Efficacy of Low-Dose Prophylactic Quetiapine on Delirium Prevention in Critically Ill Patients: A Prospective, Randomized, Double-Blind, Placebo-Controlled Study
Abstract
1. Introduction
2. Materials and Methods
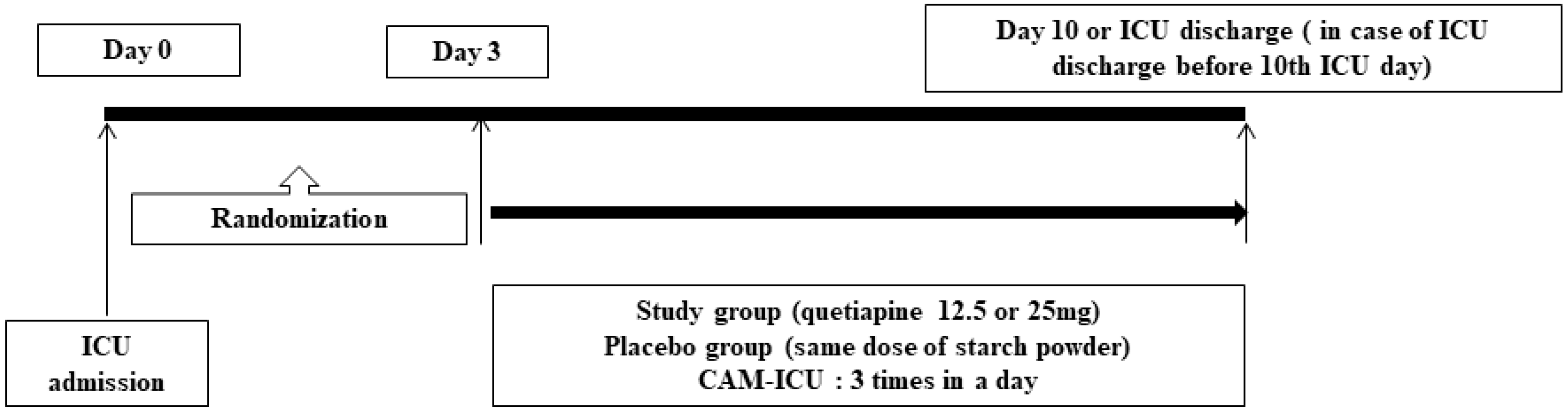
2.1. Study Design
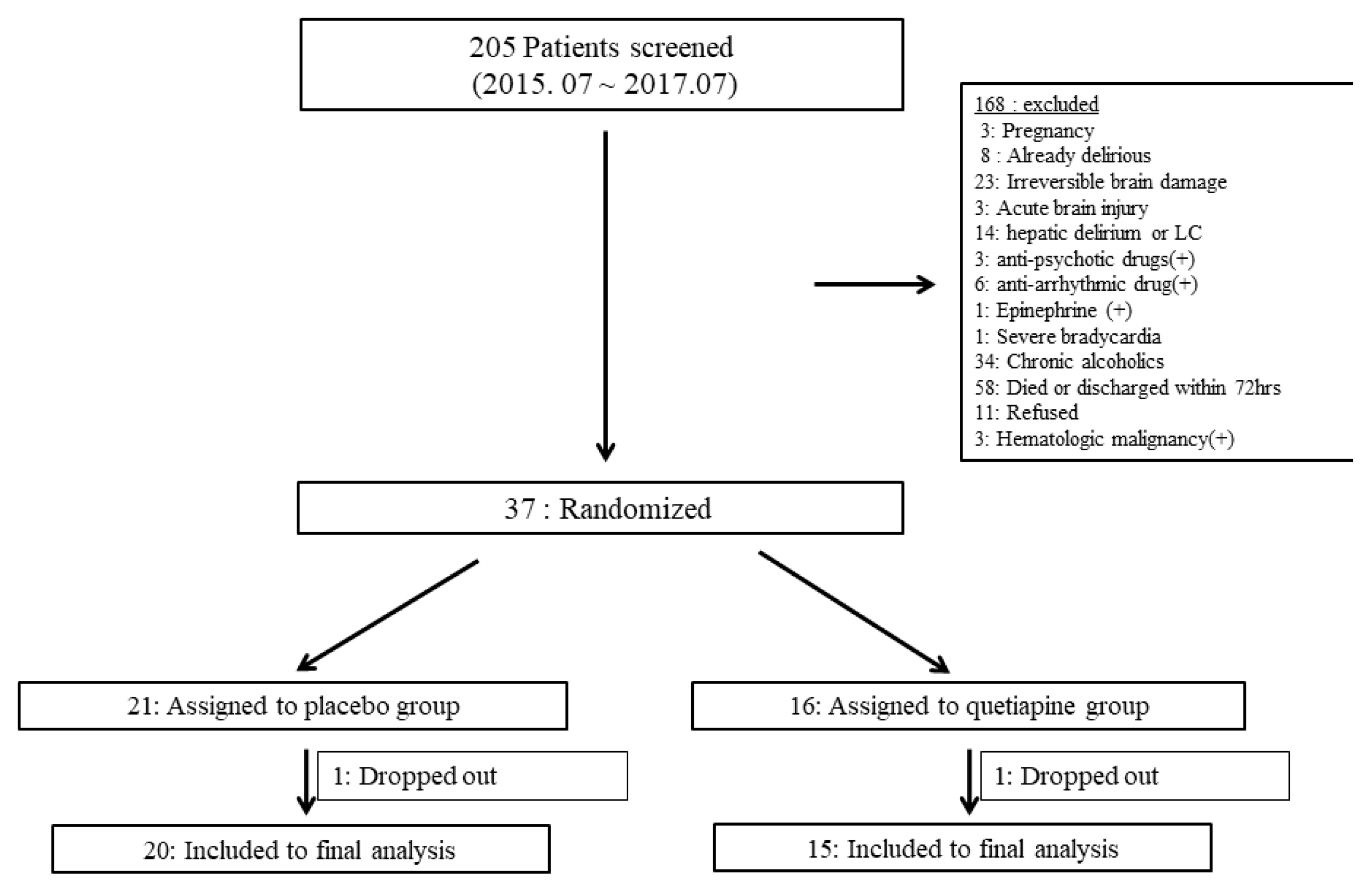
2.2. Enrollment
2.3. Randomization
2.4. Study Protocols
2.5. Outcomes and Data C ollection
2.6. Statistical Analyses
3. Results
3.1. Baseline Characteristics
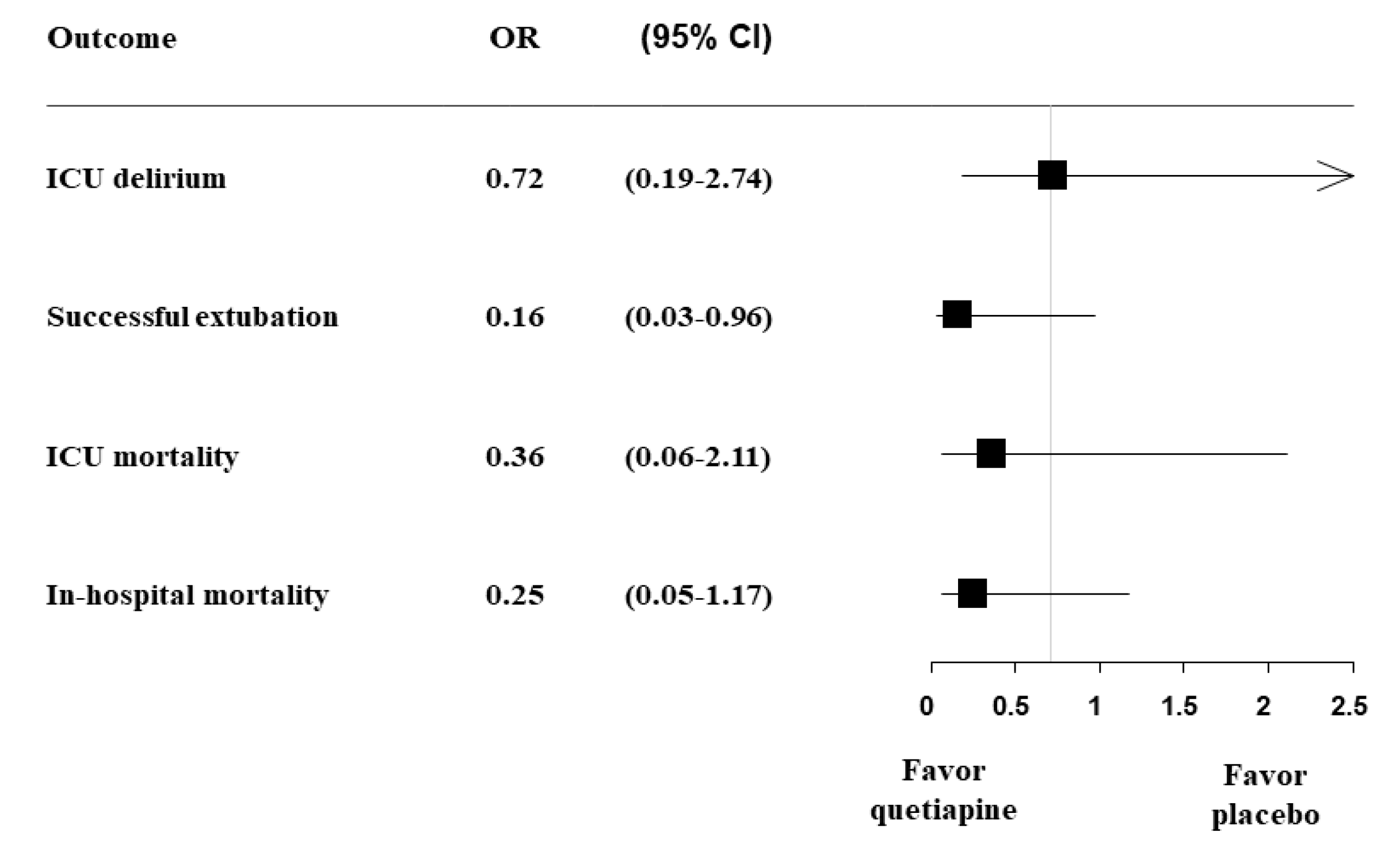
3.2. Clinical Outcomes Related to Delirium in ICU
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ICU | Intensive care unit |
| CAM-ICU | Confusion Assessment Method for the ICU |
| IRB | Institutional Review Board |
| RCTs | Randomized controlled trials |
References
- Skrobik, Y. Delirium prevention and treatment. Crit. Care Clin. 2009, 25, 585–591. [Google Scholar] [CrossRef] [PubMed]
- Ouimet, S.; Kavanagh, B.P.; Gottfried, S.B.; Skrobik, Y. Incidence, risk factors and consequences of ICU delirium. Intensive Care Med. 2007, 33, 66–73. [Google Scholar] [CrossRef] [PubMed]
- Gnanavel, S.; Robert, R.S. Diagnostic and statistical manual of mental disorders, fifth edition, and the impact of events scale-revised. Chest 2013, 144, 1974. [Google Scholar] [CrossRef]
- Ely, E.W.; Shintani, A.; Truman, B.; Speroff, T.; Gordon, S.M.; Harrell, F.E., Jr.; Inouye, S.K.; Bernard, G.R.; Dittus, R.S. Delirium as a Predictor of Mortality in Mechanically Ventilated Patients in the Intensive Care Unit. JAMA 2004, 291, 1753–1762. [Google Scholar] [CrossRef] [PubMed]
- Ely, E.W.; Inouye, S.K.; Bernard, G.R.; Gordon, S.; Francis, J.; May, L.; Truman, B.; Speroff, T.; Gautam, S.; Margolin, R.; et al. Delirium in mechanically ventilated patients: Validity and reliability of the confusion assessment method for the intensive care unit (CAM-ICU). JAMA 2001, 286, 2703–2710. [Google Scholar] [CrossRef]
- Salluh, J.I.; Soares, M.; Teles, J.M.; Ceraso, D.; Raimondi, N.; Nava, V.S.; Blasquez, P.; Ugarte, S.; Ibanez-Guzman, C.; Centeno, J.V.; et al. Delirium epidemiology in critical care (DECCA): An international study. Crit. Care 2010, 14, R210. [Google Scholar] [CrossRef]
- Shehabi, Y.; Riker, R.R.; Bokesch, P.M.; Wisemandle, W.; Shintani, A.; Ely, E.W.; Group, S.S. Delirium duration and mortality in lightly sedated, mechanically ventilated intensive care patients. Crit. Care Med. 2010, 38, 2311–2318. [Google Scholar] [CrossRef]
- Ely, E.W.; Gautam, S.; Margolin, R.; Francis, J.; May, L.; Speroff, T.; Truman, B.; Dittus, R.; Bernard, R.; Inouye, S.K. The impact of delirium in the intensive care unit on hospital length of stay. Intensive Care Med. 2001, 27, 1892–1900. [Google Scholar] [CrossRef]
- van den Boogaard, M.; Schoonhoven, L.; van der Hoeven, J.G.; van Achterberg, T.; Pickkers, P. Incidence and short-term consequences of delirium in critically ill patients: A prospective observational cohort study. Int. J. Nurs. Stud. 2012, 49, 775–783. [Google Scholar] [CrossRef]
- Heymann, A.; Radtke, F.; Schiemann, A.; Lutz, A.; MacGuill, M.; Wernecke, K.D.; Spies, C. Delayed treatment of delirium increases mortality rate in intensive care unit patients. J. Int. Med. Res. 2010, 38, 1584–1595. [Google Scholar] [CrossRef]
- Pandharipande, P.P.; Girard, T.D.; Jackson, J.C.; Morandi, A.; Thompson, J.L.; Pun, B.T.; Brummel, N.E.; Hughes, C.G.; Vasilevskis, E.E.; Shintani, A.K.; et al. Long-term cognitive impairment after critical illness. N. Engl. J. Med. 2013, 369, 1306–1316. [Google Scholar] [CrossRef] [PubMed]
- Sakusic, A.; O’Horo, J.C.; Dziadzko, M.; Volha, D.; Ali, R.; Singh, T.D.; Kashyap, R.; Farrell, A.M.; Fryer, J.D.; Petersen, R.; et al. Potentially Modifiable Risk Factors for Long-Term Cognitive Impairment After Critical Illness: A Systematic Review. Mayo Clin. Proc. 2018, 93, 68–82. [Google Scholar] [CrossRef]
- Devlin, J.W.; Skrobik, Y.; Gelinas, C.; Needham, D.M.; Slooter, A.J.C.; Pandharipande, P.P.; Watson, P.L.; Weinhouse, G.L.; Nunnally, M.E.; Rochwerg, B.; et al. Clinical Practice Guidelines for the Prevention and Management of Pain, Agitation/Sedation, Delirium, Immobility, and Sleep Disruption in Adult Patients in the ICU. Crit. Care Med. 2018, 46, e825–e873. [Google Scholar] [CrossRef] [PubMed]
- van den Boogaard, M.; Schoonhoven, L.; van Achterberg, T.; van der Hoeven, J.G.; Pickkers, P. Haloperidol prophylaxis in critically ill patients with a high risk for delirium. Crit. Care 2013, 17, R9. [Google Scholar] [CrossRef]
- Wang, W.; Li, H.L.; Wang, D.X.; Zhu, X.; Li, S.L.; Yao, G.Q.; Chen, K.S.; Gu, X.E.; Zhu, S.N. Haloperidol prophylaxis decreases delirium incidence in elderly patients after noncardiac surgery: A randomized controlled trial*. Crit. Care Med. 2012, 40, 731–739. [Google Scholar] [CrossRef] [PubMed]
- Kalisvaart, K.J.; de Jonghe, J.F.; Bogaards, M.J.; Vreeswijk, R.; Egberts, T.C.; Burger, B.J.; Eikelenboom, P.; van Gool, W.A. Haloperidol prophylaxis for elderly hip-surgery patients at risk for delirium: A randomized placebo-controlled study. J. Am. Geriatr. Soc. 2005, 53, 1658–1666. [Google Scholar] [CrossRef]
- Girard, T.D.; Pandharipande, P.P.; Carson, S.S.; Schmidt, G.A.; Wright, P.E.; Canonico, A.E.; Pun, B.T.; Thompson, J.L.; Shintani, A.K.; Meltzer, H.Y.; et al. Feasibility, efficacy, and safety of antipsychotics for intensive care unit delirium: The MIND randomized, placebo-controlled trial. Crit. Care Med. 2010, 38, 428–437. [Google Scholar] [CrossRef]
- van den Boogaard, M.; Slooter, A.J.C.; Bruggemann, R.J.M.; Schoonhoven, L.; Beishuizen, A.; Vermeijden, J.W.; Pretorius, D.; de Koning, J.; Simons, K.S.; Dennesen, P.J.W.; et al. Effect of Haloperidol on Survival Among Critically Ill Adults With a High Risk of Delirium: The REDUCE Randomized Clinical Trial. JAMA 2018, 319, 680–690. [Google Scholar] [CrossRef]
- Gamberini, M.; Bolliger, D.; Lurati Buse, G.A.; Burkhart, C.S.; Grapow, M.; Gagneux, A.; Filipovic, M.; Seeberger, M.D.; Pargger, H.; Siegemund, M.; et al. Rivastigmine for the prevention of postoperative delirium in elderly patients undergoing elective cardiac surgery–a randomized controlled trial. Crit. Care Med. 2009, 37, 1762–1768. [Google Scholar] [CrossRef]
- Prakanrattana, U.; Prapaitrakool, S. Efficacy of risperidone for prevention of postoperative delirium in cardiac surgery. Anaesth. Intensive Care 2007, 35, 714–719. [Google Scholar] [CrossRef]
- Su, X.; Meng, Z.T.; Wu, X.H.; Cui, F.; Li, H.L.; Wang, D.X.; Zhu, X.; Zhu, S.N.; Maze, M.; Ma, D. Dexmedetomidine for prevention of delirium in elderly patients after non-cardiac surgery: A randomised, double-blind, placebo-controlled trial. Lancet 2016, 388, 1893–1902. [Google Scholar] [CrossRef]
- Skrobik, Y.; Duprey, M.S.; Hill, N.S.; Devlin, J.W. Low-Dose Nocturnal Dexmedetomidine Prevents ICU Delirium. A Randomized, Placebo-controlled Trial. Am. J. Respir. Crit. Care Med. 2018, 197, 1147–1156. [Google Scholar] [CrossRef]
- Kim, H.; Bang, J.; Chang, H.W.; Kim, J.Y.; Park, K.U.; Kim, S.H.; Lee, K.J.; Cho, C.H.; Hwang, I.; Park, S.D.; et al. Anti-inflammatory effect of quetiapine on collagen-induced arthritis of mouse. Eur. J. Pharmacol. 2012, 678, 55–60. [Google Scholar] [CrossRef]
- Devlin, J.W.; Roberts, R.J.; Fong, J.J.; Skrobik, Y.; Riker, R.R.; Hill, N.S.; Robbins, T.; Garpestad, E. Efficacy and safety of quetiapine in critically ill patients with delirium: A prospective, multicenter, randomized, double-blind, placebo-controlled pilot study. Crit. Care Med. 2010, 38, 419–427. [Google Scholar] [CrossRef]
- Pandharipande, P.; Shintani, A.; Peterson, J.; Pun, B.T.; Wilkinson, G.R.; Dittus, R.S.; Bernard, G.R.; Ely, E.W. Lorazepam is an independent risk factor for transitioning to delirium in intensive care unit patients. Anesthesiology 2006, 104, 21–26. [Google Scholar] [CrossRef]
- Esmaoglu, A.; Ulgey, A.; Akin, A.; Boyaci, A. Comparison between dexmedetomidine and midazolam for sedation of eclampsia patients in the intensive care unit. J. Crit. Care 2009, 24, 551–555. [Google Scholar] [CrossRef]
- Sessler, C.N.; Gosnell, M.S.; Grap, M.J.; Brophy, G.M.; O’Neal, P.V.; Keane, K.A.; Tesoro, E.P.; Elswick, R.K. The Richmond Agitation-Sedation Scale: Validity and reliability in adult intensive care unit patients. Am. J. Respir. Crit. Care Med. 2002, 166, 1338–1344. [Google Scholar] [CrossRef]
- Van Rompaey, B.; Elseviers, M.M.; Schuurmans, M.J.; Shortridge-Baggett, L.M.; Truijen, S.; Bossaert, L. Risk factors for delirium in intensive care patients: A prospective cohort study. Crit. Care 2009, 13, R77. [Google Scholar] [CrossRef]
- Gunther, M.L.; Morandi, A.; Ely, E.W. Pathophysiology of delirium in the intensive care unit. Crit. Care Clin. 2008, 24, 45–65. [Google Scholar] [CrossRef]
- Morandi, A.; Rogers, B.P.; Gunther, M.L.; Merkle, K.; Pandharipande, P.; Girard, T.D.; Jackson, J.C.; Thompson, J.; Shintani, A.K.; Geevarghese, S.; et al. The relationship between delirium duration, white matter integrity, and cognitive impairment in intensive care unit survivors as determined by diffusion tensor imaging: The VISIONS prospective cohort magnetic resonance imaging study*. Crit. Care Med. 2012, 40, 2182–2189. [Google Scholar] [CrossRef]
- Gunther, M.L.; Morandi, A.; Krauskopf, E.; Pandharipande, P.; Girard, T.D.; Jackson, J.C.; Thompson, J.; Shintani, A.K.; Geevarghese, S.; Miller, R.R., 3rd; et al. The association between brain volumes, delirium duration, and cognitive outcomes in intensive care unit survivors: The VISIONS cohort magnetic resonance imaging study*. Crit. Care Med. 2012, 40, 2022–2032. [Google Scholar] [CrossRef] [PubMed]
- Reade, M.C.; Finfer, S. Sedation and delirium in the intensive care unit. N. Engl. J. Med. 2014, 370, 444–454. [Google Scholar] [CrossRef] [PubMed]
- Mo, Y.; Yam, F.K. Rational Use of Second-Generation Antipsychotics for the Treatment of ICU Delirium. J. Pharm. Pract. 2017, 30, 121–129. [Google Scholar] [CrossRef] [PubMed]
- Devlin, J.W.; Skrobik, Y.; Riker, R.R.; Hinderleider, E.; Roberts, R.J.; Fong, J.J.; Ruthazer, R.; Hill, N.S.; Garpestad, E. Impact of quetiapine on resolution of individual delirium symptoms in critically ill patients with delirium: A post-hoc analysis of a double-blind, randomized, placebo-controlled study. Crit. Care 2011, 15, R215. [Google Scholar] [CrossRef] [PubMed]
- Jaehne, E.J.; Corrigan, F.; Toben, C.; Jawahar, M.C.; Baune, B.T. The effect of the antipsychotic drug quetiapine and its metabolite norquetiapine on acute inflammation, memory and anhedonia. Pharmacol. Biochem. Behav. 2015, 135, 136–144. [Google Scholar] [CrossRef] [PubMed]
| Variables | Placebo Group (N = 20) | Study Group (N = 15) | p Value |
|---|---|---|---|
| Age | 69.10 ± 12.42 | 71.33 ± 10.35 | 0.576 |
| Sex, male | 11 (55.0) | 11 (73.3) | 0.226 |
| APACHE II score | 23.65 ± 7.85 | 21.53 ± 10.15 | 0.491 |
| SOFA score | 7.10 ± 4.08 | 5.60 ± 4.12 | 0.291 |
| Charlson comorbidity index | 4.70 ± 2.41 | 6.07 ± 1.94 | 0.081 |
| Intubated at study entry | 17 (85.0) | 13 (86.7) | 0.64 |
| Baseline QTc interval, ms | 449.25 ± 36.68 | 457.07 ± 30.85 | 0.510 |
| Mean RASS at study entry | −1.83 ± 2.12 | −1.43 ± 1.53 | 0.549 |
| Cause of ICU admission | 0.659 | ||
| Pneumonia (+ARDS) | 5 (25.0) | 6 (40.0) | |
| Respiratory failure | 13 (65.0) | 8 (53.3) | |
| Cardiogenic origin | 1 (5.0) | 1 (6.7) | |
| Others | 1 (5.0) | 0 (0.0) | |
| Sepsis/Septic shock | 6 (30.0) | 3 (20.0) | 0.700 |
| Sedative, analgesics within 24 h before randomization | |||
| Dexmedetomidine (mcg) | 246.40 ± 326.87 | 213.40 ± 419.83 | 0.795 |
| Midazolam (mg) | 23.24 ± 44.32 | 14.73 ± 28.95 | 0.523 |
| Remifentanil (mg) | 2.26 ± 1.98 | 2.17 ± 2.38 | 0.902 |
| Variables | Placebo Group (N = 20) | Study Group (N = 15) | p Value |
|---|---|---|---|
| Delirium incidence | 11 (55.0) | 7 (46.7) | 0.442 |
| Delirium subtype | 0.431 | ||
| Hypoactive (−5–−1) | 4 (20.0) | 1 (6.7) | |
| Hyperactive (0–+4) | 5 (25.0) | 6 (40.0) | |
| Mixed (−5–+4) | 11 (55.0) | 8 (53.3) | |
| Positive rate of CAM-ICU | 0.37 ± 0.38 | 0.14 ± 0.28 | 0.048 |
| Duration of delirium (days) | 1.83 ± 1.34 | 0.28 ± 0.52 | 0.018 |
| Drug administration days | 5.40 ± 1.70 | 3.93 ± 2.02 | 0.260 |
| Average dose of study drug (mg/day) | 21.42 ± 2.24 | 20.40 ± 2.72 | 0.233 |
| Time spent agitated, RASS > +2–+4 | |||
| Hours | 7.80 ± 13.64 | 7.20 ± 8.84 | 0.883 |
| Percent | 0.05 ± 0.08 | 0.06 ± 0.08 | 0.542 |
| Time spent deeply sedated, RASS < −2–−4 | |||
| Hours | 71.40 ± 80.31 | 32.00 ± 45.73 | 0.076 |
| Percent | 0.35 ± 0.32 | 0.24 ± 0.29 | 0.278 |
| Patient initiated device removal | |||
| Endotracheal tube | 0 | 0 | - |
| C lines, A line, or IV line | 1 (5.0) | 2 (13.3) | 0.383 |
| Levin tube | 4 (20.0) | 4 (26.7) | 0.642 |
| MV apply | 17 (85.0) | 13 (86.7) | 0.640 |
| Duration of MV (days) | 15.76 ± 24.22 | 6.43 ± 8.24 | 0.180 |
| ICU LOS (days) | 17.00 ± 22.56 | 7.47 ± 7.31 | 0.126 |
| Hospital LOS (days) | 35.25 ± 29.60 | 25.33 ± 21.84 | 0.283 |
| Successful extubation | 8 (47.1) | 11 (84.6) | 0.034 |
| ICU mortality | 6 (30.0) | 2 (13.3) | 0.245 |
| In-hospital mortality | 10 (50.0) | 3 (20.0) | 0.070 |
| Discharge to | 0.185 | ||
| Home | 7 (35.0) | 9 (60.0) | |
| Chronic facility care | 3 (15.0) | 3 (20.0) | |
| Death | 10 (50.0) | 3 (20.0) | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, Y.; Kim, H.-S.; Park, J.S.; Cho, Y.-J.; Yoon, H.I.; Lee, S.-M.; Lee, J.H.; Lee, C.-T.; Lee, Y.J. Efficacy of Low-Dose Prophylactic Quetiapine on Delirium Prevention in Critically Ill Patients: A Prospective, Randomized, Double-Blind, Placebo-Controlled Study. J. Clin. Med. 2020, 9, 69. https://doi.org/10.3390/jcm9010069
Kim Y, Kim H-S, Park JS, Cho Y-J, Yoon HI, Lee S-M, Lee JH, Lee C-T, Lee YJ. Efficacy of Low-Dose Prophylactic Quetiapine on Delirium Prevention in Critically Ill Patients: A Prospective, Randomized, Double-Blind, Placebo-Controlled Study. Journal of Clinical Medicine. 2020; 9(1):69. https://doi.org/10.3390/jcm9010069
Chicago/Turabian StyleKim, Youlim, Hyung-Sook Kim, Jong Sun Park, Young-Jae Cho, Ho Il Yoon, Sang-Min Lee, Jae Ho Lee, Choon-Taek Lee, and Yeon Joo Lee. 2020. "Efficacy of Low-Dose Prophylactic Quetiapine on Delirium Prevention in Critically Ill Patients: A Prospective, Randomized, Double-Blind, Placebo-Controlled Study" Journal of Clinical Medicine 9, no. 1: 69. https://doi.org/10.3390/jcm9010069
APA StyleKim, Y., Kim, H.-S., Park, J. S., Cho, Y.-J., Yoon, H. I., Lee, S.-M., Lee, J. H., Lee, C.-T., & Lee, Y. J. (2020). Efficacy of Low-Dose Prophylactic Quetiapine on Delirium Prevention in Critically Ill Patients: A Prospective, Randomized, Double-Blind, Placebo-Controlled Study. Journal of Clinical Medicine, 9(1), 69. https://doi.org/10.3390/jcm9010069






