Application of Impedance-Based Techniques in Hepatology Research
Abstract
1. Introduction
1.1. Common Methods of IBCA in Hepatology Research
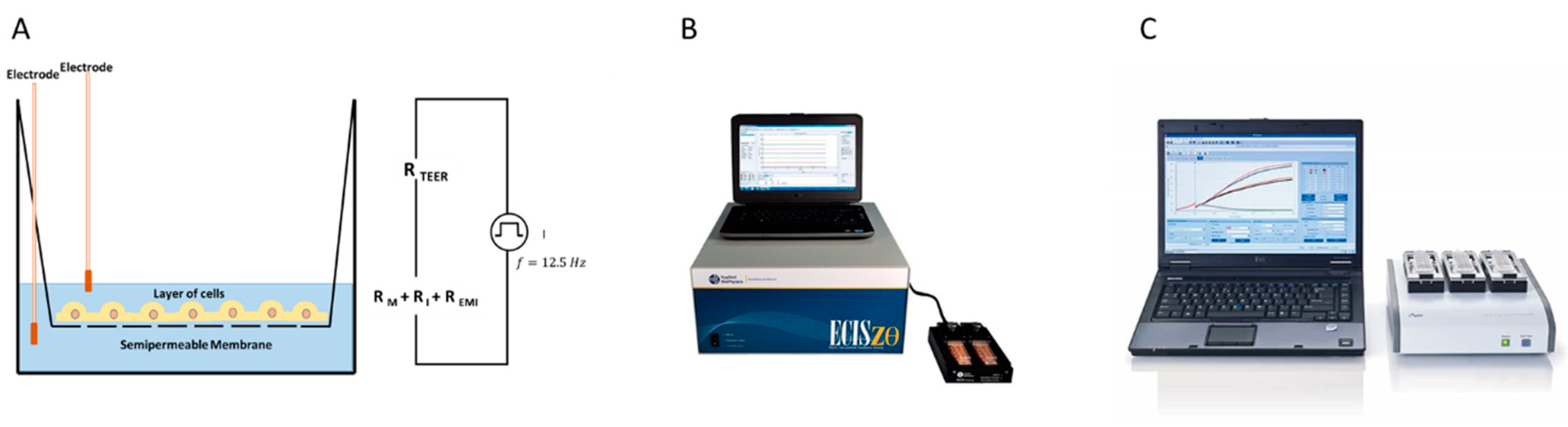
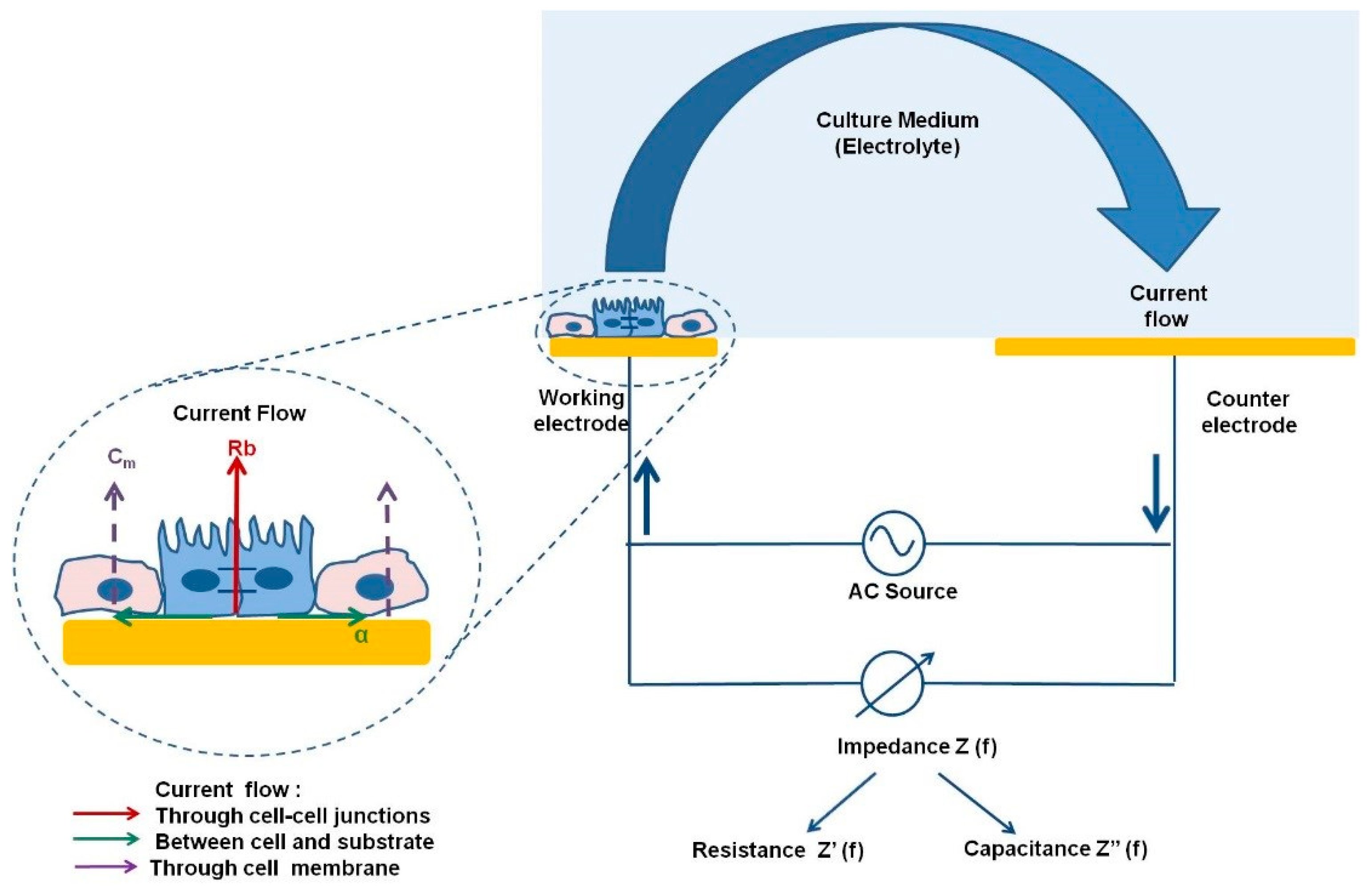
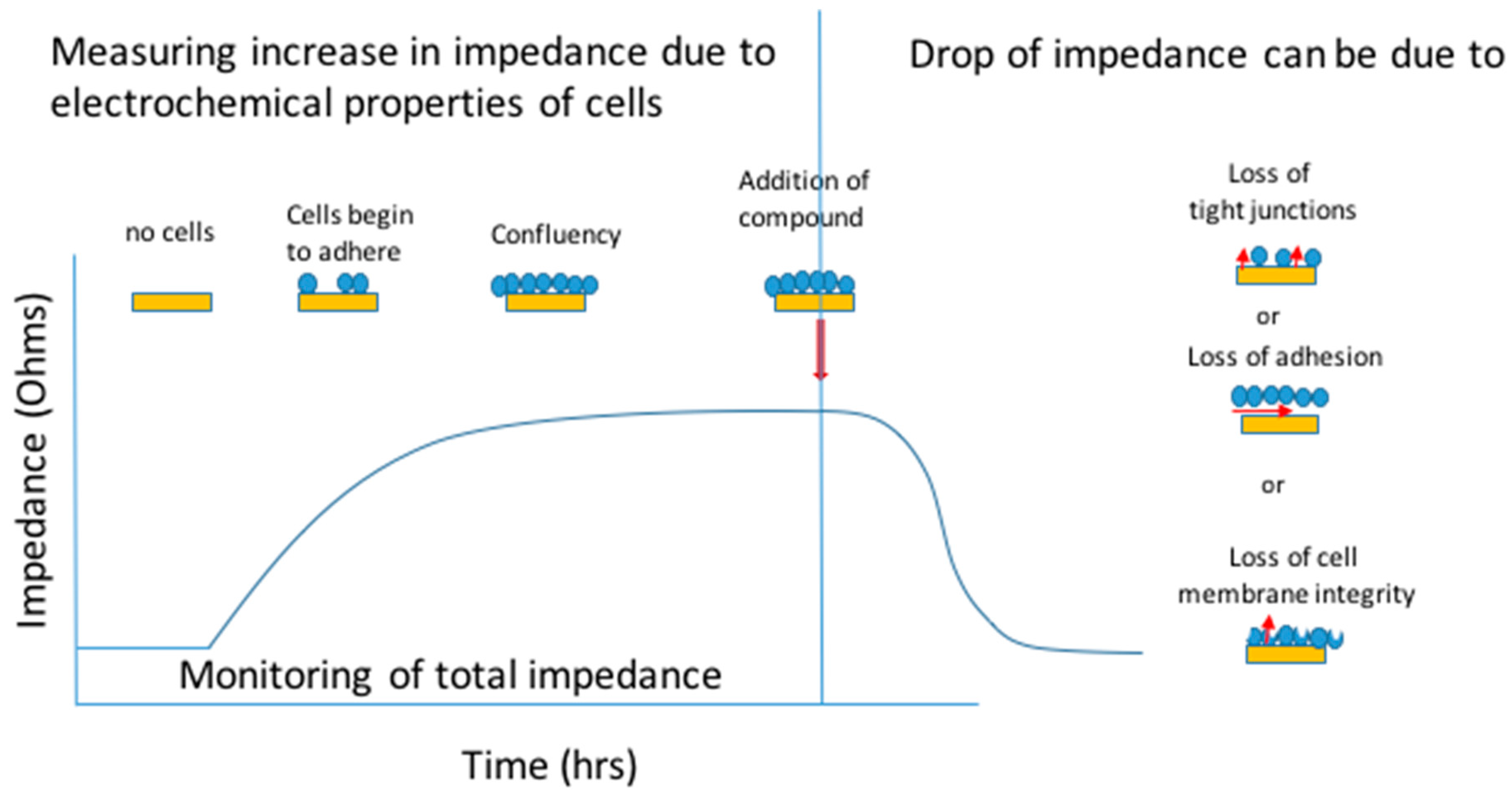
1.2. ECIS
1.3. xCELLigence™
1.4. TEER
2. Importance of Monitoring Tight/Gap Junctions and Polarity in Hepatic Cells
3. Role of Impedance Based Cellular Assays in the Investigation of Hepatic Disease
3.1. Viral Hepatitis
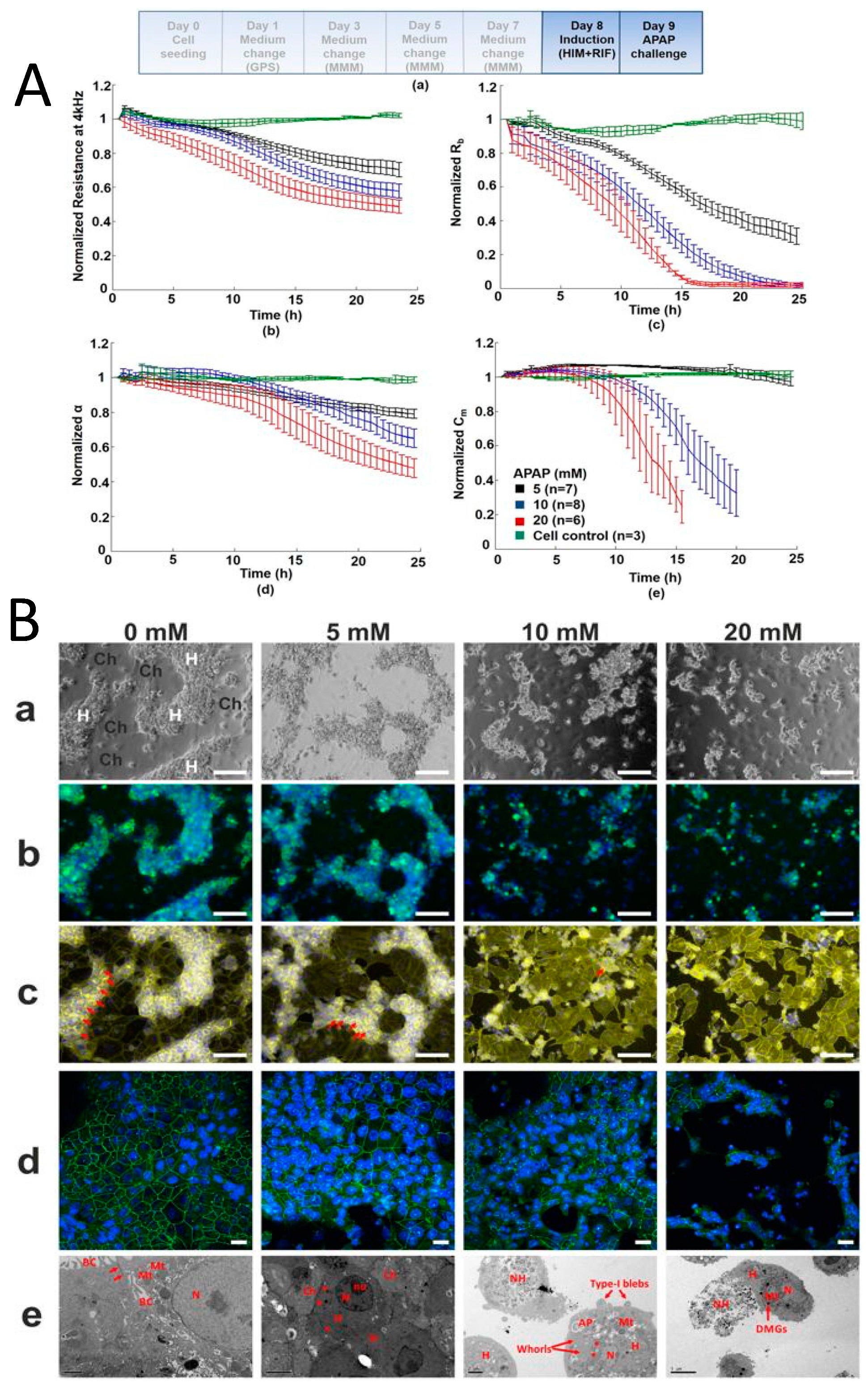
3.2. Drug Induced Liver Injury
3.3. Toxicity Assays Using HepaRG Cells
3.4. Toxicity Assays Using Pluripotent Stem Cells
4. Cholestatic Syndromes
4.1. Primary Sclerosing Cholangitis
4.2. Monitoring Hepatic Tight Junctions in Models of Cholestasis
5. Hepatocellular Cancer
5.1. Monitoring of Migration and Proliferation of Hepatocellular Cancer Cells
5.2. Role of Cellular Impedance as a Diagnostic System for HCC
6. Mechanistic Impedance Signatures
7. Cellular Impedance in Understanding the Gut/Liver Axis
8. Novel Development in Cellular Impedance
D Platforms and Microfluidic Systems
9. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Randivir, E.P.; Banks, C.E. Electrochemical impedance spectroscopy: An overview of bioanalytical applications. Anal. Methods 2013, 5, 1098–1115. [Google Scholar] [CrossRef]
- Gamal, W.; Treskes, P.; Samuel, K.; Sullivan, G.; Siller, R.; Srsen, V.; Morgan, K.; Bryans, A.; Kozlowska, A.; Underwood, I.; et al. Low-dose acetaminophen induces early disruption of cell-cell tight junctions in human hepatic cells and mouse liver. Sci. Rep. 2017, 7, 37541. [Google Scholar] [CrossRef] [PubMed]
- García-Sánchez, T.; Bragós, R.; Mir, L.M. In vitro analysis of various cell lines responses to electroporative electric pulses by means of electrical impedance spectroscopy. Biosens. Bioelectron. 2018, 117, 207–216. [Google Scholar] [CrossRef] [PubMed]
- Bagnaninchi, P.O.; Drummond, N. Real-time label-free monitoring of adipose-derived stem cell differentiation with electric cell-substrate impedance sensing. Proc. Natl. Acad. Sci. USA 2011, 108, 6462–6467. [Google Scholar] [CrossRef] [PubMed]
- Rammah, M.; Dandachi, F.; Salman, R.; Shihadeh, A.; El-Sabban, M. In vitro cytotoxicity and mutagenicity of mainstream waterpipe smoke and its functional consequences on alveolar type II derived cells. Toxicol. Lett. 2012, 211, 220–231. [Google Scholar] [CrossRef] [PubMed]
- Moodley, K.; Angel, C.E.; Glass, M.; Graham, E.S. Real-time profiling of NK cell killing of human astrocytes using xCELLigence technology. J. Neurosci. Methods 2011, 200, 173–180. [Google Scholar] [CrossRef] [PubMed]
- Sergent, J.A.; Paget, V.; Chevillar, S. Toxicity and genotoxicity of nano-SiO2 on human epithelial intestinal HT-29 cell line. Ann. Occup. Hyg. 2012, 56, 622–630. [Google Scholar]
- Diemert, S.; Dolga, A.M.; Tobaben, S.; Grohm, J.; Pfeifer, S.; Oexler, E.; Culmsee, C. Impedance measurement for real time detection of neuronal cell death. J. Neurosci. Methods 2012, 203, 69–77. [Google Scholar] [CrossRef]
- Yang, T.; Mei, H.; Xu, D.; Zhou, W.; Zhu, X.; Sun, L.; Huang, X.; Wang, X.; Shu, T.; Liu, J.; et al. Early indications of ANIT-induced cholestatic liver injury: Alteration of hepatocyte polarization and bile acid homeostasis. Food Chem. Toxicol. 2017, 110, 1–12. [Google Scholar] [CrossRef]
- Michaelis, S.; Wegener, J.; Robelek, R. Label-free monitoring of cell-based assays: Combining impedance analysis with SPR for multiparametric cell profiling. Biosens. Bioelectron. 2013, 49, 63–70. [Google Scholar] [CrossRef]
- Giaever, I.; Keese, C.R. Monitoring Fibroblast Behaviour in Tissue Culture with an Applied Electric Field. Proc. Natl. Acad. Sci. USA 1984, 81, 3761–3764. [Google Scholar] [CrossRef] [PubMed]
- Tibaldi, L.; Leyman, S.; Nicolas, A.; Notebaert, S.; Dewulf, M.; Ngo, T.H.; Zuany-Amorim, C.; Amzallag, N.; Bernard-Pierrot, I.; Sastre-Garau, X.; et al. New blocking antibodies impede adhesion, migration and survival of ovarian cancer cells, highlighting MFGE8 as a potential therapeutic target of human ovarian carcinoma. PLoS ONE 2013, 8, e72708. [Google Scholar] [CrossRef] [PubMed]
- Sansing, H.; Renner, N.; MacLean, A.G. An inverted blood-brain barrier model that permits interactions between glia and inflammatory stimuli. J. Neurosci. Methods 2012, 207, 91–96. [Google Scholar] [CrossRef] [PubMed]
- Kustermann, S.; Boess, F.; Buness, A.; Schmitz, M.; Watzele, M.; Weiser, T.; Singer, T.; Suter, L.; Roth, A. A label-free impedance-based real time assay to identify drug-induced toxicities and differentiate cytostatic from cytotoxic effects. Toxicol. In Vitro 2013, 27, 1589–1595. [Google Scholar] [CrossRef]
- Marinova, Z.; Waltiza, S.; Grunblatt, E. 5-HT2A serotonin receptor agonist DOI alleviates cytotoxicity in neuroblastoma cells: Role of the ERK pathway. Prog. Neuro Psychopharmacol. Biol. Psychiatry 2013, 44, 64–72. [Google Scholar] [CrossRef]
- Moniri, M.R.; Young, A.; Reinheimer, K.; Rayat, J.; Dai, L.J.; Warnock, G.L. Dynamic assessment of cell viability, proliferation and migration using real time cell analyser system (RTCA). Cytotechnology 2015, 67, 379–386. [Google Scholar] [CrossRef]
- Benson, K.; Cramer, S.; Galla, H.J. Impedance-Based Cell Monitoring: Barrier Properties and Beyond. Fluids Barriers CNS 2013, 10, 5. [Google Scholar] [CrossRef]
- Srinivasan, B.; Kolli, A.R.; Esch, M.B.; Abaci, H.E.; Shuler, M.L.; Hickman, J.J. TEER Measurement Techniques for In Vitro Barrier Model Systems. J. Lab. Autom. 2015, 20, 107–126. [Google Scholar] [CrossRef]
- Lee, N.P.; Luk, J.M. Hepatic tight junctions: From viral entry to cancer metastasis. World J. Gastroenterol. 2010, 16, 289–295. [Google Scholar] [CrossRef]
- Mitic, L.; Anderson, J.M. Molecular architecture of tight junctions. Annu. Rev. Physiol. 1998, 60, 121–142. [Google Scholar] [CrossRef]
- Claude, P.; Goodenough, D.A. Fracture faces of zonulae occludentes from ‘tight’ and ‘leaky’ epithelia. J. Cell Biol. 1973, 58, 390–400. [Google Scholar] [CrossRef] [PubMed]
- Kojima, T.; Yamamoto, T.; Murata, M.; Chiba, H.; Kokai, Y.; Swada, N. Regulation of the blood-biliary barrier: Interaction between gap and tight junctions in hepatocytes. Med. Electron. Microsci. 2003, 36, 157–164. [Google Scholar] [CrossRef] [PubMed]
- Nathanson, M.H.; Rios-Velez, L.; Burgstahler, A.D.; Mennone, A. Communication via gap junctions modulates bile secretion in the isolated perfused rat liver. Gastroenterology 1999, 116, 1176–1183. [Google Scholar] [CrossRef]
- Gitlin, N. The Liver Biology and Pathobiology, 4th ed.; Arias, I.M., Boyer, J.L., Chisari, F.V., Fausto, N., Schachter, D., Schafritz, D.A., Eds.; Lippincott Williams and Wilkins: Philadelphia, PA, USA, 2001; Chapter 3; pp. 29–46. ISBN 0-7817-2390-6. [Google Scholar]
- Zeisel, M.B.; Dhawan, P.; Baumert, T.F. Tight junction proteins in gastrointestinal and liver disease. Gut 2019, 68, 547–561. [Google Scholar] [CrossRef] [PubMed]
- Lódi, C.; Szabó, E.; Holczbauer, A.; Batmunkh, E.; Szíjártó, A.; Kupcsulik, P.; Kovalszky, I.; Paku, S.; Illyés, G.; Kiss, A.; et al. Claudin-4 differentiates biliary tract cancers from hepatocellular carcinomas. Mod. Pathol. 2006, 19, 460–469. [Google Scholar] [CrossRef]
- Benedicto, I.; Molina-Jimenez, F.; Bartosch, B.; Cosset, F.L.; Lavilette, D.; Prieto, J.; Moreno-Otero, R.; Valenzuela-Fernandez, A.; Aldabe, R.; Lopez-Cabrera, M.; et al. The tight junction-associated protein occludin is required for a postbinding step in hepatitis C virus entry and infection. J. Virol. 2009, 83, 8012–8020. [Google Scholar] [CrossRef]
- Mankouri, J.; Walter, C.; Stewart, H.; Bentham, M.; Park, W.S.; Do Heo, W.; Fukuda, M.; Griffin, S.; Harris, M. Release of Infectious Hepatitis C Virus from Huh7 Cells Occurs via a trans-Golgi Network-to-Endosome Pathway Independent of Very-Low-Density Lipoprotein Secretion. J. Virol. 2016, 90, 7159–7170. [Google Scholar] [CrossRef]
- Teimourpour, R.; Meshkat, Z.; Gholoubi, A.; Nomani, H.; Rostami, S. Viral Load Analysis of Hepatitis C Virus in Huh7.5 Cell Culture System. Jundishapur J. Microbiol. 2015, 8, e19279. [Google Scholar] [CrossRef]
- Sainz, B., Jr.; Barretto, N.; Uprichard, S.L. Hepatitis C virus infection in phenotypically distinct Huh7 cell lines. PLoS ONE 2009, 4, e6561. [Google Scholar] [CrossRef]
- Bartenschlager, R.; Pietschmann, T. Efficient hepatitis C virus cell culture system: What a difference the host cell makes. Proc. Natl. Acad. Sci. USA 2005, 102, 9739–9740. [Google Scholar] [CrossRef]
- Ndongo-Thiam, N.; Berthillon, P.; Errazuriz, E.; Bordes, I.; De Sequeira, S.; Trépo, C.; Petit, M.A. Long-term propagation of serum hepatitis C virus (HCV) with production of enveloped HCV particles in human HepaRG hepatocytes. Hepatology 2011, 54, 406–417. [Google Scholar] [CrossRef] [PubMed]
- Fletcher, N.F.; Clark, A.R.; Balfe, P.; McKeating, J.A. TNF superfamily members promote hepatitis C virus entry via an NF-κB and myosin light chain kinase dependent pathway. J. Gen. Virol. 2017, 98, 405–412. [Google Scholar] [CrossRef] [PubMed]
- Yoneda, M.; Hyun, J.; Jakubski, S.; Saito, S.; Nakajima, A.; Schiff, E.R.; Thomas, E. Hepatitis B Virus and DNA Stimulation Trigger a Rapid Innate Immune Response through NF-κB. J. Immunol. 2016, 197, 630–643. [Google Scholar] [CrossRef] [PubMed]
- Kasai, F.; Hirayama, N.; Ozawa, M.; Satoh, M.; Kohara, A. HuH-7 reference genome profile: Complex karyotype composed of massive loss of heterozygosity. Hum. Cell 2018, 31, 261–267. [Google Scholar] [CrossRef] [PubMed]
- Nakabayashi, H.; Miyano, K.; Sato, J.; Yamane, T.; Taketa, K. Growth of human hepatoma cell lines with differentiated functions in chemically defined medium. Cancer Res. 1982, 42, 3858–3863. [Google Scholar]
- Castell, J.V.; Gomez-Lechon Maria, J.; Ponsoda, X.; Bort, R. In vitro investigation of the molecular mechanisms of hepatotoxicity. In In Vitro Methods in Pharmaceutical Research; Academic Press Limited: London, UK, 1996; Chapter 16; pp. 375–410. [Google Scholar]
- Gerets, H.H.; Tilmant, K.; Gerin, B.; Chanteux, H.; Depelchin, B.O.; Dhalluin, S.; Atienzar, F.A. Characterization of primary human hepatocytes, HepG2 cells, and HepaRG cells at the mRNA level and CYP activity in response to inducers and their predictivity for the detection of human hepatotoxins. Cell Biol. Toxicol. 2012, 28, 69–87. [Google Scholar] [CrossRef]
- Pennington, M.R.R.; Van de Walle, G.R. Electric cell-substrate impedance sensing to monitor viral growth and study cellular responses to infection with alphaherpesviruses in real time. mSphere 2017, 2, e00039-17. [Google Scholar] [CrossRef]
- Morgan, F.; Martucci, N.; Kozlowska, A.; Gamal, W.; Brzeszczynki, F.; Treskes, P.; Samuel, K.; Hayes, P.; Nelson, L.; Bagnaninchi, P.; et al. Chlorpromazine toxicity is associated with disruption of cell membrane integrity and initiation of a pro-inflammatory response in the HepaRG hepatic cell line. Biomed. Pharmacother. 2019, 111, 1408–1416. [Google Scholar] [CrossRef]
- Atienzar, F.A.; Tilmant, K.; Gerets, H.H.; Toussaint, G.; Speeckaert, S.; Hanon, E.; Depelchin, O.; Dhalluin, S. The use of real-time cell analyser technology in drug discovery: Defining optimal cell culture conditions and assay reproducibility with different adherent cellular models. J. Biomol. Screen 2011, 16, 575–587. [Google Scholar] [CrossRef]
- Atienzar, F.A.; Gerets, H.; Tilmant, K.; Toussain, G.; Dhalluin, S. Evaluation of impedance-based label-free technology as a tool for pharmacology and toxicology investigations. Biosensors 2013, 3, 132–156. [Google Scholar] [CrossRef]
- Peyre, L.; de Sousa, G.; Barcellini-Couget, S.; Luzy, A.P.; Zucchini-Pascal, N.; Rahmini, R. High-content screening imaging and real-time cellular impedance monitoring for the assessment of chemical’s bio-activation with regards hepatotoxicity. Toxicol. In Vitro 2015, 29, 1916–1931. [Google Scholar] [CrossRef] [PubMed]
- Gripon, P.; Rumin, S.; Urban, S.; Le Seyec, J.; Glaise, D.; Cannie, I.; Guyomard, C.; Lucas, J.; Christian, T.; Guguen-Guillouzo, C. Infection of a human hepatoma cell line by hepatitis B virus. Proc. Natl. Acad. Sci. USA 2002, 99, 15655–15660. [Google Scholar] [CrossRef] [PubMed]
- Le Vee, M.; Jouan, E.; Stieger, B.; Fardel, O. Differential regulation of drug transporter expression by all-trans retinoic acid in hepatoma HepaRG cells and human hepatocytes. Eur. J. Pharm. Sci. 2013, 48, 767–774. [Google Scholar] [CrossRef] [PubMed]
- Szabo, M.; Veres, Z.; Baranyai, Z.; Jakab, F.; Jemnitz, K. Comparison of Human Hepatoma HepaRG cells with Human and Rat Hepatocytes in Uptake Transport Assays in Order to Predict a Risk of Drug Induced Hepatotoxicity. PLoS ONE 2013, 8, e59432. [Google Scholar] [CrossRef] [PubMed]
- Chun, Y.S.; Chaudhari, P.; Jan, Y.Y. Applications of Patient-Specific Induced Pluripotent Stem Cells; Focused on Disease Modeling, Drug Screening and Therapeutic Potentials for Liver Disease. Int. J. Biol. Sci. 2010, 6, 796–805. [Google Scholar] [CrossRef]
- Sullivan, G.J.; Hay, D.C.; Park, I.H.; Fletcher, J.; Hannoun, Z.; Payne, C.M.; Dalgetty, D.; Black, J.R.; Ross, J.A.; Samuel, K.; et al. Generation of Functional Human Hepatic Endoderm from Human Induced Pluripotent Stem Cells. Hepatology 2010, 51, 329–335. [Google Scholar] [CrossRef]
- Rashid, S.T.T.; Alexander, G.J.M.J. Induced pluripotent stem cells: From Nobel Prizes to clinical applications. J. Hepatol. 2013, 58, 625–629. [Google Scholar] [CrossRef][Green Version]
- Zhou, Y.; Yang, D.; Zhou, Y.; Khoo, B.L.; Han, J.; Ai, Y. Characterizing Deformability and Electrical Impedance of Cancer Cells in a Microfluidic Device. Anal. Chem. 2017, 90, 912–919. [Google Scholar] [CrossRef]
- Tabibian, J.H.; Trussoni, C.E.; O’hara, S.P.; Splinter, P.L.; Heimbach, J.K.; LaRusso, N.F. Characterization of cultured cholangiocytes isolated from livers of patients with primary sclerosing cholangitis. Lab. Investig. 2014, 94, 1126–1133. [Google Scholar] [CrossRef]
- Anthérieu, S.; Azzi, P.B.; Dumont, J.; Abdel-Razzak, Z.; Guguen-Guillouzo, C.; Fromenty, B.; Robin, M.A.; Guillouzo, A. Oxidative stress plays a major role in chlorpromazine-induced cholestasis in human HepaRG cells. Hepatology 2013, 57, 1518–1529. [Google Scholar] [CrossRef]
- Anthérieu, S.; Chesné, C.; Li, R.; Guguen-Guillouzo, C.; Guillouzo, A. Optimization of the HepaRG cell model for drug metabolism and toxicity studies. Toxicol. In Vitro 2012, 26, 1278–1285. [Google Scholar] [CrossRef] [PubMed]
- Akerboom, T.; Schnieder, I.; vom Dahl, S.; Sies, H. Cholestasis and changes of portal pressure caused by chlorpromazine in the perfused rat liver. Hepatology 1991, 13, 216–221. [Google Scholar] [CrossRef] [PubMed]
- Zimmerman, H.J.; Lewis, J.H. Drug-induced cholestasis. Med. Toxicol. 1987, 2, 112–160. [Google Scholar] [CrossRef] [PubMed]
- Moradpour, D.; Altorfer, J.; Flury, R.; Greminger, P.; Meyenberger, C.; Schmid, M. Chlorpromazine-induced vanishing bile duct syndrome leading to biliary cirrhosis. Hepatology 1994, 20, 1437–1441. [Google Scholar] [CrossRef]
- Baba, A.I.; Câtoi, C. Comparative Oncology; Chapter 3, Tumor Cell Morphology; The Publishing House of the Romanian Academy: Bucharest, Romania, 2007; Available online: https://www.ncbi.nlm.nih.gov/books/NBK9553/ (accessed on 12 February 2019).
- Zou, Y.; Guo, Z. A review of electrical impedance techniques for breast cancer detection. Med. Eng. Phys. 2003, 25, 79–90. [Google Scholar] [CrossRef]
- Hong, J.; Kandasamy, K.; Marimuthu, M.; Choi, C.S.; Kim, S. Electrical cell-substrate impedance sensing as a non-invasive tool for cancer cell study. Analyst 2011, 136, 237–245. [Google Scholar] [CrossRef]
- Saxena, N.K.; Sharma, D.; Ding, X.; Lin, S.; Marra, F.; Merlin, D.; Anania, F.A. Concomitant activation of the JAK/STAT, PI3K/AKT, and ERK signaling is involved in leptin-mediated promotion of invasion and migration of hepatocellular carcinoma cells. Cancer Res. 2007, 67, 2497–2507. [Google Scholar] [CrossRef]
- Wang, L.; Yin, H.; Xing, W.; Yu, Z.; Guo, M.; Cheng, J. Real-time, label-free monitoring of the cell cycle with a cellular impedance sensing chip. Biosens. Bioelectron. 2010, 25, 990–995. [Google Scholar] [CrossRef]
- Porter, K.; Prescott, D.; Frye, J. Changes in surface morphology of Chinese hamster ovary cells during the cell cycle. J. Cell Biol. 1973, 57, 815–836. [Google Scholar] [CrossRef]
- Chen, D.; Shen, M.; Cao, Y.; Bo, B.; Chen, Z.; Shu, Y.; Li, G. Electrochemical identification of hepatocellular carcinoma based on the assay of human cervical cancer oncoprotein-1 in serum. Electrochem. Commun. 2013, 27, 38–41. [Google Scholar] [CrossRef]
- Abassi, Y.A.; Xi, B.; Zhang, W.; Ye, P.; Kirstein, S.L.; Gaylord, M.R.; Feinstein, S.C.; Wang, X.; Xu, X. Kinetic cell-based morphological screening: Prediction of mechanism of compound action and off-targets effects. Chem. Biol. 2009, 16, 712–723. [Google Scholar] [CrossRef]
- Bustin, S.A.; Benes, V.; Garson, J.A.; Hellemans, J.; Huggett, J.; Kubista, M.; Mueller, R.; Nolan, T.; Pfaffl, M.W.; Shipley, G.L.; et al. The MIQE guidelines: Minimum information for publication of quantitative real-time PCR experiments. Clin. Chem. 2009, 55, 611–622. [Google Scholar] [CrossRef] [PubMed]
- Szabo, G. Gut-Liver axis in alcoholic liver disease. Gastroenterology 2015, 148, 30–36. [Google Scholar] [CrossRef] [PubMed]
- Weist, R.; Abillos, A.; Trauner, M.; Bajaj, J.S.; Jilan, R. Targeting the gut-liver axis in liver disease. J. Hepatol. 2017, 67, 1084–1103. [Google Scholar] [CrossRef] [PubMed]
- Tan, H.-Y.; Trier, S.; Rahbek, U.L.; Dufva, M.; Kutter, J.P.; Andresen, T.L. A multi-chamber microfluidic intestinal barrier model using Caco-2 cells for drug transport studies. PLoS ONE 2018, 13, e0197101. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.Y.; Sung, J.H. Gut-liver on a chip toward an in vitro model of hepatic steatosis. Biotechnol. Bioeng. 2018, 115, 2817–2827. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Yang, Y.; Bagnaninchi, P.; Jia, J. Electrical impedance tomography for real-time and label-free cellular viability assays of 3D tumour spheroids. Analyst 2018, 143, 4189–4198. [Google Scholar] [CrossRef]
- Wu, H.; Zhou, W.; Yang, Y.; Jia, J. Exploring the Potential of Electrical Impedance Tomography for Tissue Engineering Applications. Materials 2018, 11, 930. [Google Scholar] [CrossRef]
- Yang, Y.; Jia, J.; Smith, S.; Jamil, N.; Gamal, W.; Bagnaninchi, P.O. A Miniature Electrical Impedance Tomography Sensor and 3-D Image Reconstruction for Cell Imaging. IEEE Sens. J. 2017, 17, 514–523. [Google Scholar] [CrossRef]
- Tran, T.B.; Cho, S.; Min, J. Hydrogel-based diffusion chip with Electric Cell-substrate Impedance Sensing (ECIS) integration for cell viability assay and drug toxicity screening. Biosens. Bioelectron. 2013, 50, 453–459. [Google Scholar] [CrossRef]
- Hilber, W.; Lornejad-Schäfer, M.R.; Schäfer, C.; Lederer, T.; Schröder, K.; Jakoby, B. Impedance Spectroscopy of a Human Hepatic 3D Cell Model in-Vitro: A Comparative Study with Differently Shaped Electrodes. Procedia Eng. 2011, 25, 1000–1003. [Google Scholar] [CrossRef][Green Version]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morgan, K.; Gamal, W.; Samuel, K.; Morley, S.D.; Hayes, P.C.; Bagnaninchi, P.; Plevris, J.N. Application of Impedance-Based Techniques in Hepatology Research. J. Clin. Med. 2020, 9, 50. https://doi.org/10.3390/jcm9010050
Morgan K, Gamal W, Samuel K, Morley SD, Hayes PC, Bagnaninchi P, Plevris JN. Application of Impedance-Based Techniques in Hepatology Research. Journal of Clinical Medicine. 2020; 9(1):50. https://doi.org/10.3390/jcm9010050
Chicago/Turabian StyleMorgan, Katie, Wesam Gamal, Kay Samuel, Steven D. Morley, Peter C. Hayes, Pierre Bagnaninchi, and John N. Plevris. 2020. "Application of Impedance-Based Techniques in Hepatology Research" Journal of Clinical Medicine 9, no. 1: 50. https://doi.org/10.3390/jcm9010050
APA StyleMorgan, K., Gamal, W., Samuel, K., Morley, S. D., Hayes, P. C., Bagnaninchi, P., & Plevris, J. N. (2020). Application of Impedance-Based Techniques in Hepatology Research. Journal of Clinical Medicine, 9(1), 50. https://doi.org/10.3390/jcm9010050





