Independent Impact of Gynoid Fat Distribution and Free Testosterone on Circulating Levels of N-Terminal Pro-Brain Natriuretic Peptide (NT-proBNP) in Humans
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Data Collection and Assays
2.3. Ethical Issues
2.4. Statistical Analysis
3. Results
4. Discussion
4.1. NT-proBNP Serum Levels in Relation to Gender
4.2. Impact of Clinical Factors on NT-proBNP
4.3. Impact of Obesity and Fat Tissue Distribution on NT-proBNP Concentration
4.4. Impact of Gender and Steroid Hormones on NT-proBNP
4.5. Possible Mechanism
4.6. Limitation
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Daniels, L.B.; Maisel, A.S. Natriuretic peptides. J. Am. Coll. Cardiol. 2007, 50, 2357–2368. [Google Scholar] [CrossRef] [PubMed]
- De Lemos, J.A.; McGuire, D.K.; Drazner, M.H. B-type natriuretic peptide in cardiovascular disease. Lancet 2003, 362, 316–322. [Google Scholar] [CrossRef]
- Hunt, P.J.; Richards, A.M.; Nicholls, M.G.; Yandle, T.G.; Doughty, R.N.; Espiner, E.A. Immunoreactive amino-terminal pro-brain natriuretic peptide (NT-PROBNP): A new marker of cardiac impairment. Clin. Endocrinol. 1997, 47, 287–296. [Google Scholar] [CrossRef] [PubMed]
- Groenning, B.A.; Nilsson, J.C.; Sondergaard, L.; Pedersen, F.; Trawinski, J.; Baumann, M.; Larsson, H.B.; Hildebrandt, P.R. Detection of left ventricular enlargement and impaired systolic function with plasma N-terminal pro brain natriuretic peptide concentrations. Am. Heart J. 2002, 143, 923–929. [Google Scholar] [CrossRef]
- Groenning, B.A.; Raymond, I.; Hildebrandt, P.R.; Nilsson, J.C.; Baumann, M.; Pedersen, F. Diagnostic and prognostic evaluation of left ventricular systolic heart failure by plasma N-terminal pro-brain natriuretic peptide concentrations in a large sample of the general population. Heart 2004, 90, 297–303. [Google Scholar] [CrossRef]
- Januzzi, J.L., Jr.; Camargo, C.A.; Anwaruddin, S.; Baggish, A.L.; Chen, A.A.; Krauser, D.G.; Tung, R.; Cameron, R.; Nagurney, J.T.; Chae, C.U.; et al. The N-terminal Pro-BNP investigation of dyspnea in the emergency department (PRIDE) study. Am. J. Cardiol. 2005, 95, 948–954. [Google Scholar] [CrossRef]
- Wang, T.J.; Larson, M.G.; Levy, D.; Leip, E.P.; Benjamin, E.J.; Wilson, P.W.; Sutherland, P.; Omland, T.; Vasan, R.S. Impact of age and sex on plasma natriuretic peptide levels in healthy adults. Am. J. Cardiol. 2002, 90, 254–258. [Google Scholar] [CrossRef]
- Redfield, M.M.; Rodeheffer, R.J.; Jacobsen, S.J.; Mahoney, D.W.; Bailey, K.R.; Burnett, J.C., Jr. Plasma brain natriuretic peptide concentration: Impact of age and gender. J. Am. Coll. Cardiol. 2002, 40, 976–982. [Google Scholar] [CrossRef]
- Das, S.R.; Drazner, M.H.; Dries, D.L.; Vega, G.L.; Stanek, H.G.; Abdullah, S.M.; Canham, R.M.; Chung, A.K.; Leonard, D.; Wians, F.H., Jr.; et al. Impact of body mass and body composition on circulating levels of natriuretic peptides: Results from the Dallas Heart Study. Circulation 2005, 112, 2163–2168. [Google Scholar] [CrossRef]
- Schou, M.; Gustafsson, F.; Kistorp, C.N.; Corell, P.; Kjaer, A.; Hildebrandt, P.R. Effects of body mass index and age on N-terminal pro brain natriuretic peptide are associated with glomerular filtration rate in chronic heart failure patients. Clin. Chem. 2007, 53, 1928–1935. [Google Scholar] [CrossRef]
- Wang, T.J.; Larson, M.G.; Keyes, M.J.; Levy, D.; Benjamin, E.J.; Vasan, R.S. Association of plasma natriuretic peptide levels with metabolic risk factors in ambulatory individuals. Circulation 2007, 115, 1345–1353. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.; Fox, C.S.; Larson, M.G.; Massaro, J.M.; McCabe, E.L.; Khan, A.M.; Levy, D.; Hoffmann, U.; O’Donnell, C.J.; Miller, K.K.; et al. Relation of visceral adiposity to circulating natriuretic peptides in ambulatory individuals. Am. J. Cardiol. 2011, 108, 979–984. [Google Scholar] [CrossRef] [PubMed]
- Bann, D.; Wu, F.C.; Keevil, B.; Lashen, H.; Adams, J.; Hardy, R.; Muniz, G.; Kuh, D.; Ben-Shlomo, Y.; Ong, K.K. Changes in testosterone related to body composition in late midlife: Findings from the 1946 British birth cohort study. Obesity 2015, 23, 1486–1492. [Google Scholar] [CrossRef] [PubMed]
- Ly, L.P.; Handelsman, D.J. Empirical estimation of free testosterone from testosterone and sex hormone-binding globulin immunoassays. Eur. J. Endocrinol. 2005, 152, 471–478. [Google Scholar] [CrossRef] [PubMed]
- Nagueh, S.F.; Smiseth, O.A.; Appleton, C.P.; Byrd, B.F.; Dokainish, H.; Edvardsen, T.; Flachskampf, F.A.; Gillebert, T.C.; Klein, A.L.; Lancellotti, P.; et al. Recommendations for the Evaluation of Left Ventricular Diastolic Function by Echocardiography: An Update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur. J. Echocardiogr. 2016, 17, 1321–1360. [Google Scholar] [CrossRef]
- De Lemos, J.A.; McGuire, D.K.; Khera, A.; Das, S.R.; Murphy, S.A.; Omland, T.; Drazner, M.H. Screening the population for left ventricular hypertrophy and left ventricular systolic dysfunction using natriuretic peptides: Results from the Dallas Heart Study. Am. Heart J. 2009, 157, 746–753. [Google Scholar] [CrossRef]
- Galasko, G.I.; Lahiri, A.; Barnes, S.C.; Collinson, P.; Senior, R. What is the normal range for N-terminal pro-brain natriuretic peptide? How well does this normal range screen for cardiovascular disease? Eur. Heart J. 2005, 26, 2269–2276. [Google Scholar] [CrossRef]
- Raymond, I.; Groenning, B.A.; Hildebrandt, P.R.; Nilsson, J.C.; Baumann, M.; Trawinski, J.; Pedersen, F. The influence of age, sex and other variables on the plasma level of N-terminal pro brain natriuretic peptide in a large sample of the general population. Heart 2003, 89, 745–751. [Google Scholar] [CrossRef]
- Costello-Boerrigter, L.C.; Boerrigter, G.; Redfield, M.M.; Rodeheffer, R.J.; Urban, L.H.; Mahoney, D.W.; Jacobsen, S.J.; Heublein, D.M.; Burnett, J.C., Jr. Amino-terminal pro-B-type natriuretic peptide and B-type natriuretic peptide in the general community: Determinants and detection of left ventricular dysfunction. J. Am. Coll. Cardiol. 2006, 47, 345–353. [Google Scholar] [CrossRef]
- Daniels, L.B.; Clopton, P.; deFilippi, C.R.; Sanchez, O.A.; Bahrami, H.; Lima, J.A.; Tracy, R.P.; Siscovick, D.; Bertoni, A.G.; Greenland, P.; et al. Serial measurement of N-terminal pro-B-type natriuretic peptide and cardiac troponin T for cardiovascular disease risk assessment in the Multi-Ethnic Study of Atherosclerosis (MESA). Am. Heart J. 2015, 170, 1170–1183. [Google Scholar] [CrossRef]
- Wang, T.J.; Larson, M.G.; Levy, D.; Benjamin, E.J.; Leip, E.P.; Wilson, P.W.; Vasan, R.S. Impact of obesity on plasma natriuretic peptide levels. Circulation 2004, 109, 594–600. [Google Scholar] [CrossRef] [PubMed]
- Fox, E.R.; Musani, S.K.; Bidulescu, A.; Nagarajarao, H.S.; Samdarshi, T.E.; Gebreab, S.Y.; Sung, J.H.; Steffes, M.W.; Wang, T.J.; Taylor, H.A.; et al. Relation of obesity to circulating B-type natriuretic peptide concentrations in blacks: The Jackson Heart Study. Circulation 2011, 124, 1021–1027. [Google Scholar] [CrossRef] [PubMed]
- Sarzani, R.; Paci, V.M.; Dessi-Fulgheri, P.; Espinosa, E.; Rappelli, A. Comparative analysis of atrial natriuretic peptide receptor expression in rat tissues. J. Hypertens. 1993, 11, S214–S215. [Google Scholar] [CrossRef]
- Sarzani, R.; Dessi-Fulgheri, P.; Paci, V.M.; Espinosa, E.; Rappelli, A. Expression of natriuretic peptide receptors in human adipose and other tissues. J. Endocrinol. Investig. 1996, 19, 581–585. [Google Scholar] [CrossRef] [PubMed]
- Sarzani, R.; Paci, V.M.; Zingaretti, C.M.; Pierleoni, C.; Cinti, S.; Cola, G.; Rappelli, A.; Dessi-Fulgheri, P. Fasting inhibits natriuretic peptides clearance receptor expression in rat adipose tissue. J. Hypertens. 1995, 13, 1241–1246. [Google Scholar] [CrossRef] [PubMed]
- Sengenes, C.; Berlan, M.; De Glisezinski, I.; Lafontan, M.; Galitzky, J. Natriuretic peptides: A new lipolytic pathway in human adipocytes. FASEB J. 2000, 14, 1345–1351. [Google Scholar] [CrossRef]
- Engeli, S.; Birkenfeld, A.L.; Badin, P.M.; Bourlier, V.; Louche, K.; Viguerie, N.; Thalamas, C.; Montastier, E.; Larrouy, D.; Harant, I.; et al. Natriuretic peptides enhance the oxidative capacity of human skeletal muscle. J. Clin. Investig. 2012, 122, 4675–4679. [Google Scholar] [CrossRef]
- Birkenfeld, A.L.; Boschmann, M.; Moro, C.; Adams, F.; Heusser, K.; Franke, G.; Berlan, M.; Luft, F.C.; Lafontan, M.; Jordan, J. Lipid mobilization with physiological atrial natriuretic peptide concentrations in humans. J. Clin. Endocrinol. Metab. 2005, 90, 3622–3628. [Google Scholar] [CrossRef]
- Moro, C.; Crampes, F.; Sengenes, C.; De Glisezinski, I.; Galitzky, J.; Thalamas, C.; Lafontan, M.; Berlan, M. Atrial natriuretic peptide contributes to physiological control of lipid mobilization in humans. FASEB J. 2004, 18, 908–910. [Google Scholar] [CrossRef]
- Sarzani, R.; Marcucci, P.; Salvi, F.; Bordicchia, M.; Espinosa, E.; Mucci, L.; Lorenzetti, B.; Minardi, D.; Muzzonigro, G.; Dessi-Fulgheri, P.; et al. Angiotensin II stimulates and atrial natriuretic peptide inhibits human visceral adipocyte growth. Int. J. Obes. 2008, 32, 259–267. [Google Scholar] [CrossRef][Green Version]
- Abdullah, S.M.; Khera, A.; Das, S.R.; Stanek, H.G.; Canham, R.M.; Chung, A.K.; Morrow, D.A.; Drazner, M.H.; McGuire, D.K.; de Lemos, J.A. Relation of coronary atherosclerosis determined by electron beam computed tomography and plasma levels of n-terminal pro-brain natriuretic peptide in a multiethnic population-based sample (the Dallas Heart Study). Am. J. Cardiol. 2005, 96, 1284–1289. [Google Scholar] [CrossRef] [PubMed]
- Manolopoulos, K.N.; Karpe, F.; Frayn, K.N. Gluteofemoral body fat as a determinant of metabolic health. Int. J. Obes. 2010, 34, 949–959. [Google Scholar] [CrossRef] [PubMed]
- Jackson, A.S.; Stanforth, P.R.; Gagnon, J.; Rankinen, T.; Leon, A.S.; Rao, D.C.; Skinner, J.S.; Bouchard, C.; Wilmore, J.H. The effect of sex, age and race on estimating percentage body fat from body mass index: The Heritage Family Study. Int. J. Obes. 2002, 26, 789–796. [Google Scholar] [CrossRef] [PubMed]
- Seidell, J.C.; Oosterlee, A.; Deurenberg, P.; Hautvast, J.G.; Ruijs, J.H. Abdominal fat depots measured with computed tomography: Effects of degree of obesity, sex, and age. Eur. J. Clin. Nutr. 1988, 42, 805–815. [Google Scholar]
- Haupt, A.; Thamer, C.; Heni, M.; Machicao, F.; Machann, J.; Schick, F.; Stefan, N.; Fritsche, A.; Haring, H.U.; Staiger, H. Novel obesity risk loci do not determine distribution of body fat depots: A whole-body MRI/MRS study. Obesity 2010, 18, 1212–1217. [Google Scholar] [CrossRef]
- Ludescher, B.; Najib, A.; Baar, S.; Machann, J.; Thamer, C.; Schick, F.; Buchkremer, G.; Claussen, C.D.; Eschweiler, G.W. Gender specific correlations of adrenal gland size and body fat distribution: A whole body MRI study. Horm. Metab. Res. 2007, 39, 515–518. [Google Scholar] [CrossRef]
- Thomas, E.L.; Saeed, N.; Hajnal, J.V.; Brynes, A.; Goldstone, A.P.; Frost, G.; Bell, J.D. Magnetic resonance imaging of total body fat. J. Appl. Physiol. 1998, 85, 1778–1785. [Google Scholar] [CrossRef]
- Karastergiou, K.; Smith, S.R.; Greenberg, A.S.; Fried, S.K. Sex differences in human adipose tissues - the biology of pear shape. Biol. Sex Differ. 2012, 3, 13. [Google Scholar] [CrossRef]
- Maynard, L.M.; Wisemandle, W.; Roche, A.F.; Chumlea, W.C.; Guo, S.S.; Siervogel, R.M. Childhood body composition in relation to body mass index. Pediatrics 2001, 107, 344–350. [Google Scholar] [CrossRef]
- Hattori, K.; Tahara, Y.; Moji, K.; Aoyagi, K.; Furusawa, T. Chart analysis of body composition change among pre- and postadolescent Japanese subjects assessed by underwater weighing method. Int. J. Obes. 2004, 28, 520–524. [Google Scholar] [CrossRef][Green Version]
- Wells, J.C. Sexual dimorphism of body composition. Best Pract. Res. Clin. Endocrinol. Metab. 2007, 21, 415–430. [Google Scholar] [CrossRef] [PubMed]
- Svendsen, O.L.; Hassager, C.; Christiansen, C. Age- and menopause-associated variations in body composition and fat distribution in healthy women as measured by dual-energy X-ray absorptiometry. Metabolism 1995, 44, 369–373. [Google Scholar] [CrossRef]
- Toth, M.J.; Tchernof, A.; Sites, C.K.; Poehlman, E.T. Effect of menopausal status on body composition and abdominal fat distribution. Int. J. Obes. 2000, 24, 226–231. [Google Scholar] [CrossRef] [PubMed]
- Lovejoy, J.C.; Champagne, C.M.; de Jonge, L.; Xie, H.; Smith, S.R. Increased visceral fat and decreased energy expenditure during the menopausal transition. Int. J. Obes. 2008, 32, 949–958. [Google Scholar] [CrossRef] [PubMed]
- Allan, C.A.; Strauss, B.J.; Burger, H.G.; Forbes, E.A.; McLachlan, R.I. Testosterone therapy prevents gain in visceral adipose tissue and loss of skeletal muscle in nonobese aging men. J. Clin. Endocrinol. Metab. 2008, 93, 139–146. [Google Scholar] [CrossRef] [PubMed]
- Stevens, J.; Katz, E.G.; Huxley, R.R. Associations between gender, age and waist circumference. Eur. J. Clin. Nutr. 2010, 64, 6–15. [Google Scholar] [CrossRef]
- Koch, A.; Singer, H. Normal values of B type natriuretic peptide in infants, children, and adolescents. Heart 2003, 89, 875–878. [Google Scholar] [CrossRef]
- Maffei, S.; Del Ry, S.; Prontera, C.; Clerico, A. Increase in circulating levels of cardiac natriuretic peptides after hormone replacement therapy in postmenopausal women. Clin. Sci. 2001, 101, 447–453. [Google Scholar] [CrossRef]
- Chang, A.Y.; Abdullah, S.M.; Jain, T.; Stanek, H.G.; Das, S.R.; McGuire, D.K.; Auchus, R.J.; de Lemos, J.A. Associations among androgens, estrogens, and natriuretic peptides in young women: Observations from the Dallas Heart Study. J. Am. Coll. Cardiol. 2007, 49, 109–116. [Google Scholar] [CrossRef]
- Lam, C.S.; Cheng, S.; Choong, K.; Larson, M.G.; Murabito, J.M.; Newton-Cheh, C.; Bhasin, S.; McCabe, E.L.; Miller, K.K.; Redfield, M.M.; et al. Influence of sex and hormone status on circulating natriuretic peptides. J. Am. Coll. Cardiol. 2011, 58, 618–626. [Google Scholar] [CrossRef]
- Tran, T.T.; Kahn, C.R. Transplantation of adipose tissue and stem cells: Role in metabolism and disease. Nat. Rev. Endocrinol. 2010, 6, 195–213. [Google Scholar] [CrossRef] [PubMed]
- Tran, T.T.; Yamamoto, Y.; Gesta, S.; Kahn, C.R. Beneficial effects of subcutaneous fat transplantation on metabolism. Cell Metab. 2008, 7, 410–420. [Google Scholar] [CrossRef] [PubMed]
- Fried, S.K.; Kral, J.G. Sex differences in regional distribution of fat cell size and lipoprotein lipase activity in morbidly obese patients. Int. J. Obes. 1987, 11, 129–140. [Google Scholar] [PubMed]
- Edens, N.K.; Fried, S.K.; Kral, J.G.; Hirsch, J.; Leibel, R.L. In vitro lipid synthesis in human adipose tissue from three abdominal sites. Am. J. Physiol. Endocrinol. Metab. 1993, 265, E374–E379. [Google Scholar] [CrossRef]
- Foley, J.E.; Kashiwagi, A.; Chang, H.; Huecksteadt, T.P.; Lillioja, S.; Verso, M.A.; Reaven, G. Sex difference in insulin-stimulated glucose transport in rat and human adipocytes. Am. J. Physiol. Endocrinol. Metab. 1984, 246, E211–E215. [Google Scholar] [CrossRef]
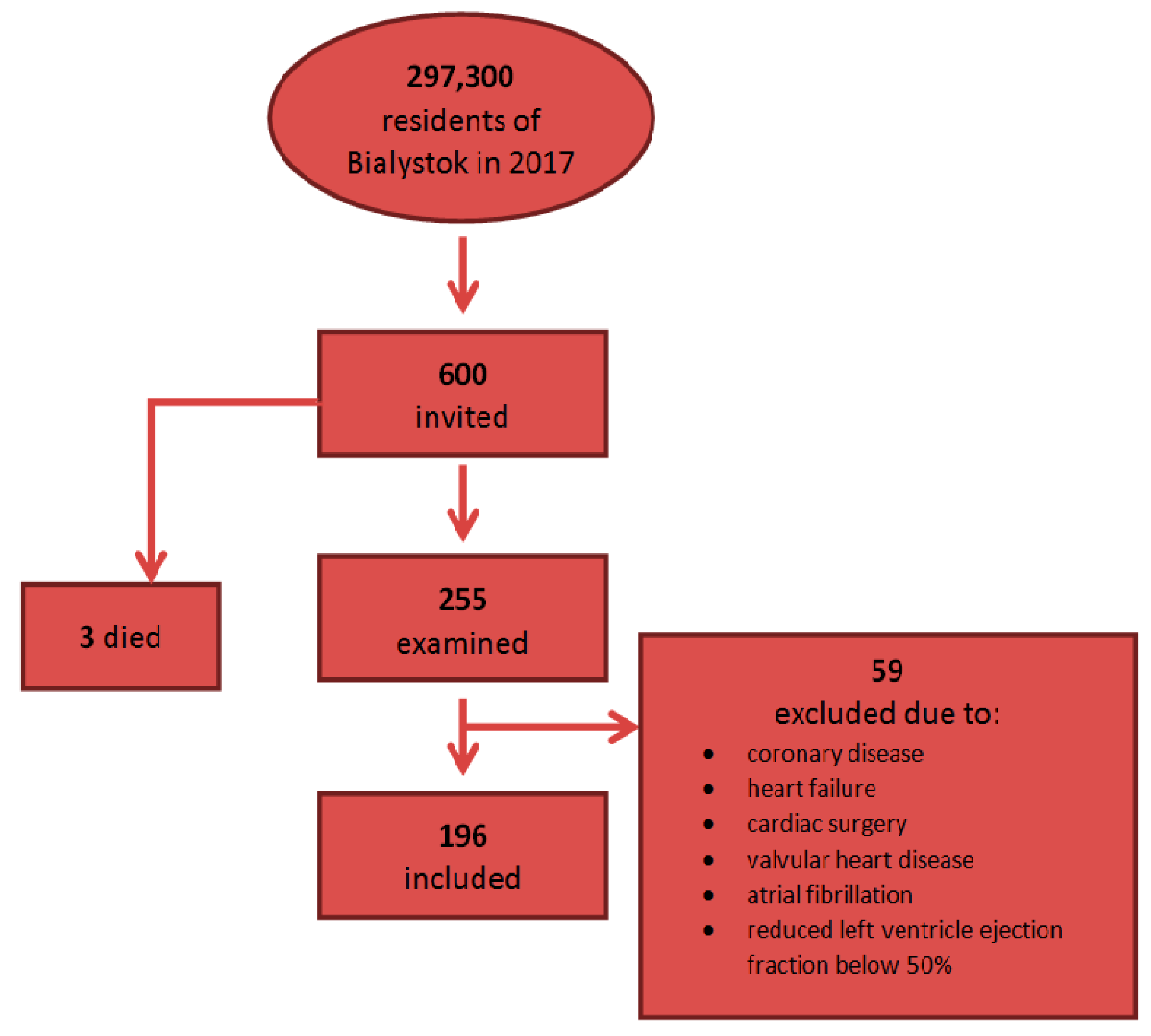
| Variable | Value (n = 196) |
|---|---|
| Age, years | 47.83 ± 14.97 |
| Male sex | 70 (35.7) |
| BMI, kg/m2 | 26.28 ± 4.80 |
| BMI 25–29.99 kg/m2 | 70 (35.7) |
| BMI ≥ 30 kg/m2 | 45 (23.0) |
| NT-proBNP, pg/mL | 72.59 ± 67.15 |
| hs-TnT, pg/mL | 6.02 ± 3.36 |
| Diastolic dysfunction of left ventricle | 19 (9.7) |
| LVMI, g/m2 | 82.01 ± 22.38 |
| LAVI, mL/m2 | 22.88 ± 7.09 |
| Creatinine, μmol/L | 75.90 ± 16.71 |
| GFR, mL/min/1.73 m2 | 103.63 ± 31.42 |
| BPs, mmHg | 121.28 ± 16.72 |
| BPd, mmHg | 81.60 ± 10.49 |
| BP ≥ 140 and/or ≥ 90 mmHg | 42 (21.8) |
| HR, bpm | 73.68 ± 11.66 |
| History of hypertension | 56 (28.9) |
| History of diabetes | 13 (6.7) |
| Currently smoking | 47 (24.0) |
| Variables | Woman | Man | p-Values |
|---|---|---|---|
| Number of subjects | 126 (64.29) | 70 (35.71) | |
| Age, years | 48.25 ± 15.05 | 47.07 ± 14.89 | 0.493 |
| BMI, kg/m2 | 25.82 ± 5.13 | 27.11 ± 4.04 | 0.035 |
| NT-proBNP, pg/mL | 79.69 ± 53.73 | 59.81 ± 79.00 | <0.001 |
| hs-TnT, pg/mL | 5.40 ± 3.40 | 7.12 ± 3.02 | <0.001 |
| Creatinine, μmol/L | 68.93 ± 13.08 | 88.35 ± 15.25 | <0.001 |
| GFR, mL/min/1.73 m2 | 99.77 ± 32.66 | 110.53 ± 27.96 | 0.003 |
| HbA1c, % | 5.44 ± 0.48 | 5.51 ± 0.50 | 0.398 |
| TT, ng/mL | 0.20 ± 0.13 | 4.53 ± 1.89 | <0.001 |
| CFT, pmol/L | 5.52 ± 5.34 | 229.74 ± 91.12 | <0.001 |
| SHBG, nmol/L | 69.25 ± 34.25 | 40.95 ± 19.60 | <0.001 |
| LVMI, g/m2 | 74.78 ± 18.88 | 95.86 ± 22.16 | <0.001 |
| LVMI, ≥95 g/m2 women, ≥115 g/m2 men | 17 (15.0) | 10 (16.9) | 0.744 |
| LAVI, mL/m2 | 21.98 ± 6.65 | 24.58 ± 7.63 | 0.031 |
| LAVI, >34 mL/m2 | 6 (5.5) | 7 (12.1) | 0.127 |
| Diastolic dysfunction of left ventricle | 16 (12.7) | 3 (4.3) | 0.060 |
| BPs, mmHg | 116.73 ± 15.91 | 129.66 ± 14.91 | <0.001 |
| BPd, mmHg | 80.04 ± 9.61 | 84.47 ± 11.47 | 0.010 |
| BP ≥ 140 or/and ≥90, mmHg | 20 (16.0) | 22 (32.4) | 0.009 |
| HR, bpm | 74.03 ± 10.58 | 73.04 ± 13.49 | 0.465 |
| Total fat mass, kg | 26.23 ± 9.38 | 23.72 ± 8.51 | 0.136 |
| Total lean mass, kg | 41.51 ± 5.03 | 57.54 ± 7.15 | <0.001 |
| Android fat mass, kg | 2.11 ± 1.15 | 2.45 ± 1.23 | 0.055 |
| Android lean mass, kg | 2.86 ± 0.37 | 3.83 ± 0.54 | <0.001 |
| Gynoid fat mass, kg | 4.47 ± 1.4 | 3.26 ± 1.12 | <0.001 |
| Gynoid lean mass, kg | 6.22 ± 0.75 | 8.53 ± 1.28 | <0.001 |
| Visceral mass, kg | 0.81 ± 0.68 | 1.59 ± 1.05 | <0.001 |
| History of hypertension | 34 (27.2) | 22 (31.9) | 0.491 |
| History of blood pressure medicine | 33 (26.2) | 23 (32.9) | 0.322 |
| History of diabetes | 7 (5.6) | 6 (8.7) | 0.418 |
| Current smoking | 28 (22.22) | 19 (27.14) | 0.897 |
| Variables | Univariate Analysis | Model 1 | Model 2 | Model 3 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| B | p | R2 | B | p | Adjuste R2 | B | p | adjusted R2 | B | p | Adjusted R2 | |
| Age, years | 2.084 | <0.001 | 0.216 | - | - | - | - | - | - | - | - | - |
| Gender, male | −19.885 | 0.047 | 0.020 | - | - | - | - | - | - | - | - | - |
| GFR, mL/min/1.73 m2 | −0.713 | <0.001 | 0.112 | - | - | - | - | - | - | - | - | - |
| BMI, kg/m2 | 1.294 | 0.197 | 0.009 | - | - | - | - | - | - | - | - | - |
| BPs, mmHg | 0.818 | 0.005 | 0.041 | - | - | - | - | - | - | - | - | - |
| BPd, mmHg | 0.020 | 0.965 | 0.000 | - | - | - | - | - | - | - | - | - |
| BP ≥ 140 and/or ≥90 mmHg | 31.997 | 0.006 | 0.038 | - | - | - | - | - | - | - | - | - |
| WHR | 1.780 | 0.973 | 0.000 | −81.088 | 0.372 | 0.232 | −109.141 | 0.222 | 0.265 | −88.341 | 0.316 | 0.292 |
| HR, bpm | 0.055 | 0.896 | 0.000 | 0.299 | 0.427 | 0.232 | 0.307 | 0.405 | 0.262 | 0.278 | 0.442 | 0.290 |
| LVMI, g/m2 | 0.360 | 0.126 | 0.014 | 0.101 | 0.727 | 0.209 | 0.023 | 0.937 | 0.233 | −0.008 | 0.976 | 0.257 |
| LVMI, ≥95 g/m2 women, ≥115 g/m2 men | 29.355 | 0.042 | 0.024 | 8.276 | 0.562 | 0.210 | 5.984 | 0.671 | 0.234 | 4.044 | 0.771 | 0.258 |
| LAVI, mL/m2 | 1.576 | 0.036 | 0.026 | 1.122 | 0.111 | 0.250 | 1.043 | 0.1135 | 0.267 | 1.132 | 0.097 | 0.300 |
| LAVI, >34 mL/m2 | 3.227 | 0.872 | 0.000 | −0.948 | 0.958 | 0.238 | −4.344 | 0.807 | 0.257 | −0.529 | 0.976 | 0.287 |
| Diastolic dysfunction of left ventricle | 20.492 | 0.207 | 0.008 | −3.407 | 0.824 | 0.226 | 0.884 | 0.953 | 0.256 | −1.350 | 0.928 | 0.285 |
| Fasting glucose, mg/dL | 1.052 | 0.002 | 0.050 | 0.302 | 0.433 | 0.232 | 0.358 | 0.343 | 0.263 | 0.434 | 0.242 | 0.293 |
| 2-h glucose, mg/dL | 0.235 | 0.021 | 0.031 | −0.024 | 0.815 | 0.252 | −0.011 | 0.912 | 0.274 | −0.027 | 0.781 | 0.334 |
| HOMA-IR | 1.559 | 0.628 | 0.001 | −2.750 | 0.446 | 0.231 | −2.908 | 0.411 | 0.262 | −2.878 | 0.406 | 0.291 |
| HbA1c, % | 29.227 | 0.004 | 0.044 | −6.112 | 0.596 | 0.233 | −5.577 | 0.621 | 0.262 | −1.453 | 0.896 | 0.291 |
| DHEA-s, µg/dL | −0.184 | <0.001 | 0.111 | −0.003 | 0.946 | 0.229 | −0.020 | 0.693 | 0.260 | 0.001 | 0.980 | 0.288 |
| TT, ng/mL | −5.855 | 0.003 | 0.047 | −9.371 | 0.015 | 0.234 | - | - | - | - | - | - |
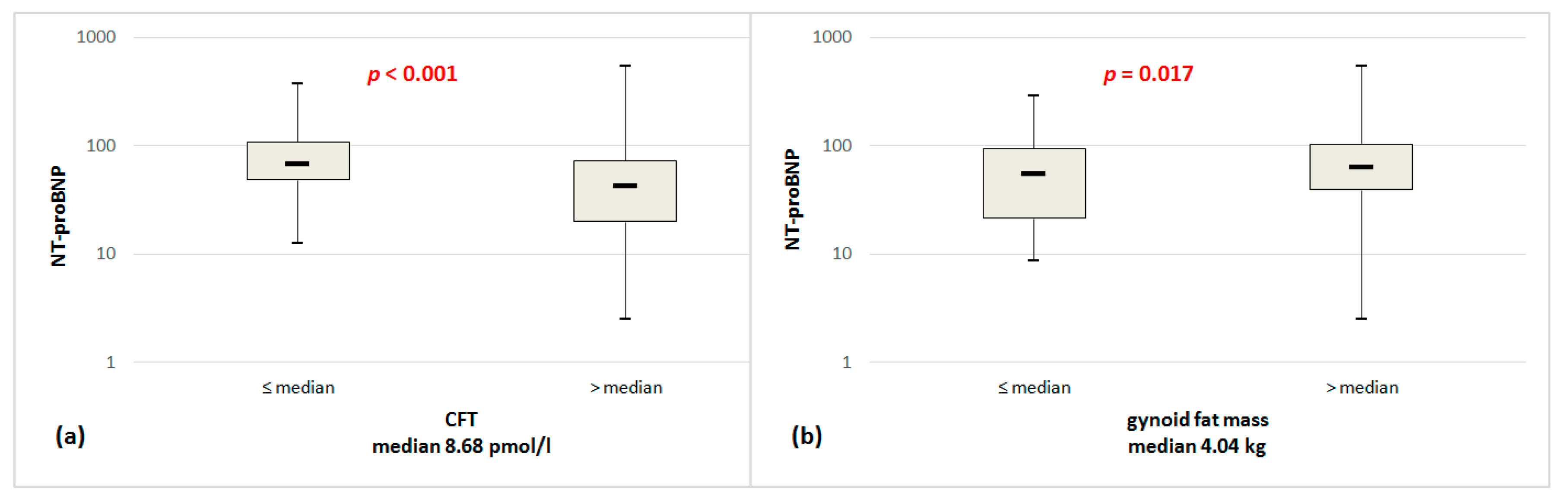
| CFT, pmol/L | −0.145 | <0.001 | 0.066 | −0.221 | 0.004 | 0.263 | - | - | - | - | - | - |
| SHBG, nmol/L | 0.433 | 0.003 | 0.045 | 0.394 | 0.010 | 0.257 | 0.430 | 0.004 | 0.292 | - | - | - |
| Total fat mass, kg | 0.001 | 0.096 | 0.014 | 0.001 | 0.455 | 0.229 | 0.000 | 0.777 | 0.257 | 9.27 | 0.948 | 0.286 |
| Total lean mass, kg | −0.001 | 0.004 | 0.042 | 0.000 | 0.846 | 0.227 | 0.000 | 0.714 | 0.257 | 0.001 | 0.578 | 0.287 |
| Android fat mass, kg | 0.005 | 0.194 | 0.009 | −0.002 | 0.861 | 0.227 | −0.009 | 0.351 | 0.260 | −0.008 | 0.377 | 0.289 |
| Android lean mass, kg | −0.011 | 0.133 | 0.012 | 0.008 | 0.557 | 0.228 | 0.003 | 0.803 | 0.257 | 0.007 | 0.594 | 0.287 |
| Gynoid fat mass, kg | 0.007 | 0.041 | 0.021 | 0.019 | 0.004 | 0.261 | 0.017 | 0.009 | 0.285 | 0.015 | 0.028 | 0.305 |
| Gynoid lean mass, kg | −0.009 | 0.004 | 0.043 | 0.000 | 0.969 | 0.226 | 0.001 | 0.884 | 0.257 | 0.002 | 0.728 | 0.286 |
| Visceral mass, kg | 0.005 | 0.311 | 0.005 | −0.017 | 0.073 | 0.240 | −0.024 | 0.014 | 0.282 | −0.023 | 0.015 | 0.309 |
| A/G fat mass ratio | 1.956 | 0.928 | 0.000 | −90.002 | 0.014 | 0.252 | −108.543 | 0.003 | 0.294 | −93.035 | 0.010 | 0.312 |
| G/TF mass ratio | 129.221 | 0.451 | −0.002 | 874.08 | 0.001 | 0.275 | 874.040 | <0.001 | 0.306 | 772.721 | 0.002 | 0.323 |
| Handgrip strength test, max | −1.487 | <0.001 | 0.100 | −0.801 | 0.070 | 0.243 | −0.778 | 0.073 | 0.272 | −0.742 | 0.081 | 0.300 |
| History of hypertension | 43.278 | <0.001 | 0.085 | - | - | - | - | - | - | - | - | - |
| History of hypertension treatment | 41.406 | <0.001 | 0.078 | - | - | - | - | - | - | - | - | - |
| Variables | Full Model | Final Model | ||||
|---|---|---|---|---|---|---|
| B | p | R2 | B | p | R2 | |
| Age, year | 1.926 | <0.001 | 0.323 | 2.447 | <0.001 | 0.326 |
| Gender, male | 71.641 | 0.001 | 0.323 | 72.909 | <0.001 | 0.326 |
| G/TF mass ratio | 772.721 | 0.002 | 0.323 | 659.928 | 0.004 | 0.326 |
| CFT, pmol/L | −0.235 | 0.002 | 0.323 | −0.245 | 0.001 | 0.326 |
| SHBG, nmol/L | 0.349 | 0.020 | 0.323 | 0.359 | 0.012 | 0.326 |
| BMI, kg/m2 | 1.035 | 0.476 | 0.323 | - | - | - |
| GFR, mL/min/1.73 m2 | −0.145 | 0.488 | 0.323 | - | - | - |
| History of hypertension | 12.034 | 0.302 | 0.323 | - | - | - |
| BP ≥ 140 and/or ≥90 mmHg | 12.425 | 0.247 | 0.323 | - | - | - |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chlabicz, M.; Jamiołkowski, J.; Paniczko, M.; Sowa, P.; Łapińska, M.; Szpakowicz, M.; Jurczuk, N.; Kondraciuk, M.; Raczkowski, A.; Sawicka, E.; et al. Independent Impact of Gynoid Fat Distribution and Free Testosterone on Circulating Levels of N-Terminal Pro-Brain Natriuretic Peptide (NT-proBNP) in Humans. J. Clin. Med. 2020, 9, 74. https://doi.org/10.3390/jcm9010074
Chlabicz M, Jamiołkowski J, Paniczko M, Sowa P, Łapińska M, Szpakowicz M, Jurczuk N, Kondraciuk M, Raczkowski A, Sawicka E, et al. Independent Impact of Gynoid Fat Distribution and Free Testosterone on Circulating Levels of N-Terminal Pro-Brain Natriuretic Peptide (NT-proBNP) in Humans. Journal of Clinical Medicine. 2020; 9(1):74. https://doi.org/10.3390/jcm9010074
Chicago/Turabian StyleChlabicz, Małgorzata, Jacek Jamiołkowski, Marlena Paniczko, Paweł Sowa, Magda Łapińska, Małgorzata Szpakowicz, Natalia Jurczuk, Marcin Kondraciuk, Andrzej Raczkowski, Emilia Sawicka, and et al. 2020. "Independent Impact of Gynoid Fat Distribution and Free Testosterone on Circulating Levels of N-Terminal Pro-Brain Natriuretic Peptide (NT-proBNP) in Humans" Journal of Clinical Medicine 9, no. 1: 74. https://doi.org/10.3390/jcm9010074
APA StyleChlabicz, M., Jamiołkowski, J., Paniczko, M., Sowa, P., Łapińska, M., Szpakowicz, M., Jurczuk, N., Kondraciuk, M., Raczkowski, A., Sawicka, E., & Kamiński, K. A. (2020). Independent Impact of Gynoid Fat Distribution and Free Testosterone on Circulating Levels of N-Terminal Pro-Brain Natriuretic Peptide (NT-proBNP) in Humans. Journal of Clinical Medicine, 9(1), 74. https://doi.org/10.3390/jcm9010074






