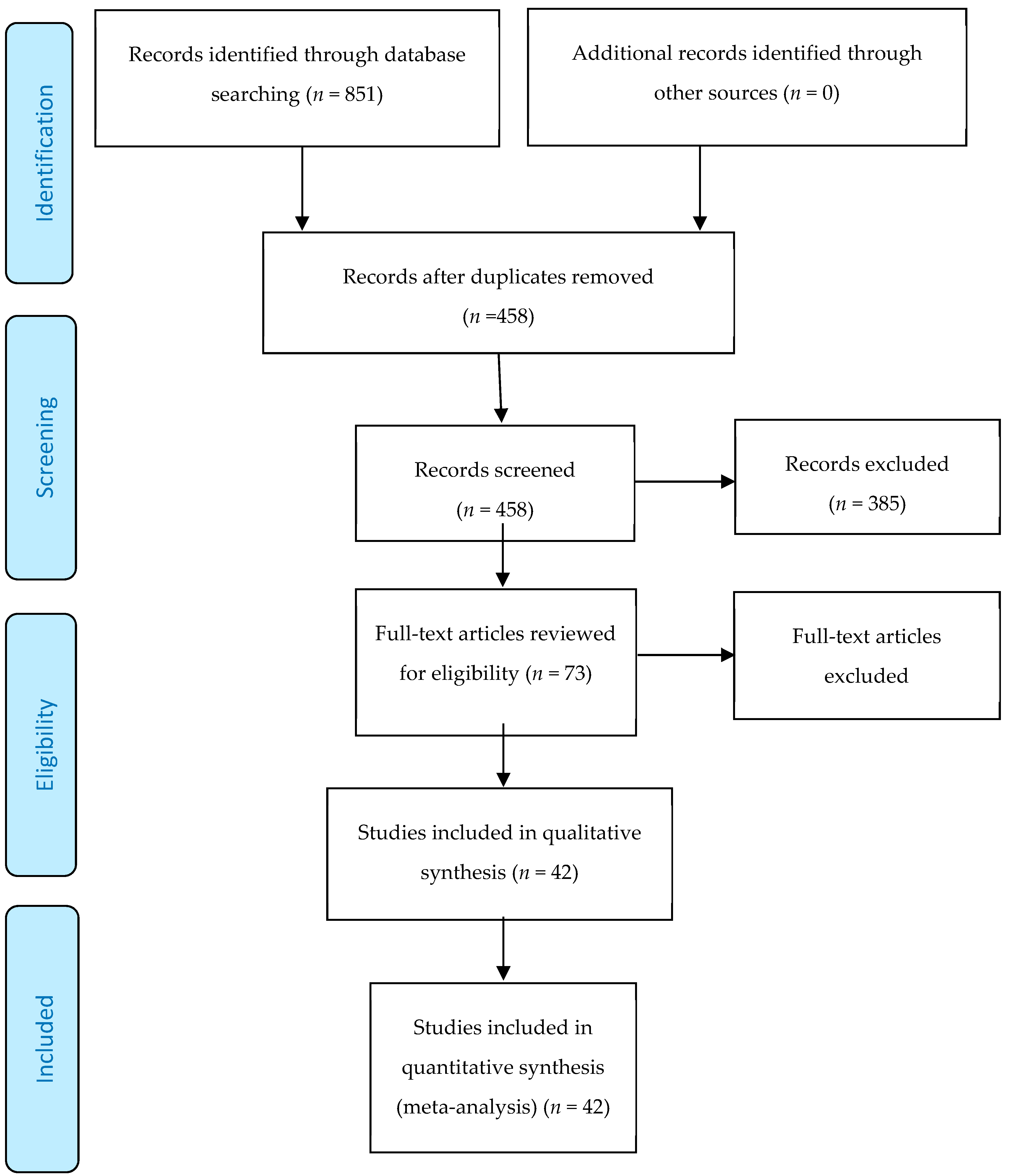
2.1. Search Strategy and Data Extraction
2.2. Eligibility Criteria
2.3. Screening
2.4. Endpoints
2.5. Subgroup Analyses
2.6. Statistical Analysis
3. Results
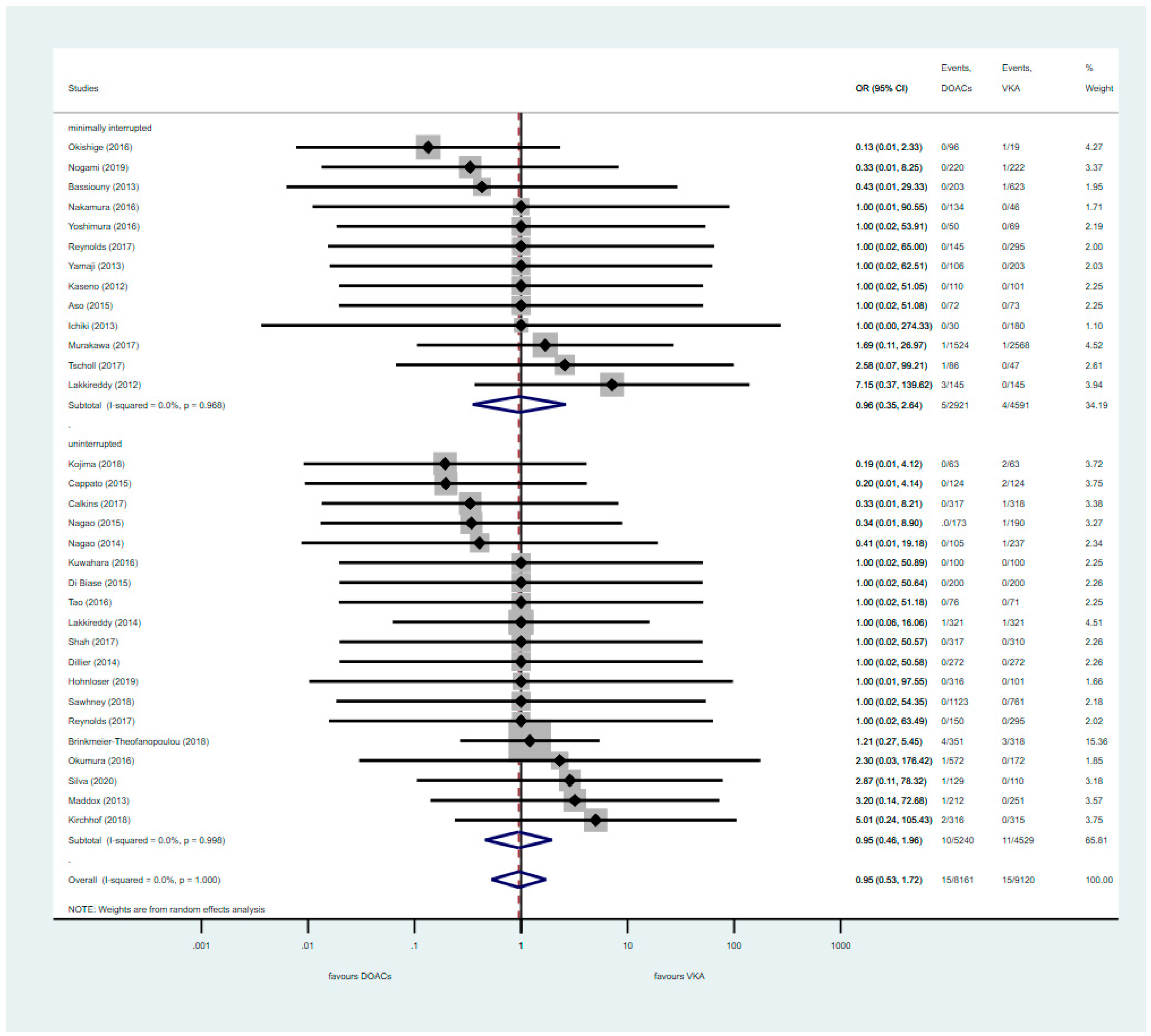
3.1. Analysis of RCTs
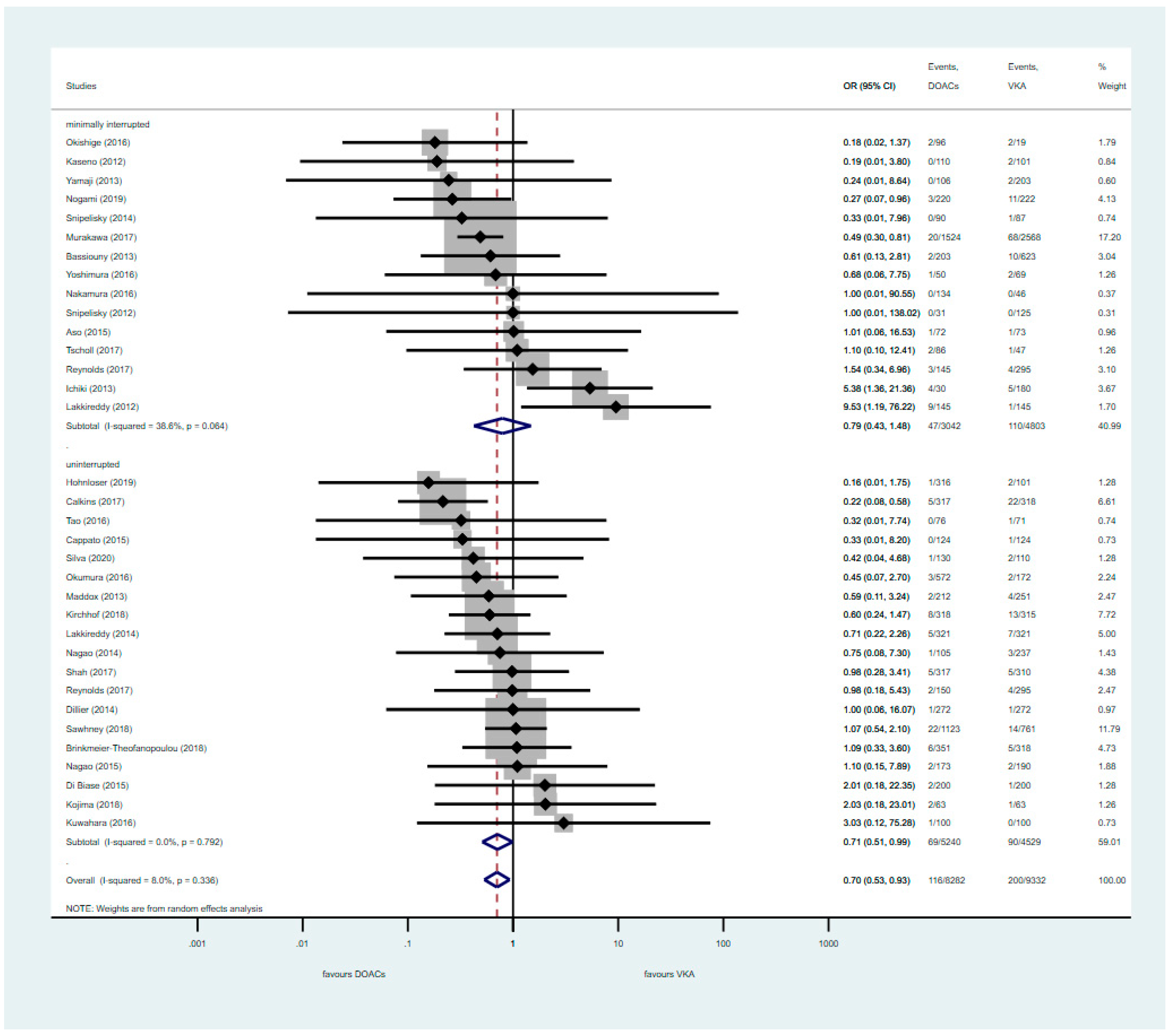
3.2. Pooled Analysis of Uninterrupted and Minimally Interrupted DOAC Studies
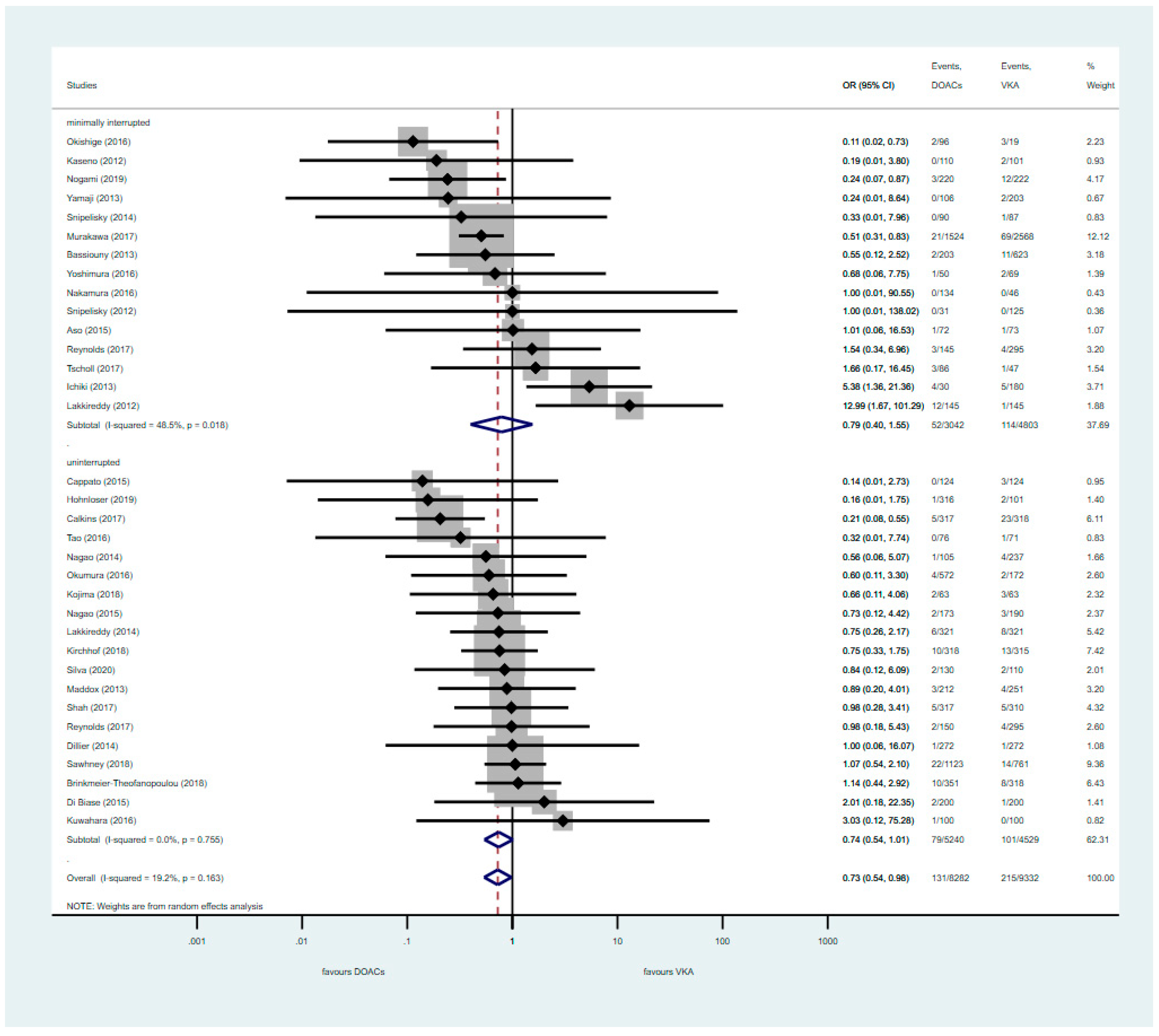
3.3. Analysis of Nterrupted DOAC Studies
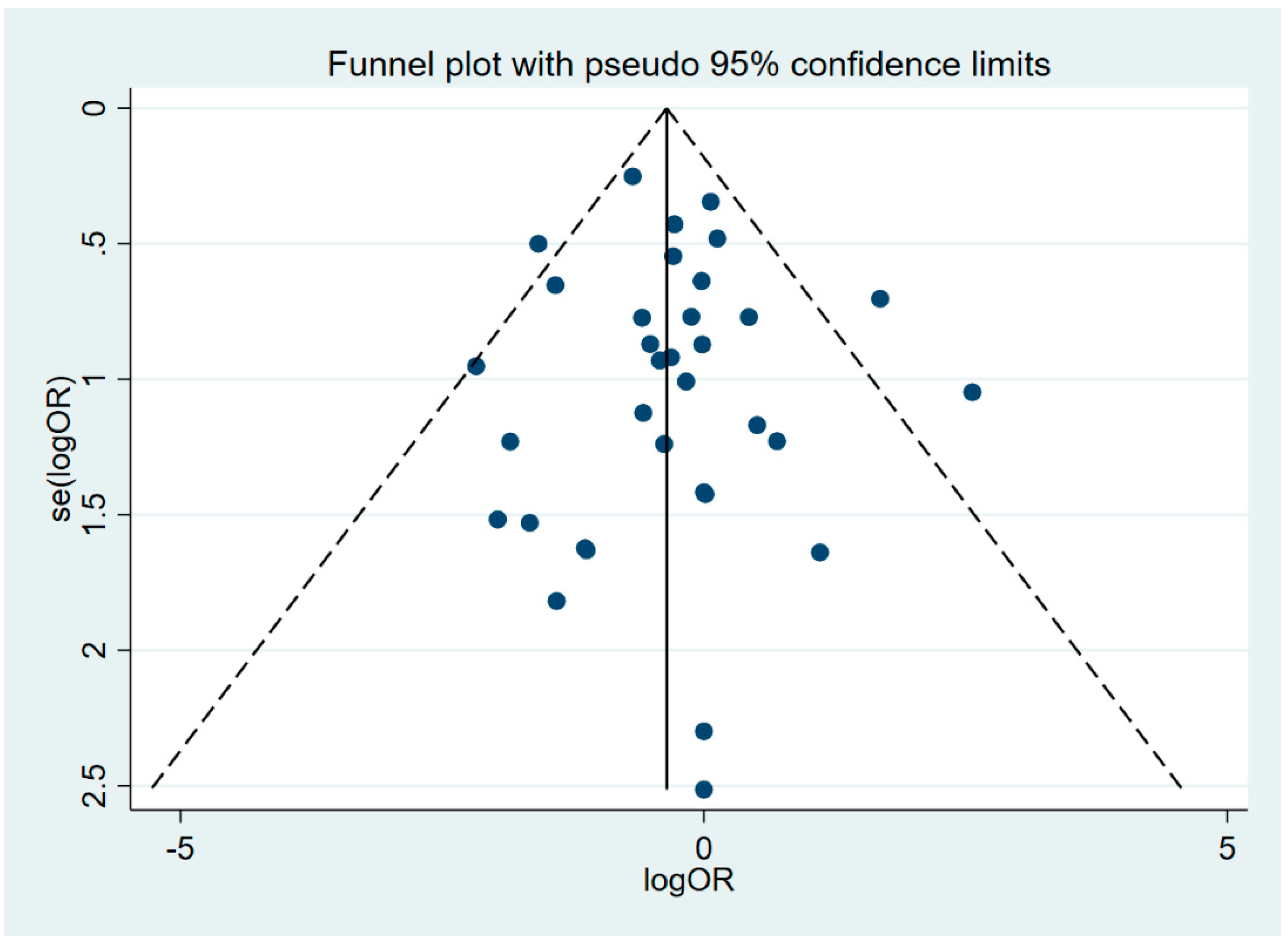
3.4. Quality Assessment
4. Discussion
5. Conclusions
6. Limitations
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chugh, S.S.; Havmoeller, R.; Narayanan, K.; Singh, D.; Rienstra, M.; Benjamin, E.J.; Gillum, R.F.; Kim, Y.H.; McAnulty, J.H.; Zheng, Z.-J.; et al. Worldwide epidemiology of atrial fibrillation: A global burden of disease 2010 study. Circulation 2013, 129, 837–847. [Google Scholar] [CrossRef]
- Wolf, P.A.; Abbott, R.D.; Kannel, W.B. Atrial fibrillation as an independent risk factor for stroke: The Framingham Study. Stroke 1991, 22, 983–988. [Google Scholar] [CrossRef] [PubMed]
- Hart, R.G.; Pearce, L.A.; Aguilar, M.I. Meta-analysis: Antithrombotic therapy to prevent stroke in patients who have nonvalvular atrial fibrillation. Ann. Intern. Med. 2007, 146, 857–867. [Google Scholar] [CrossRef] [PubMed]
- Granger, C.B.; Alexander, J.H.; McMurray, J.J.; Lopes, R.D.; Hylek, E.M.; Hanna, M.; Al-Khalidi, H.R.; Ansell, J.; Atar, D.; Avezum, Á.; et al. Apixaban versus warfarin in patients with atrial fibrillation. N. Engl. J. Med. 2011, 365, 981–992. [Google Scholar] [CrossRef] [PubMed]
- Giugliano, R.P.; Ruff, C.T.; Braunwald, E.; Murphy, S.A.; Wiviott, S.D.; Halperin, J.L.; Waldo, A.L.; Ezekowitz, M.D.; Weitz, J.I.; Špinar, J.; et al. Edoxaban versus warfarin in patients with atrial fibrillation. N. Engl. J. Med. 2013, 369, 2093–2104. [Google Scholar] [CrossRef]
- Connolly, S.J.; Ezekowitz, M.D.; Yusuf, S.; Eikelboom, J.W.; Oldgren, J.; Parekh, A.; Pogue, J.; Reilly, P.A.; Themeles, E.; Varrone, J.; et al. Dabigatran versus warfarin in patients with atrial fibrillation. N. Engl. J. Med. 2009, 361, 1139–1151. [Google Scholar] [CrossRef]
- Patel, M.R.; Mahaffey, K.W.; Garg, J.; Pan, G.; Singer, D.E.; Hacke, W.; Breithardt, G.; Halperin, J.L.; Hankey, G.J.; Piccini, J.P.; et al. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N. Engl. J. Med. 2011, 365, 883–891. [Google Scholar] [CrossRef]
- Al-Khatib, S.M.; Lapointe, N.M.A.; Povsic, T.J.; Raju, S.S.; Shah, B.; Kosinski, A.S.; McBroom, A.J.; Sanders, G.D.; Chatterjee, R.; Crowley, M.J.; et al. Rate- and rhythm-control therapies in patients with atrial fibrillation: A systematic review. Ann. Intern. Med. 2014, 160, 760–773. [Google Scholar] [CrossRef]
- Noheria, A.; Shrader, P.; Thomas, L.E.; Peterson, E.D.; Gersh, B.J.; Piccini, J.P.; Fonarow, G.C.; Kowey, P.R.; Mahaffey, K.W.; Naccarelli, G.; et al. Rhythm control versus rate control and clinical outcomes in patients with atrial fibrillation: Results from the ORBIT-AF registry. JACC Clin. Electrophysiol. 2016, 2, 221–229. [Google Scholar] [CrossRef]
- Hindricks, G.; Potpara, T.; Dagres, N.; Arbelo, E.; Bax, J.J.; Lundquist, C.B.; Boriani, G.; Castella, M.; Dan, G.A.; Dilaveris, P.; et al. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association of Cardio-Thoracic Surgery (EACTS): The Task Force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) Developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur. Heart J. 2020, ehaa612. [Google Scholar] [CrossRef]
- Deshmukh, A.J.; Patel, N.J.; Pant, S.; Shah, N.; Chothani, A.; Mehta, K.; Grover, P.; Singh, V.; Vallurupalli, S.; Savani, G.T.; et al. In-Hospital complications associated with catheter ablation of atrial fibrillation in the United States between 2000 and 2010: Analysis of 93,801 procedures. Circulation 2013, 128, 2104–2112. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Perera, T.; Ganesan, A.; Sullivan, T.; Lau, D.S.; Roberts-Thomson, K.C.; Brooks, A.G.; Sanders, P. Complications of catheter ablation of atrial fibrillation: A systematic review. Circ. Arrhythm. Electrophysiol. 2013, 6, 1082–1088. [Google Scholar] [CrossRef] [PubMed]
- Providência, R.; Marijon, E.; Albenque, J.-P.; Combes, S.; Jourda, F.; Hireche, H.; Morais, J.; Boveda, S. Rivaroxaban and dabigatran in patients undergoing catheter ablation of atrial fibrillation. Europace 2014, 16, 1137–1144. [Google Scholar] [CrossRef] [PubMed]
- Bassiouny, M.; Saliba, W.; Rickard, J.; Shao, M.; Sey, A.; Diab, M.; Martin, D.O.; Hussein, A.; Khoury, M.; Abi-Saleh, B.; et al. Use of dabigatran for periprocedural anticoagulation in patients undergoing catheter ablation for atrial fibrillation. Circ. Arrhythm. Electrophysiol. 2013, 6, 460–466. [Google Scholar] [CrossRef]
- Winkle, R.A.; Mead, R.H.; Engel, G.; Kong, M.H.; Patrawala, R.A. Peri-procedural interrupted oral anticoagulation for atrial fibrillation ablation: Comparison of aspirin, warfarin, dabigatran, and rivaroxaban. Europace 2014, 16, 1443–1449. [Google Scholar] [CrossRef]
- Armbruster, H.L.; Lindsley, J.P.; Moranville, M.P.; Habibi, M.; Khurram, I.M.; Spragg, D.D.; Berger, R.D.; Calkins, H.; Marine, J.E. Safety of novel oral anticoagulants compared with uninterrupted warfarin for catheter ablation of atrial fibrillation. Ann. Pharm. 2015, 49, 278–284. [Google Scholar] [CrossRef]
- Potpara, T.S.; Larsen, T.B.; Deharo, J.C.; Rossvoll, O.; Dagres, N.; Todd, D.; Pison, L.; Proclemer, A.; Purefellner, H.; Blomström-Lundqvist, C. Oral anticoagulant therapy for stroke prevention in patients with atrial fibrillation undergoing ablation: Results from the first European Snapshot Survey on procedural routines for atrial fibrillation ablation (ESS-PRAFA). Europace 2015, 17, 986–993. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; The PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. BMJ 2009, 339, b2535. [Google Scholar] [CrossRef]
- Mehran, R.; Rao, S.V.; Bhatt, D.L.; Gibson, C.M.; Caixeta, A.; Eikelboom, J.W.; Kaul, S.; Wiviott, S.D.; Menon, V.; Nikolsky, E.; et al. Standardized bleeding definitions for cardiovascular clinical trials: A consensus report from the bleeding academic research consortium. Circulation 2011, 123, 2736–2747. [Google Scholar] [CrossRef]
- Schulman, S.; Kearon, C.; Subcommittee on Control of Anticoagulation of the Scientific and Standardization Committee of the International Society on Thrombosis and Haemostasis. Definition of major bleeding in clinical investigations of antihemostatic medicinal products in nonsurgical patients. J. Thromb. Haemost. 2005, 3, 692–694. [Google Scholar] [CrossRef]
- Nogami, A.; Harada, T.; Sekiguchi, Y.; Otani, R.; Yoshida, Y.; Yoshida, K.; Nakano, Y.; Nuruki, N.; Nakahara, S.; Goya, M.; et al. Safety and Efficacy of Minimally Interrupted Dabigatran vs Uninterrupted Warfarin Therapy in Adults Undergoing Atrial Fibrillation Catheter Ablation: A Randomized Clinical Trial. JAMA Netw. Open. 2019, 2, e191994. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.U.; Rahman, H.; Talluri, S.; Kaluski, E. The clinical benefits and mortality reduction associated with catheter ablation in subjects with atrial fibrillation: A systematic review and meta-analysis. JACC Clin. Electrophysiol. 2018, 4, 626–635. [Google Scholar] [CrossRef] [PubMed]
- Packer, D.L.; Mark, D.B.; Robb, R.A.; Monahan, K.H.; Bahnson, T.D.; Poole, J.E.; Noseworthy, P.A.; Rosenberg, Y.D.; Jeffries, N.; Mitchell, L.B.; et al. Effect of catheter ablation vs antiarrhythmic drug therapy on mortality, stroke, bleeding, and cardiac arrest among patients with atrial ibrillation: The CABANA Randomized Clinical Trial. JAMA 2019, 321, 1261–1274. [Google Scholar] [CrossRef] [PubMed]
- Friberg, L.; Tabrizi, F.; Englund, A. Catheter ablation for atrial fibrillation is associated with lower incidence of stroke and death: Data from Swedish health registries. Eur. Heart J. 2016, 37, 2478–2487. [Google Scholar] [CrossRef]
- Kirchhof, P.; Camm, A.J.; Goette, A.; Brandes, A.; Eckardt, L.; Elvan, A.; Fetsch, T.; Van Gelder, I.C.; Haase, D.; Haegeli, L.M.; et al. Early rhythm-control therapy in patients with atrial fibrillation. N. Engl. J. Med. 2020. [Google Scholar] [CrossRef]
- Marrouche, N.F.; Brachmann, J.; Andresen, D.; Siebels, J.; Boersma, L.; Jordaens, L.; Merkely, B.; Pokushalov, E.; Sanders, P.; Proff, J.; et al. Catheter ablation for atrial fibrillation with heart failure. N. Engl. J. Med. 2018, 378, 417–427. [Google Scholar] [CrossRef]
- Ha, F.J.; Barra, S.; Brown, A.J.; Begley, D.A.; Grace, A.A.; Agarwal, S. Continuous and minimally-interrupted direct oral anticoagulant are both safe compared with vitamin K antagonist for atrial fibrillation ablation: An updated meta-analysis. Int. J. Cardiol. 2018, 262, 51–56. [Google Scholar] [CrossRef]
- Cardoso, R.N.; Knijnik, L.; Bhonsale, A.; Miller, J.; Nasi, G.; Rivera, M.; Blumer, V.; Calkins, H. An updated meta-analysis of novel oral anticoagulants versus vitamin K antagonists for uninterrupted anticoagulation in atrial fibrillation catheter ablation. Heart Rhythm 2018, 15, 107–115. [Google Scholar] [CrossRef]
- Romero, J.; Cerrud-Rodriguez, R.C.; Díaz, J.C.; Michaud, G.F.; Taveras, J.; Alviz, I.; Grupposo, V.; Cerna, L.; Avendano, R.; Kumar, S.; et al. Uninterrupted direct oral anticoagulants vs. uninterrupted vitamin K antagonists during catheter ablation of non-valvular atrial fibrillation: A systematic review and meta-analysis of randomized controlled trials. Europace 2018, 20, 1612–1620. [Google Scholar] [CrossRef]
- Nakamura, K.; Naito, S.; Sasaki, T.; Take, Y.; Minami, K.; Kitagawa, Y.; Motoda, H.; Inoue, M.; Otsuka, Y.; Niijima, K.; et al. Uninterrupted vs. interrupted periprocedural direct oral anticoagulants for catheter ablation of atrial fibrillation: A prospective randomized single-centre study on post-ablation thrombo-embolic and haemorrhagic events. Europace 2018, 21, 259–267. [Google Scholar] [CrossRef]
- Calkins, H.; Willems, S.; Gerstenfeld, E.P.; Verma, A.; Schilling, R.; Hohnloser, S.H.; Okumura, K.; Serota, H.; Nordaby, M.; Guiver, K.; et al. Uninterrupted dabigatran versus warfarin for ablation in atrial fibrillation. N. Engl. J. Med. 2017, 376, 1627–1636. [Google Scholar] [CrossRef] [PubMed]
- Goya, M.; Nogami, A.; Hirao, K.; Aonuma, K. Ablation perioperative dabigatran in use envisioning in Japan: The ABRIDGE-J Study Design. J. Cardiol. 2016, 68, 236–240. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Reynolds, M.R.; Allison, J.S.; Natale, A.; Weisberg, I.L.; Ellenbogen, K.A.; Richards, M.; Hsieh, W.-H.; Sutherland, J.; Cannon, C.P. A Prospective randomized trial of apixaban dosing during atrial fibrillation ablation. JACC Clin. Electrophysiol. 2018, 4, 580–588. [Google Scholar] [CrossRef] [PubMed]
- Calkins, H.; Hindricks, G.; Cappato, R. 2017 HRS/EHRA/ECAS/APHRS/SOLAECE expert consensus statement on catheter and surgical ablation of atrial fibrillation. Heart Rhythm 2017, 14, e275–e444. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ottóffy, M.; Mátrai, P.; Farkas, N.; Hegyi, P.; Czopf, L.; Márta, K.; Garami, A.; Balaskó, M.; Pótóné-Oláh, E.; Mikó, A.; et al. Uninterrupted or Minimally Interrupted Direct Oral Anticoagulant Therapy is a Safe Alternative to Vitamin K Antagonists in Patients Undergoing Catheter Ablation for Atrial Fibrillation: An Updated Meta-Analysis. J. Clin. Med. 2020, 9, 3073. https://doi.org/10.3390/jcm9103073
Ottóffy M, Mátrai P, Farkas N, Hegyi P, Czopf L, Márta K, Garami A, Balaskó M, Pótóné-Oláh E, Mikó A, et al. Uninterrupted or Minimally Interrupted Direct Oral Anticoagulant Therapy is a Safe Alternative to Vitamin K Antagonists in Patients Undergoing Catheter Ablation for Atrial Fibrillation: An Updated Meta-Analysis. Journal of Clinical Medicine. 2020; 9(10):3073. https://doi.org/10.3390/jcm9103073
Chicago/Turabian StyleOttóffy, Máté, Péter Mátrai, Nelli Farkas, Péter Hegyi, László Czopf, Katalin Márta, András Garami, Márta Balaskó, Emőke Pótóné-Oláh, Alexandra Mikó, and et al. 2020. "Uninterrupted or Minimally Interrupted Direct Oral Anticoagulant Therapy is a Safe Alternative to Vitamin K Antagonists in Patients Undergoing Catheter Ablation for Atrial Fibrillation: An Updated Meta-Analysis" Journal of Clinical Medicine 9, no. 10: 3073. https://doi.org/10.3390/jcm9103073
APA StyleOttóffy, M., Mátrai, P., Farkas, N., Hegyi, P., Czopf, L., Márta, K., Garami, A., Balaskó, M., Pótóné-Oláh, E., Mikó, A., Rostás, I., Wobbe, B., & Habon, T. (2020). Uninterrupted or Minimally Interrupted Direct Oral Anticoagulant Therapy is a Safe Alternative to Vitamin K Antagonists in Patients Undergoing Catheter Ablation for Atrial Fibrillation: An Updated Meta-Analysis. Journal of Clinical Medicine, 9(10), 3073. https://doi.org/10.3390/jcm9103073






