Immunological and Clinical Impact of Manipulated and Unmanipulated DLI after Allogeneic Stem Cell Transplantation of AML Patients
Abstract
1. Introduction
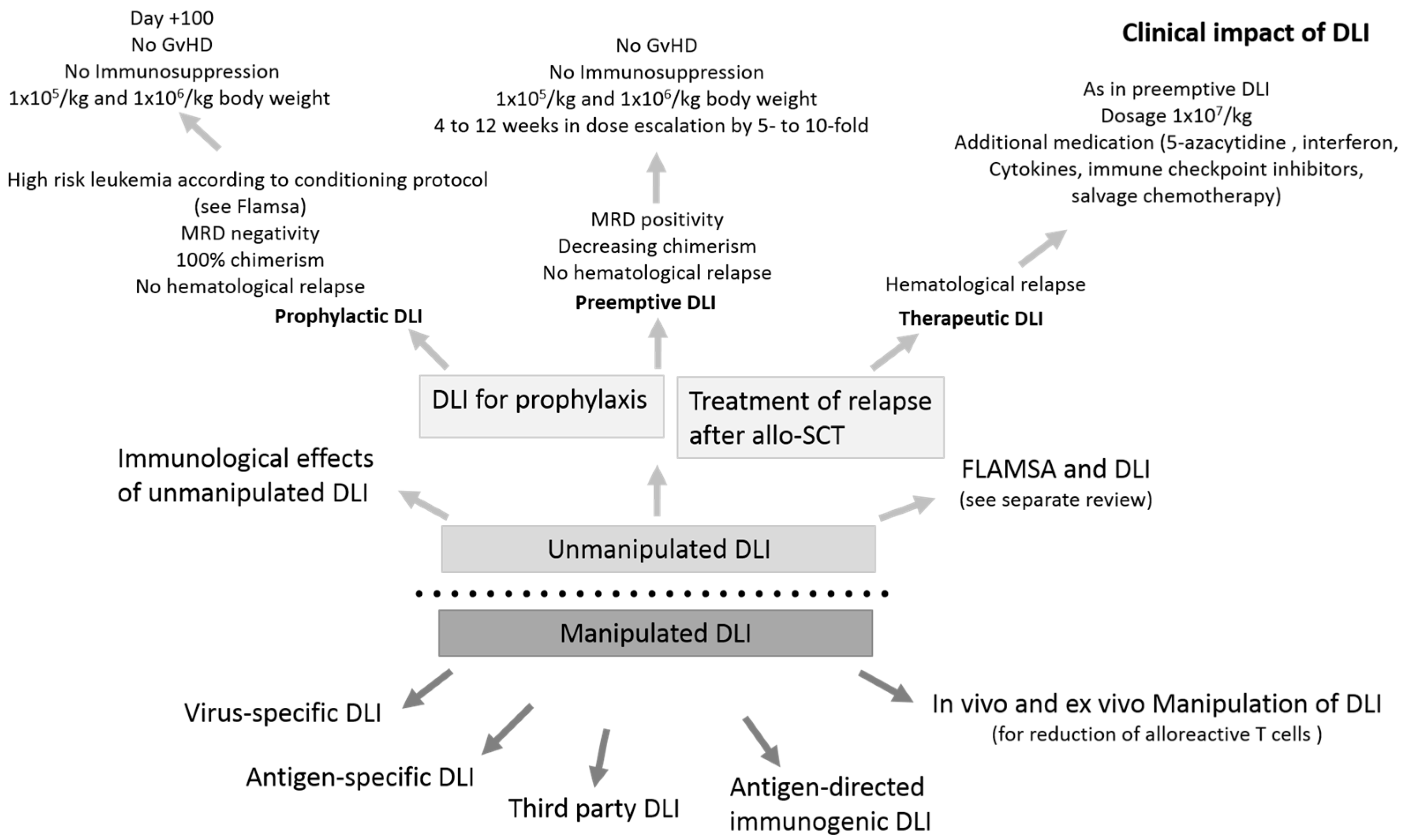
2. Understanding the Functionality of DLI—Immunological Effects of Unmanipulated DLI
3. Clinical Impact of Unmanipulated DLI in AML
3.1. Therapeutic DLI for the Treatment of Morphological Relapse
3.2. Biology of Therapeutic DLI
3.3. Prophylactic Use of DLI in AML/MDS
FLAMSA-RIC and DLI
3.4. Preemptive Use of DLI in AML/MDS
3.5. Biology of Preemptive/Prophylactic DLI
4. Antigen-Directed Immunogenic DLI
5. Specifically Stimulated and Modified DLI
5.1. Virus-Specific Donor T Cells for Cytomegalovirus (CMV)
5.2. Virus-Specific Donor T Cells for Epstein-Barr Virus (EBV)
5.3. Third Party DLI
6. In Vivo and Ex Vivo Manipulation of DLI for the Reduction of Alloreactive T Cells
6.1. In Vivo T Cell Depletion
6.2. Ex Vivo T Cell Depletion
7. Five-Year View
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Kolb, H.J.; Mittermuller, J.; Clemm, C.; Holler, E.; Ledderose, G.; Brehm, G.; Heim, M.; Wilmanns, W. Donor leukocyte transfusions for treatment of recurrent chronic myelogenous leukemia in marrow transplant patients. Blood 1990, 76, 2462–2465. [Google Scholar] [CrossRef] [PubMed]
- Kolb, H.J.; Schattenberg, A.; Goldman, J.M.; Hertenstein, B.; Jacobsen, N.; Arcese, W.; Ljungman, P.; Ferrant, A.; Verdonck, L.; Niederwieser, D.; et al. Graft-versus-leukemia effect of donor lymphocyte transfusions in marrow grafted patients. Blood 1995, 86, 2041–2050. [Google Scholar] [CrossRef] [PubMed]
- Barrett, A.J.; Mavroudis, D.; Tisdale, J.; Molldrem, J.; Clave, E.; Dunbar, C.; Cottler-Fox, M.; Phang, S.; Carter, C.; Okunnieff, P.; et al. T cell-depleted bone marrow transplantation and delayed T cell add-back to control acute GVHD and conserve a graft-versus-leukemia effect. Bone Marrow Transplant. 1998, 21, 543–551. [Google Scholar] [CrossRef] [PubMed]
- Rapoport, A.P.; Stadtmauer, E.A.; Binder-Scholl, G.K.; Goloubeva, O.; Vogl, D.T.; Lacey, S.F.; Badros, A.Z.; Garfall, A.; Weiss, B.; Finklestein, J.; et al. NY-ESO-1-specific TCR-engineered T cells mediate sustained antigen-specific antitumor effects in myeloma. Nat. Med. 2015, 21, 914–921. [Google Scholar] [CrossRef]
- Tawara, I.M.M.; Kageyama, S.; Nishida, T.; Terakura, S.; Murata, M.; Fujiwara, H.; Akatsuka, Y.; Ikeda, H.; Miyahara, Y.; Tomura, D.; et al. Adoptive Transfer of WT1-Specific TCR Gene-Transduced Lymphocytes in Patients with Myelodysplastic Syndrome and Acute Myeloid Leukemia. Blood 2015, 126, 97. [Google Scholar] [CrossRef]
- Perret, R.; Valliant-Saunders, K.; Cao, J.W.; Greenberg, P.D. Expanding the scope of WT1-and cyclin A1-specific TCR gene therapy for AML and other cancers. J. Immunol. 2016, 143, 145. [Google Scholar]
- Schmitt, T.M.; Aggen, D.H.; Stromnes, I.M.; Dossett, M.L.; Richman, S.A.; Kranz, D.M.; Greenberg, P.D. Enhanced-affinity murine T-cell receptors for tumor/self-antigens can be safe in gene therapy despite surpassing the threshold for thymic selection. Blood 2013, 122, 348–356. [Google Scholar] [CrossRef]
- Kochenderfer, J.N.; Dudley, M.E.; Kassim, S.H.; Somerville, R.P.; Carpenter, R.O.; Stetler-Stevenson, M.; Yang, J.C.; Phan, G.Q.; Hughes, M.S.; Sherry, R.M.; et al. Chemotherapy-refractory diffuse large B-cell lymphoma and indolent B-cell malignancies can be effectively treated with autologous T cells expressing an anti-CD19 chimeric antigen receptor. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2015, 33, 540–549. [Google Scholar] [CrossRef]
- Brudno, J.N.; Somerville, R.P.; Shi, V.; Rose, J.J.; Halverson, D.C.; Fowler, D.H.; Gea-Banacloche, J.C.; Pavletic, S.Z.; Hickstein, D.D.; Lu, T.L.; et al. Allogeneic T Cells That Express an Anti-CD19 Chimeric Antigen Receptor Induce Remissions of B-Cell Malignancies That Progress After Allogeneic Hematopoietic Stem-Cell Transplantation Without Causing Graft-Versus-Host Disease. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2016, 34, 1112–1121. [Google Scholar] [CrossRef]
- Yun, H.D.; Waller, E.K. Finding the sweet spot for donor lymphocyte infusions. Biol. Blood Marrow Transplant. J. Am. Soc. Blood Marrow Transplant. 2013, 19, 507–508. [Google Scholar] [CrossRef]
- Alyea, E.P.; DeAngelo, D.J.; Moldrem, J.; Pagel, J.M.; Przepiorka, D.; Sadelin, M.; Young, J.W.; Giralt, S.; Bishop, M.; Riddell, S. NCI First International Workshop on The Biology, Prevention and Treatment of Relapse after Allogeneic Hematopoietic Cell Transplantation: Report from the committee on prevention of relapse following allogeneic cell transplantation for hematologic malignancies. Biol. Blood Marrow Transplant. J. Am. Soc. Blood Marrow Transplant. 2010, 16, 1037–1069. [Google Scholar] [CrossRef]
- Jedlickova, Z.; Schmid, C.; Koenecke, C.; Hertenstein, B.; Baurmann, H.; Schwerdtfeger, R.; Tischer, J.; Kolb, H.J.; Schleuning, M. Long-term results of adjuvant donor lymphocyte transfusion in AML after allogeneic stem cell transplantation. Bone Marrow Transplant. 2016, 51, 663–667. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, S.; Schmitt, M.; Gotz, M.; Dohner, H.; Wiesneth, M.; Bunjes, D.; Greiner, J. Donor lymphocyte infusion leads to diversity of specific T cell responses and reduces regulatory T cell frequency in clinical responders. Int. J. Cancer J. Int. du Cancer 2019, 144, 1135–1146. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, A.; Sakaguchi, S. Regulatory T cells in cancer immunotherapy. Cell Res. 2017, 27, 109–118. [Google Scholar] [CrossRef] [PubMed]
- Gjerdrum, L.M.; Woetmann, A.; Odum, N.; Burton, C.M.; Rossen, K.; Skovgaard, G.L.; Ryder, L.P.; Ralfkiaer, E. FOXP3+ regulatory T cells in cutaneous T-cell lymphomas: Association with disease stage and survival. Leukemia 2007, 21, 2512–2518. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Xu, Y. Clinical significance of Treg cell frequency in acute myeloid leukemia. Int. J. Hematol. 2013, 98, 558–562. [Google Scholar] [CrossRef]
- D’Arena, G.; Laurenti, L.; Minervini, M.M.; Deaglio, S.; Bonello, L.; De Martino, L.; De Padua, L.; Savino, L.; Tarnani, M.; De Feo, V.; et al. Regulatory T-cell number is increased in chronic lymphocytic leukemia patients and correlates with progressive disease. Leuk. Res. 2011, 35, 363–368. [Google Scholar] [CrossRef]
- Mailloux, A.W.; Sugimori, C.; Komrokji, R.S.; Yang, L.; Maciejewski, J.P.; Sekeres, M.A.; Paquette, R.; Loughran, T.P., Jr.; List, A.F.; Epling-Burnette, P.K. Expansion of effector memory regulatory T cells represents a novel prognostic factor in lower risk myelodysplastic syndrome. J. Immunol. 2012, 189, 3198–3208. [Google Scholar] [CrossRef]
- Idris, S.Z.; Hassan, N.; Lee, L.J.; Md Noor, S.; Osman, R.; Abdul-Jalil, M.; Nordin, A.J.; Abdullah, M. Increased regulatory T cells in acute lymphoblastic leukaemia patients. Hematology 2016, 21, 206–212. [Google Scholar] [CrossRef]
- Simpson, T.R.; Li, F.; Montalvo-Ortiz, W.; Sepulveda, M.A.; Bergerhoff, K.; Arce, F.; Roddie, C.; Henry, J.Y.; Yagita, H.; Wolchok, J.D.; et al. Fc-dependent depletion of tumor-infiltrating regulatory T cells co-defines the efficacy of anti-CTLA-4 therapy against melanoma. J. Exp. Med. 2013, 210, 1695–1710. [Google Scholar] [CrossRef]
- Hofmann, S.; Gotz, M.; Schneider, V.; Guillaume, P.; Bunjes, D.; Dohner, H.; Wiesneth, M.; Greiner, J. Donor lymphocyte infusion induces polyspecific CD8(+) T-cell responses with concurrent molecular remission in acute myeloid leukemia with NPM1 mutation. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2013, 31, e44–e47. [Google Scholar] [CrossRef] [PubMed]
- Greiner, J.; Schneider, V.; Schmitt, M.; Gotz, M.; Dohner, K.; Wiesneth, M.; Dohner, H.; Hofmann, S. Immune responses against the mutated region of cytoplasmatic NPM1 might contribute to the favorable clinical outcome of AML patients with NPM1 mutations (NPM1mut). Blood 2013, 122, 1087–1088. [Google Scholar] [CrossRef] [PubMed]
- Snyder, A.; Makarov, V.; Merghoub, T.; Yuan, J.; Zaretsky, J.M.; Desrichard, A.; Walsh, L.A.; Postow, M.A.; Wong, P.; Ho, T.S.; et al. Genetic basis for clinical response to CTLA-4 blockade in melanoma. N. Engl. J. Med. 2014, 371, 2189–2199. [Google Scholar] [CrossRef]
- McGranahan, N.; Furness, A.J.; Rosenthal, R.; Ramskov, S.; Lyngaa, R.; Saini, S.K.; Jamal-Hanjani, M.; Wilson, G.A.; Birkbak, N.J.; Hiley, C.T.; et al. Clonal neoantigens elicit T cell immunoreactivity and sensitivity to immune checkpoint blockade. Science 2016, 351, 1463–1469. [Google Scholar] [CrossRef] [PubMed]
- Moore, T.; Wagner, C.R.; Scurti, G.M.; Hutchens, K.A.; Godellas, C.; Clark, A.L.; Kolawole, E.M.; Hellman, L.M.; Singh, N.K.; Huyke, F.A.; et al. Clinical and immunologic evaluation of three metastatic melanoma patients treated with autologous melanoma-reactive TCR-transduced T cells. Cancer Immunol. Immunother. CII 2018, 67, 311–325. [Google Scholar] [CrossRef]
- Rambaldi, A.; Biagi, E.; Bonini, C.; Biondi, A.; Introna, M. Cell-based strategies to manage leukemia relapse: Efficacy and feasibility of immunotherapy approaches. Leukemia 2015, 29, 1–10. [Google Scholar] [CrossRef]
- Collins, R.H., Jr.; Shpilberg, O.; Drobyski, W.R.; Porter, D.L.; Giralt, S.; Champlin, R.; Goodman, S.A.; Wolff, S.N.; Hu, W.; Verfaillie, C.; et al. Donor leukocyte infusions in 140 patients with relapsed malignancy after allogeneic bone marrow transplantation. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 1997, 15, 433–444. [Google Scholar] [CrossRef]
- Posthuma, E.F.M.; Marijt, E.W.A.F.; Barge, R.M.Y.; van Soest, R.A.; Baas, I.O.; Starrenburg, C.W.J.I.; van Zelderen-Bhola, S.L.; Fibbe, W.E.; Smit, W.M.; Willemze, R.; et al. α-Interferon with very-low-dose donor lymphocyte infusion for hematologic or cytogenetic relapse of chronic myeloid leukemia induces rapid and durable complete remissions and is associated with acceptable graft-versus-host disease. Biol. Blood Marrow Transplant. 2004, 10, 204–212. [Google Scholar] [CrossRef]
- Verdonck, L.F.; Petersen, E.J.; Lokhorst, H.M.; Nieuwenhuis, H.K.; Dekker, A.W.; Tilanus, M.G.; de Weger, R.A. Donor leukocyte infusions for recurrent hematologic malignancies after allogeneic bone marrow transplantation: Impact of infused and residual donor T cells. Bone Marrow Transplant. 1998, 22, 1057–1063. [Google Scholar] [CrossRef][Green Version]
- Bao, H.; Wu, D. Current Status of Leukemia Cytotherapy-Exploitation with Immune Cells. Curr. Stem Cell Res. Ther. 2017, 12, 188–196. [Google Scholar] [CrossRef]
- Kongtim, P.; Lee, D.A.; Cooper, L.J.; Kebriaei, P.; Champlin, R.E.; Ciurea, S.O. Haploidentical Hematopoietic Stem Cell Transplantation as a Platform for Post-Transplantation Cellular Therapy. Biol. Blood Marrow Transplant. J. Am. Soc. Blood Marrow Transplant. 2015, 21, 1714–1720. [Google Scholar] [CrossRef] [PubMed]
- Bleakley, M.; Heimfeld, S.; Loeb, K.R.; Jones, L.A.; Chaney, C.; Seropian, S.; Gooley, T.A.; Sommermeyer, F.; Riddell, S.R.; Shlomchik, W.D. Outcomes of acute leukemia patients transplanted with naive T cell-depleted stem cell grafts. J. Clin. Investig. 2015, 125, 2677–2689. [Google Scholar] [CrossRef] [PubMed]
- De Lima, M.; Porter, D.L.; Battiwalla, M.; Bishop, M.R.; Giralt, S.A.; Hardy, N.M.; Kroger, N.; Wayne, A.S.; Schmid, C. Proceedings from the National Cancer Institute’s Second International Workshop on the Biology, Prevention, and Treatment of Relapse After Hematopoietic Stem Cell Transplantation: Part III. Prevention and treatment of relapse after allogeneic transplantation. Biol. Blood Marrow Transplant. J. Am. Soc. Blood Marrow Transplant. 2014, 20, 4–13. [Google Scholar] [CrossRef]
- Kolb, H.J. Hematopoietic stem cell transplantation and cellular therapy. HLA 2017, 89, 267–277. [Google Scholar] [CrossRef]
- Cooper, N.; Rao, K.; Goulden, N.; Amrolia, P.; Veys, P. Alpha interferon augments the graft-versus-leukaemia effect of second stem cell transplants and donor lymphocyte infusions in high-risk paediatric leukaemias. Br. J. Haematol. 2012, 156, 550–552. [Google Scholar] [CrossRef] [PubMed]
- Schmid, C.; Labopin, M.; Nagler, A.; Bornhauser, M.; Finke, J.; Fassas, A.; Volin, L.; Gurman, G.; Maertens, J.; Bordigoni, P.; et al. Donor lymphocyte infusion in the treatment of first hematological relapse after allogeneic stem-cell transplantation in adults with acute myeloid leukemia: A retrospective risk factors analysis and comparison with other strategies by the EBMT Acute Leukemia Working Party. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2007, 25, 4938–4945. [Google Scholar] [CrossRef]
- Schmid, C.; Labopin, M.; Nagler, A.; Niederwieser, D.; Castagna, L.; Tabrizi, R.; Stadler, M.; Kuball, J.; Cornelissen, J.; Vorlicek, J.; et al. Treatment, risk factors, and outcome of adults with relapsed AML after reduced intensity conditioning for allogeneic stem cell transplantation. Blood 2012, 119, 1599–1606. [Google Scholar] [CrossRef]
- Zeiser, R.; Vago, L. Mechanisms of immune escape after allogeneic hematopoietic cell transplantation. Blood 2019, 133, 1290–1297. [Google Scholar] [CrossRef]
- Rautenberg, C.; Nachtkamp, K.; Dienst, A.; Schmidt, P.V.; Heyn, C.; Kondakci, M.; Germing, U.; Haas, R.; Kobbe, G.; Schroeder, T. Sorafenib and azacitidine as salvage therapy for relapse of FLT3-ITD mutated AML after allo-SCT. Eur. J. Haematol. 2017, 98, 348–354. [Google Scholar] [CrossRef]
- De Freitas, T.; Marktel, S.; Piemontese, S.; Carrabba, M.G.; Tresoldi, C.; Messina, C.; Lupo Stanghellini, M.T.; Assanelli, A.; Corti, C.; Bernardi, M.; et al. High rate of hematological responses to sorafenib in FLT3-ITD acute myeloid leukemia relapsed after allogeneic hematopoietic stem cell transplantation. Eur. J. Haematol. 2016, 96, 629–636. [Google Scholar] [CrossRef]
- Sommer, S.; Cruijsen, M.; Claus, R.; Bertz, H.; Wasch, R.; Marks, R.; Zeiser, R.; Bogatyreva, L.; Blijlevens, N.M.A.; May, A.; et al. Decitabine in combination with donor lymphocyte infusions can induce remissions in relapsed myeloid malignancies with higher leukemic burden after allogeneic hematopoietic cell transplantation. Leuk. Res. 2018, 72, 20–26. [Google Scholar] [CrossRef] [PubMed]
- Schroeder, T.; Rautenberg, C.; Kruger, W.; Platzbecker, U.; Bug, G.; Steinmann, J.; Klein, S.; Hopfer, O.; Nachtkamp, K.; Kondakci, M.; et al. Treatment of relapsed AML and MDS after allogeneic stem cell transplantation with decitabine and DLI-a retrospective multicenter analysis on behalf of the German Cooperative Transplant Study Group. Ann. Hematol. 2018, 97, 335–342. [Google Scholar] [CrossRef] [PubMed]
- Ghobadi, A.; Choi, J.; Fiala, M.A.; Fletcher, T.; Liu, J.; Eissenberg, L.G.; Abboud, C.; Cashen, A.; Vij, R.; Schroeder, M.A.; et al. Phase I study of azacitidine following donor lymphocyte infusion for relapsed acute myeloid leukemia post allogeneic stem cell transplantation. Leuk. Res. 2016, 49, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Steinmann, J.; Bertz, H.; Wasch, R.; Marks, R.; Zeiser, R.; Bogatyreva, L.; Finke, J.; Lubbert, M. 5-Azacytidine and DLI can induce long-term remissions in AML patients relapsed after allograft. Bone Marrow Transplant. 2015, 50, 690–695. [Google Scholar] [CrossRef] [PubMed]
- Schroeder, T.; Rachlis, E.; Bug, G.; Stelljes, M.; Klein, S.; Steckel, N.K.; Wolf, D.; Ringhoffer, M.; Czibere, A.; Nachtkamp, K.; et al. Treatment of acute myeloid leukemia or myelodysplastic syndrome relapse after allogeneic stem cell transplantation with azacitidine and donor lymphocyte infusions–A retrospective multicenter analysis from the German Cooperative Transplant Study Group. Biol. Blood Marrow Transplant. J. Am. Soc. Blood Marrow Transplant. 2015, 21, 653–660. [Google Scholar] [CrossRef] [PubMed]
- Tessoulin, B.; Delaunay, J.; Chevallier, P.; Loirat, M.; Ayari, S.; Peterlin, P.; Le Gouill, S.; Gastinne, T.; Moreau, P.; Mohty, M.; et al. Azacitidine salvage therapy for relapse of myeloid malignancies following allogeneic hematopoietic SCT. Bone Marrow Transplant. 2014, 49, 567–571. [Google Scholar] [CrossRef]
- Motabi, I.H.; Ghobadi, A.; Liu, J.; Schroeder, M.; Abboud, C.N.; Cashen, A.F.; Stockler-Goldstein, K.E.; Uy, G.L.; Vij, R.; Westervelt, P.; et al. Chemotherapy versus Hypomethylating Agents for the Treatment of Relapsed Acute Myeloid Leukemia and Myelodysplastic Syndrome after Allogeneic Stem Cell Transplant. Biol. Blood Marrow Transplant. J. Am. Soc. Blood Marrow Transplant. 2016, 22, 1324–1329. [Google Scholar] [CrossRef]
- Levine, J.E.; Braun, T.; Penza, S.L.; Beatty, P.; Cornetta, K.; Martino, R.; Drobyski, W.R.; Barrett, A.J.; Porter, D.L.; Giralt, S.; et al. Prospective trial of chemotherapy and donor leukocyte infusions for relapse of advanced myeloid malignancies after allogeneic stem-cell transplantation. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2002, 20, 405–412. [Google Scholar] [CrossRef]
- Guieze, R.; Damaj, G.; Pereira, B.; Robin, M.; Chevallier, P.; Michallet, M.; Vigouroux, S.; Beguin, Y.; Blaise, D.; El Cheikh, J.; et al. Management of Myelodysplastic Syndrome Relapsing after Allogeneic Hematopoietic Stem Cell Transplantation: A Study by the French Society of Bone Marrow Transplantation and Cell Therapies. Biol. Blood Marrow Transplant. J. Am. Soc. Blood Marrow Transplant. 2016, 22, 240–247. [Google Scholar] [CrossRef]
- Cornelissen, J.J.; Blaise, D. Hematopoietic stem cell transplantation for patients with AML in first complete remission. Blood 2016, 127, 62–70. [Google Scholar] [CrossRef]
- Dietz, A.C.; Wayne, A.S. Cells to prevent/treat relapse following allogeneic stem cell transplantation. Hematol. Educ. Program Am. Soc. Hematol. Am. Soc. Hematol. Educ. Program 2017, 2017, 708–715. [Google Scholar] [CrossRef]
- Sun, C.; Dotti, G.; Savoldo, B. Utilizing cell-based therapeutics to overcome immune evasion in hematologic malignancies. Blood 2016, 127, 3350–3359. [Google Scholar] [CrossRef] [PubMed]
- Orr, M.T.; Lanier, L.L. Natural killer cell education and tolerance. Cell 2010, 142, 847–856. [Google Scholar] [CrossRef] [PubMed]
- Handgretinger, R.; Schilbach, K. The potential role of gammadelta T cells after allogeneic HCT for leukemia. Blood 2018, 131, 1063–1072. [Google Scholar] [CrossRef] [PubMed]
- Deol, A.; Lum, L.G. Role of donor lymphocyte infusions in relapsed hematological malignancies after stem cell transplantation revisited. Cancer Treat. Rev. 2010, 36, 528–538. [Google Scholar] [CrossRef] [PubMed]
- Tsirigotis, P.; Byrne, M.; Schmid, C.; Baron, F.; Ciceri, F.; Esteve, J.; Gorin, N.C.; Giebel, S.; Mohty, M.; Savani, B.N.; et al. Relapse of AML after hematopoietic stem cell transplantation: Methods of monitoring and preventive strategies. A review from the ALWP of the EBMT. Bone Marrow Transplant. 2016, 51, 1431–1438. [Google Scholar] [CrossRef] [PubMed]
- Schneidawind, C.; Jahnke, S.; Schober-Melms, I.; Schumm, M.; Handgretinger, R.; Faul, C.; Kanz, L.; Bethge, W.; Schneidawind, D. G-CSF administration prior to donor lymphocyte apheresis promotes anti-leukaemic effects in allogeneic HCT patients. Br. J. Haematol. 2019, 186, 60–71. [Google Scholar] [CrossRef]
- Zhao, X.S.; Wang, Y.; Yan, C.H.; Wang, J.Z.; Zhang, X.H.; Xu, L.P.; Liu, K.Y.; Huang, X.J. The cell composition of infused donor lymphocyte has different impact in different types of allogeneic hematopoietic stem cell transplantation. Clin. Transplant. 2014, 28, 926–934. [Google Scholar] [CrossRef]
- Vago, L.; Perna, S.K.; Zanussi, M.; Mazzi, B.; Barlassina, C.; Stanghellini, M.T.; Perrelli, N.F.; Cosentino, C.; Torri, F.; Angius, A.; et al. Loss of mismatched HLA in leukemia after stem-cell transplantation. N. Engl. J. Med. 2009, 361, 478–488. [Google Scholar] [CrossRef]
- Goldsmith, S.R.; Slade, M.; DiPersio, J.F.; Westervelt, P.; Schroeder, M.A.; Gao, F.; Romee, R. Donor-lymphocyte infusion following haploidentical hematopoietic cell transplantation with peripheral blood stem cell grafts and PTCy. Bone Marrow Transplant. 2017, 52, 1623–1628. [Google Scholar] [CrossRef]
- Miyamoto, T.; Fukuda, T.; Nakashima, M.; Henzan, T.; Kusakabe, S.; Kobayashi, N.; Sugita, J.; Mori, T.; Kurokawa, M.; Mori, S.I. Donor Lymphocyte Infusion for Relapsed Hematological Malignancies after Unrelated Allogeneic Bone Marrow Transplantation Facilitated by the Japan Marrow Donor Program. Biol. Blood Marrow Transplant. J. Am. Soc. Blood Marrow Transplant. 2017, 23, 938–944. [Google Scholar] [CrossRef] [PubMed]
- Mo, X.D.; Zhang, X.H.; Xu, L.P.; Wang, Y.; Yan, C.H.; Chen, H.; Chen, Y.H.; Han, W.; Wang, F.R.; Wang, J.Z.; et al. Comparison of outcomes after donor lymphocyte infusion with or without prior chemotherapy for minimal residual disease in acute leukemia/myelodysplastic syndrome after allogeneic hematopoietic stem cell transplantation. Ann. Hematol. 2017, 96, 829–838. [Google Scholar] [CrossRef] [PubMed]
- Schmid, C.; Labopin, M.; Schaap, N.; Veelken, H.; Schleuning, M.; Stadler, M.; Finke, J.; Hurst, E.; Baron, F.; Ringden, O.; et al. Prophylactic donor lymphocyte infusion after allogeneic stem cell transplantation in acute leukaemia-a matched pair analysis by the Acute Leukaemia Working Party of EBMT. Br. J. Haematol. 2019, 184, 782–787. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.N.; Lin, J.; Wang, S.H.; Huang, W.R.; Li, F.; Li, H.H.; Chen, J.; Wang, L.J.; Gao, C.J.; Yu, L.; et al. Donor lymphocyte infusion for prevention of relapse after unmanipulated haploidentical PBSCT for very high-risk hematologic malignancies. Ann. Hematol. 2019, 98, 185–193. [Google Scholar] [CrossRef] [PubMed]
- Cauchois, R.; Castagna, L.; Pagliardini, T.; Harbi, S.; Calmels, B.; Bramanti, S.; Granata, A.; Lemarie, C.; Maisano, V.; Legrand, F.; et al. Prophylactic donor lymphocyte infusions after haploidentical haematopoietic stem cell transplantation for high risk haematological malignancies: A retrospective bicentric analysis of serial infusions of increasing doses of CD3(+) cells. Br. J. Haematol. 2018. [Google Scholar] [CrossRef] [PubMed]
- Eefting, M.; Halkes, C.J.; de Wreede, L.C.; van Pelt, C.M.; Kersting, S.; Marijt, E.W.; von dem Borne, P.A.; Willemze, R.; Veelken, H.; Falkenburg, J.H. Myeloablative T cell-depleted alloSCT with early sequential prophylactic donor lymphocyte infusion is an efficient and safe post-remission treatment for adult ALL. Bone Marrow Transplant. 2014, 49, 287–291. [Google Scholar] [CrossRef]
- Ljungman, P.; Brand, R.; Einsele, H.; Frassoni, F.; Niederwieser, D.; Cordonnier, C. Donor CMV serologic status and outcome of CMV-seropositive recipients after unrelated donor stem cell transplantation: An EBMT megafile analysis. Blood 2003, 102, 4255–4260. [Google Scholar] [CrossRef]
- Xuan, L.; Fan, Z.; Zhang, Y.; Zhou, H.; Huang, F.; Dai, M.; Nie, D.; Lin, D.; Xu, N.; Guo, X.; et al. Sequential intensified conditioning followed by prophylactic DLI could reduce relapse of refractory acute leukemia after allo-HSCT. Oncotarget 2016, 7, 32579–32591. [Google Scholar] [CrossRef]
- Michallet, M.; Sobh, M.; Detrait, M.Y.; Labussiere-Wallet, H.; Hayette, S.; Tigaud, I.; Elhamri, M.; Gilis, L.; Lebras, L.L.; Barraco, F.; et al. Flamsa Sequential Chemotherapy Followed By Reduced Intensity Conditioning and Allogeneic Hematopoietic Transplantation for High Risk Acute Myeloid Leukemia Patients. Blood 2014, 124, 3892. [Google Scholar] [CrossRef]
- Yan, C.H.; Liu, D.H.; Liu, K.Y.; Xu, L.P.; Liu, Y.R.; Chen, H.; Han, W.; Wang, Y.; Qin, Y.Z.; Huang, X.J. Risk stratification-directed donor lymphocyte infusion could reduce relapse of standard-risk acute leukemia patients after allogeneic hematopoietic stem cell transplantation. Blood 2012, 119, 3256–3262. [Google Scholar] [CrossRef]
- Chiappinelli, K.B.; Strissel, P.L.; Desrichard, A.; Li, H.; Henke, C.; Akman, B.; Hein, A.; Rote, N.S.; Cope, L.M.; Snyder, A.; et al. Inhibiting DNA Methylation Causes an Interferon Response in Cancer via dsRNA Including Endogenous Retroviruses. Cell 2015, 162, 974–986. [Google Scholar] [CrossRef] [PubMed]
- Platzbecker, U.; Wermke, M.; Radke, J.; Oelschlaegel, U.; Seltmann, F.; Kiani, A.; Klut, I.M.; Knoth, H.; Rollig, C.; Schetelig, J.; et al. Azacitidine for treatment of imminent relapse in MDS or AML patients after allogeneic HSCT: Results of the RELAZA trial. Leukemia 2012, 26, 381–389. [Google Scholar] [CrossRef] [PubMed]
- Platzbecker, U.; Schetelig, J.; Finke, J.; Trenschel, R.; Scott, B.L.; Kobbe, G.; Schaefer-Eckart, K.; Bornhauser, M.; Itzykson, R.; Germing, U.; et al. Allogeneic hematopoietic cell transplantation in patients age 60–70 years with de novo high-risk myelodysplastic syndrome or secondary acute myelogenous leukemia: Comparison with patients lacking donors who received azacitidine. Biol. Blood Marrow Transplant. J. Am. Soc. Blood Marrow Transplant. 2012, 18, 1415–1421. [Google Scholar] [CrossRef]
- Mo, X.D.; Zhang, X.H.; Xu, L.P.; Wang, Y.; Yan, C.H.; Chen, H.; Chen, Y.H.; Han, W.; Wang, F.R.; Wang, J.Z.; et al. Salvage chemotherapy followed by granulocyte colony-stimulating factor-primed donor leukocyte infusion with graft-vs.-host disease control for minimal residual disease in acute leukemia/myelodysplastic syndrome after allogeneic hematopoietic stem cell transplantation: Prognostic factors and clinical outcomes. Eur. J. Haematol. 2016, 96, 297–308. [Google Scholar] [CrossRef] [PubMed]
- Horowitz, M.M.; Gale, R.P.; Sondel, P.M.; Goldman, J.M.; Kersey, J.; Kolb, H.J.; Rimm, A.A.; Ringden, O.; Rozman, C.; Speck, B.; et al. Graft-versus-leukemia reactions after bone marrow transplantation. Blood 1990, 75, 555–562. [Google Scholar] [CrossRef] [PubMed]
- Malard, F.; Labopin, M.; Stuhler, G.; Bittenbring, J.; Ganser, A.; Tischer, J.; Michallet, M.; Kroger, N.; Schmid, C.; Huynh, A.; et al. Sequential Intensified Conditioning Regimen Allogeneic Hematopoietic Stem Cell Transplantation in Adult Patients with Intermediate-or High-Risk Acute Myeloid Leukemia in Complete Remission: A Study from the Acute Leukemia Working Party of the European Group for Blood and Marrow Transplantation. Biol. Blood Marrow Transplant. J. Am. Soc. Blood Marrow Transplant. 2017, 23, 278–284. [Google Scholar] [CrossRef]
- Maury, S.; Lemoine, F.M.; Hicheri, Y.; Rosenzwajg, M.; Badoual, C.; Cherai, M.; Beaumont, J.L.; Azar, N.; Dhedin, N.; Sirvent, A.; et al. CD4+CD25+ regulatory T cell depletion improves the graft-versus-tumor effect of donor lymphocytes after allogeneic hematopoietic stem cell transplantation. Sci. Transl. Med. 2010, 2, 41ra52. [Google Scholar] [CrossRef] [PubMed]
- Peggs, K.S.; Thomson, K.; Hart, D.P.; Geary, J.; Morris, E.C.; Yong, K.; Goldstone, A.H.; Linch, D.C.; Mackinnon, S. Dose-escalated donor lymphocyte infusions following reduced intensity transplantation: Toxicity, chimerism, and disease responses. Blood 2004, 103, 1548–1556. [Google Scholar] [CrossRef]
- Lemieux, J.; Jobin, C.; Simard, C.; Neron, S. A global look into human T cell subsets before and after cryopreservation using multiparametric flow cytometry and two-dimensional visualization analysis. J. Immunol. Methods 2016, 434, 73–82. [Google Scholar] [CrossRef]
- Groger, M.; Gagelmann, N.; Wolschke, C.; von Pein, U.M.; Klyuchnikov, E.; Christopeit, M.; Zander, A.; Ayuk, F.; Kroger, N. Long-Term Results of Prophylactic Donor Lymphocyte Infusions for Patients with Multiple Myeloma after Allogeneic Stem Cell Transplantation. Biol. Blood Marrow Transplant. J. Am. Soc. Blood Marrow Transplant. 2018, 24, 1399–1405. [Google Scholar] [CrossRef]
- Falkenburg, J.H.; Wafelman, A.R.; Joosten, P.; Smit, W.M.; van Bergen, C.A.; Bongaerts, R.; Lurvink, E.; van der Hoorn, M.; Kluck, P.; Landegent, J.E.; et al. Complete remission of accelerated phase chronic myeloid leukemia by treatment with leukemia-reactive cytotoxic T lymphocytes. Blood 1999, 94, 1201–1208. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Schmitt, A.; Chen, B.; Xu, X.; Mani, J.; Linnebacher, M.; Freund, M.; Schmitt, M. Streptamer-based selection of WT1-specific CD8+ T cells for specific donor lymphocyte infusions. Exp. Hematol. 2010, 38, 1066–1073. [Google Scholar] [CrossRef] [PubMed]
- Bae, J.; Samur, M.; Richardson, P.; Munshi, N.C.; Anderson, K.C. Selective targeting of multiple myeloma by B cell maturation antigen (BCMA)-specific central memory CD8(+) cytotoxic T lymphocytes: Immunotherapeutic application in vaccination and adoptive immunotherapy. Leukemia 2019. [Google Scholar] [CrossRef] [PubMed]
- Tawara, I.; Kageyama, S.; Miyahara, Y.; Fujiwara, H.; Nishida, T.; Akatsuka, Y.; Ikeda, H.; Tanimoto, K.; Terakura, S.; Murata, M.; et al. Safety and persistence of WT1-specific T-cell receptor gene-transduced lymphocytes in patients with AML and MDS. Blood 2017, 130, 1985–1994. [Google Scholar] [CrossRef]
- Boeckh, M.; Murphy, W.J.; Peggs, K.S. Recent advances in cytomegalovirus: An update on pharmacologic and cellular therapies. Biol. Blood Marrow Transplant. J. Am. Soc. Blood Marrow Transplant. 2015, 21, 24–29. [Google Scholar] [CrossRef]
- Ljungman, P.; Boeckh, M.; Hirsch, H.H.; Josephson, F.; Lundgren, J.; Nichols, G.; Pikis, A.; Razonable, R.R.; Miller, V.; Griffiths, P.D.; et al. Definitions of Cytomegalovirus Infection and Disease in Transplant Patients for Use in Clinical Trials. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2017, 64, 87–91. [Google Scholar] [CrossRef]
- Quinnan, G.V., Jr.; Kirmani, N.; Rook, A.H.; Manischewitz, J.F.; Jackson, L.; Moreschi, G.; Santos, G.W.; Saral, R.; Burns, W.H. Cytotoxic t cells in cytomegalovirus infection: HLA-restricted T-lymphocyte and non-T-lymphocyte cytotoxic responses correlate with recovery from cytomegalovirus infection in bone-marrow-transplant recipients. N. Engl. J. Med. 1982, 307, 7–13. [Google Scholar] [CrossRef]
- Reusser, P.; Riddell, S.R.; Meyers, J.D.; Greenberg, P.D. Cytotoxic T-lymphocyte response to cytomegalovirus after human allogeneic bone marrow transplantation: Pattern of recovery and correlation with cytomegalovirus infection and disease. Blood 1991, 78, 1373–1380. [Google Scholar] [CrossRef]
- Gillespie, G.M.; Wills, M.R.; Appay, V.; O’Callaghan, C.; Murphy, M.; Smith, N.; Sissons, P.; Rowland-Jones, S.; Bell, J.I.; Moss, P.A. Functional heterogeneity and high frequencies of cytomegalovirus-specific CD8(+) T lymphocytes in healthy seropositive donors. J. Virol. 2000, 74, 8140–8150. [Google Scholar] [CrossRef]
- Ma, C.K.K.; Clancy, L.; Simms, R.; Burgess, J.; Deo, S.; Blyth, E.; Micklethwaite, K.P.; Gottlieb, D.J. Adjuvant Peptide Pulsed Dendritic Cell Vaccination in Addition to T Cell Adoptive Immunotherapy for Cytomegalovirus Infection in Allogeneic Hematopoietic Stem Cell Transplantation Recipients. Biol. Blood Marrow Transplant. J. Am. Soc. Blood Marrow Transplant. 2018, 24, 71–77. [Google Scholar] [CrossRef]
- Meyers, J.D.; Flournoy, N.; Thomas, E.D. Risk factors for cytomegalovirus infection after human marrow transplantation. J. Infect. Dis. 1986, 153, 478–488. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, A.; Tonn, T.; Busch, D.H.; Grigoleit, G.U.; Einsele, H.; Odendahl, M.; Germeroth, L.; Ringhoffer, M.; Ringhoffer, S.; Wiesneth, M.; et al. Adoptive transfer and selective reconstitution of streptamer-selected cytomegalovirus-specific CD8+ T cells leads to virus clearance in patients after allogeneic peripheral blood stem cell transplantation. Transfusion 2011, 51, 591–599. [Google Scholar] [CrossRef] [PubMed]
- Papadopoulos, E.B.; Ladanyi, M.; Emanuel, D.; Mackinnon, S.; Boulad, F.; Carabasi, M.H.; Castro-Malaspina, H.; Childs, B.H.; Gillio, A.P.; Small, T.N.; et al. Infusions of donor leukocytes to treat Epstein-Barr virus-associated lymphoproliferative disorders after allogeneic bone marrow transplantation. N. Engl. J. Med. 1994, 330, 1185–1191. [Google Scholar] [CrossRef] [PubMed]
- Einsele, H.; Roosnek, E.; Rufer, N.; Sinzger, C.; Riegler, S.; Loffler, J.; Grigoleit, U.; Moris, A.; Rammensee, H.G.; Kanz, L.; et al. Infusion of cytomegalovirus (CMV)-specific T cells for the treatment of CMV infection not responding to antiviral chemotherapy. Blood 2002, 99, 3916–3922. [Google Scholar] [CrossRef]
- Walter, E.A.; Greenberg, P.D.; Gilbert, M.J.; Finch, R.J.; Watanabe, K.S.; Thomas, E.D.; Riddell, S.R. Reconstitution of cellular immunity against cytomegalovirus in recipients of allogeneic bone marrow by transfer of T-cell clones from the donor. N. Engl. J. Med. 1995, 333, 1038–1044. [Google Scholar] [CrossRef]
- Moosmann, A.; Bigalke, I.; Tischer, J.; Schirrmann, L.; Kasten, J.; Tippmer, S.; Leeping, M.; Prevalsek, D.; Jaeger, G.; Ledderose, G.; et al. Effective and long-term control of EBV PTLD after transfer of peptide-selected T cells. Blood 2010, 115, 2960–2970. [Google Scholar] [CrossRef]
- Styczynski, J.; Einsele, H.; Gil, L.; Ljungman, P. Outcome of treatment of Epstein-Barr virus-related post-transplant lymphoproliferative disorder in hematopoietic stem cell recipients: A comprehensive review of reported cases. Transpl. Infect. Dis. Off. J. Transplant. Soc. 2009, 11, 383–392. [Google Scholar] [CrossRef]
- Tzannou, I.; Papadopoulou, A.; Naik, S.; Leung, K.; Martinez, C.A.; Ramos, C.A.; Carrum, G.; Sasa, G.; Lulla, P.; Watanabe, A.; et al. Off-the-Shelf Virus-Specific T Cells to Treat BK Virus, Human Herpesvirus 6, Cytomegalovirus, Epstein-Barr Virus, and Adenovirus Infections After Allogeneic Hematopoietic Stem-Cell Transplantation. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2017, 35, 3547–3557. [Google Scholar] [CrossRef]
- Muranski, P.; Davies, S.I.; Ito, S.; Koklanaris, E.; Superata, J.; Yu, Q.; Highfill, S.L.; Sabatino, M.; Stroncek, D.F.; Battiwalla, M.; et al. Very Early Adoptive Transfer of Ex Vivo Generated Multi-Virus Specific T Cells Is a Safe Strategy for Prevention of Viral Infection after Allogeneic T Cell Depleted Stem Cell Transplantation. Blood 2018, 132, 812. [Google Scholar] [CrossRef]
- Kanate, A.S.; Craig, M.; Cumpston, A.; Saad, A.; Hobbs, G.; Leadmon, S.; Bunner, P.; Watkins, K.; Bulian, D.; Gibson, L.; et al. Higher infused CD34+ cell dose and overall survival in patients undergoing in vivo T-cell depleted, but not t-cell repleted, allogeneic peripheral blood hematopoietic cell transplantation. Hematol. Oncol. Stem Cell Ther. 2011, 4, 149–156. [Google Scholar] [CrossRef]
- Chakrabarti, S.; MacDonald, D.; Hale, G.; Holder, K.; Turner, V.; Czarnecka, H.; Thompson, J.; Fegan, C.; Waldmann, H.; Milligan, D.W. T-cell depletion with Campath-1H “in the bag” for matched related allogeneic peripheral blood stem cell transplantation is associated with reduced graft-versus-host disease, rapid immune constitution and improved survival. Br. J. Haematol. 2003, 121, 109–118. [Google Scholar] [CrossRef] [PubMed]
- Malard, F.; Labopin, M.; Cho, C.; Blaise, D.; Papadopoulos, E.B.; Passweg, J.; O’Reilly, R.; Forcade, E.; Maloy, M.; Volin, L.; et al. Ex Vivo and In Vivo T cell-depleted allogeneic stem cell transplantation in patients with acute myeloid leukemia in first complete remission resulted in similar overall survival: On behalf of the ALWP of the EBMT and the MSKCC. J. Hematol. Oncol. 2018, 11, 127. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.J.; Savani, B.N.; Mohty, M.; Labopin, M.; Ruggeri, A.; Schmid, C.; Baron, F.; Esteve, J.; Gorin, N.C.; Giebel, S.; et al. Haploidentical hematopoietic cell transplantation for adult acute myeloid leukemia: A position statement from the Acute Leukemia Working Party of the European Society for Blood and Marrow Transplantation. Haematologica 2017, 102, 1810–1822. [Google Scholar] [CrossRef] [PubMed]
- Russo, A.; Oliveira, G.; Berglund, S.; Greco, R.; Gambacorta, V.; Cieri, N.; Toffalori, C.; Zito, L.; Lorentino, F.; Piemontese, S.; et al. NK cell recovery after haploidentical HSCT with posttransplant cyclophosphamide: Dynamics and clinical implications. Blood 2018, 131, 247–262. [Google Scholar] [CrossRef]
- Chapuis, A.G.; Ragnarsson, G.B.; Nguyen, H.N.; Chaney, C.N.; Pufnock, J.S.; Schmitt, T.M.; Duerkopp, N.; Roberts, I.M.; Pogosov, G.L.; Ho, W.Y.; et al. Transferred WT1-reactive CD8+ T cells can mediate antileukemic activity and persist in post-transplant patients. Sci. Transl. Med. 2013, 5, 174ra127. [Google Scholar] [CrossRef]
- Van Heijst, J.W.; Ceberio, I.; Lipuma, L.B.; Samilo, D.W.; Wasilewski, G.D.; Gonzales, A.M.; Nieves, J.L.; van den Brink, M.R.; Perales, M.A.; Pamer, E.G. Quantitative assessment of T cell repertoire recovery after hematopoietic stem cell transplantation. Nat. Med. 2013, 19, 372–377. [Google Scholar] [CrossRef]
- Stenger, E.O.; Turnquist, H.R.; Mapara, M.Y.; Thomson, A.W. Dendritic cells and regulation of graft-versus-host disease and graft-versus-leukemia activity. Blood 2012, 119, 5088–5103. [Google Scholar] [CrossRef]
- Boelens, J.J.; Admiraal, R.; Kuball, J.; Nierkens, S. Fine-Tuning Antithymocyte Globulin Dosing and Harmonizing Clinical Trial Design. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2018, 36, 1175–1176. [Google Scholar] [CrossRef]
- Admiraal, R.; Nierkens, S.; de Witte, M.A.; Petersen, E.J.; Fleurke, G.J.; Verrest, L.; Belitser, S.V.; Bredius, R.G.M.; Raymakers, R.A.P.; Knibbe, C.A.J.; et al. Association between anti-thymocyte globulin exposure and survival outcomes in adult unrelated haemopoietic cell transplantation: A multicentre, retrospective, pharmacodynamic cohort analysis. Lancet Haematol. 2017, 4, e183–e191. [Google Scholar] [CrossRef]
- Pasquini, M.C.; Devine, S.; Mendizabal, A.; Baden, L.R.; Wingard, J.R.; Lazarus, H.M.; Appelbaum, F.R.; Keever-Taylor, C.A.; Horowitz, M.M.; Carter, S.; et al. Comparative outcomes of donor graft CD34+ selection and immune suppressive therapy as graft-versus-host disease prophylaxis for patients with acute myeloid leukemia in complete remission undergoing HLA-matched sibling allogeneic hematopoietic cell transplantation. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2012, 30, 3194–3201. [Google Scholar] [CrossRef]
- Mielcarek, M.; Furlong, T.; O’Donnell, P.V.; Storer, B.E.; McCune, J.S.; Storb, R.; Carpenter, P.A.; Flowers, M.E.; Appelbaum, F.R.; Martin, P.J. Posttransplantation cyclophosphamide for prevention of graft-versus-host disease after HLA-matched mobilized blood cell transplantation. Blood 2016, 127, 1502–1508. [Google Scholar] [CrossRef] [PubMed]
- Locatelli, F.; Merli, P.; Pagliara, D.; Li Pira, G.; Falco, M.; Pende, D.; Rondelli, R.; Lucarelli, B.; Brescia, L.P.; Masetti, R.; et al. Outcome of children with acute leukemia given HLA-haploidentical HSCT after alphabeta T-cell and B-cell depletion. Blood 2017, 130, 677–685. [Google Scholar] [CrossRef] [PubMed]
- Kanakry, C.G.; Coffey, D.G.; Towlerton, A.M.; Vulic, A.; Storer, B.E.; Chou, J.; Yeung, C.C.; Gocke, C.D.; Robins, H.S.; O’Donnell, P.V.; et al. Origin and evolution of the T cell repertoire after posttransplantation cyclophosphamide. JCI Insight 2016, 1. [Google Scholar] [CrossRef] [PubMed]
- Yew, P.Y.; Alachkar, H.; Yamaguchi, R.; Kiyotani, K.; Fang, H.; Yap, K.L.; Liu, H.T.; Wickrema, A.; Artz, A.; van Besien, K.; et al. Quantitative characterization of T-cell repertoire in allogeneic hematopoietic stem cell transplant recipients. Bone Marrow Transplant. 2015, 50, 1227–1234. [Google Scholar] [CrossRef]
- Van Bergen, C.A.; van Luxemburg-Heijs, S.A.; de Wreede, L.C.; Eefting, M.; von dem Borne, P.A.; van Balen, P.; Heemskerk, M.H.; Mulder, A.; Claas, F.H.; Navarrete, M.A.; et al. Selective graft-versus-leukemia depends on magnitude and diversity of the alloreactive T cell response. J. Clin. Investig. 2017. [Google Scholar] [CrossRef]
- Ravens, S.; Schultze-Florey, C.; Raha, S.; Sandrock, I.; Drenker, M.; Oberdorfer, L.; Reinhardt, A.; Ravens, I.; Beck, M.; Geffers, R.; et al. Publisher Correction: Human gammadelta T cells are quickly reconstituted after stem-cell transplantation and show adaptive clonal expansion in response to viral infection. Nat. Immunol. 2018, 19, 1037. [Google Scholar] [CrossRef]
| Situation | Patients, Study Type | Strategy | Response | Reference |
|---|---|---|---|---|
| prophylactic | retrospective, matched n = 46, high-risk AML/MDS | DLI, +120 d post allo-SCT | 7-yr OS, 67% vs 31% (p < 0.001) OS in the DLI group was significantly improved | Jedlickova et al. BMT, 2016 |
| retrospective, high-risk AML | DLI, post allo-SCT | OS in DLI group was improved 70% vs. 40% (p = 0.027) | Schmid et al. Br J Haematol, 2019 | |
| preemptive | n = 105, prospective standard-risk AML, ALL, MDS | 49 low-dose IL-2 56 DLI | 3-yr OS: DLI: 58%, IL-2: 28% | Yan et al. Blood, 2012 |
| n = 101, MDS/AML | preemptive chemotherapy in combination with DLI application | CIR, NRM and DFS 39.5%, 9.6%, and 51.7% | MO et al. Eur J Haematol, 2016 | |
| prospective phase II study n = 20, MDS/ AML | > 100 d post allo-SCT four azacytidine cycles (75 mg/m2/d for 7 days) | hematological relapse in 13 patients (65%) | Platzbecker et al. Leukemia, 2012 | |
| therapeutic | retrospective, 399 patients | 177 DLI, 228 no DLI | 2 yr OS 21% with DLI, 9% without DLI (p = 0.04) | Schmid et al. J Clin Oncol, 2007 |
| n = 263, retrospective | cytoreductive therapy, followed by DLI or second HSCT | CR was reinduced in 32%; 2 yr OS was 14% | Schmid et al. Blood, 2012 | |
| n = 57, prospective | cytarabine-based therapy and DLI | 2 yr OS 19% | Levine et al. J Clin Oncol, 2002 | |
| prospective phase I study AML | azacytidine post DLI | CR (6/8) | Ghobadi et al. Leuk Res, 2016 | |
| retrospective, MDS/AML | azacytidine/DLI | Overall response was 33% OS after 2 yrs 29% | Schroeder et al. BBMT, 2015 | |
| retrospective, AML | Sorafenib, in combination with hypomethylating agents and DLI | 38% CR | De Freitas et al. Eur J Haematol, 2016 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Greiner, J.; Götz, M.; Bunjes, D.; Hofmann, S.; Wais, V. Immunological and Clinical Impact of Manipulated and Unmanipulated DLI after Allogeneic Stem Cell Transplantation of AML Patients. J. Clin. Med. 2020, 9, 39. https://doi.org/10.3390/jcm9010039
Greiner J, Götz M, Bunjes D, Hofmann S, Wais V. Immunological and Clinical Impact of Manipulated and Unmanipulated DLI after Allogeneic Stem Cell Transplantation of AML Patients. Journal of Clinical Medicine. 2020; 9(1):39. https://doi.org/10.3390/jcm9010039
Chicago/Turabian StyleGreiner, Jochen, Marlies Götz, Donald Bunjes, Susanne Hofmann, and Verena Wais. 2020. "Immunological and Clinical Impact of Manipulated and Unmanipulated DLI after Allogeneic Stem Cell Transplantation of AML Patients" Journal of Clinical Medicine 9, no. 1: 39. https://doi.org/10.3390/jcm9010039
APA StyleGreiner, J., Götz, M., Bunjes, D., Hofmann, S., & Wais, V. (2020). Immunological and Clinical Impact of Manipulated and Unmanipulated DLI after Allogeneic Stem Cell Transplantation of AML Patients. Journal of Clinical Medicine, 9(1), 39. https://doi.org/10.3390/jcm9010039






