Incidence, Management, Prevention and Outcome of Post-Operative Atrial Fibrillation in Thoracic Surgical Oncology
Abstract
1. Introduction
2. Definitions
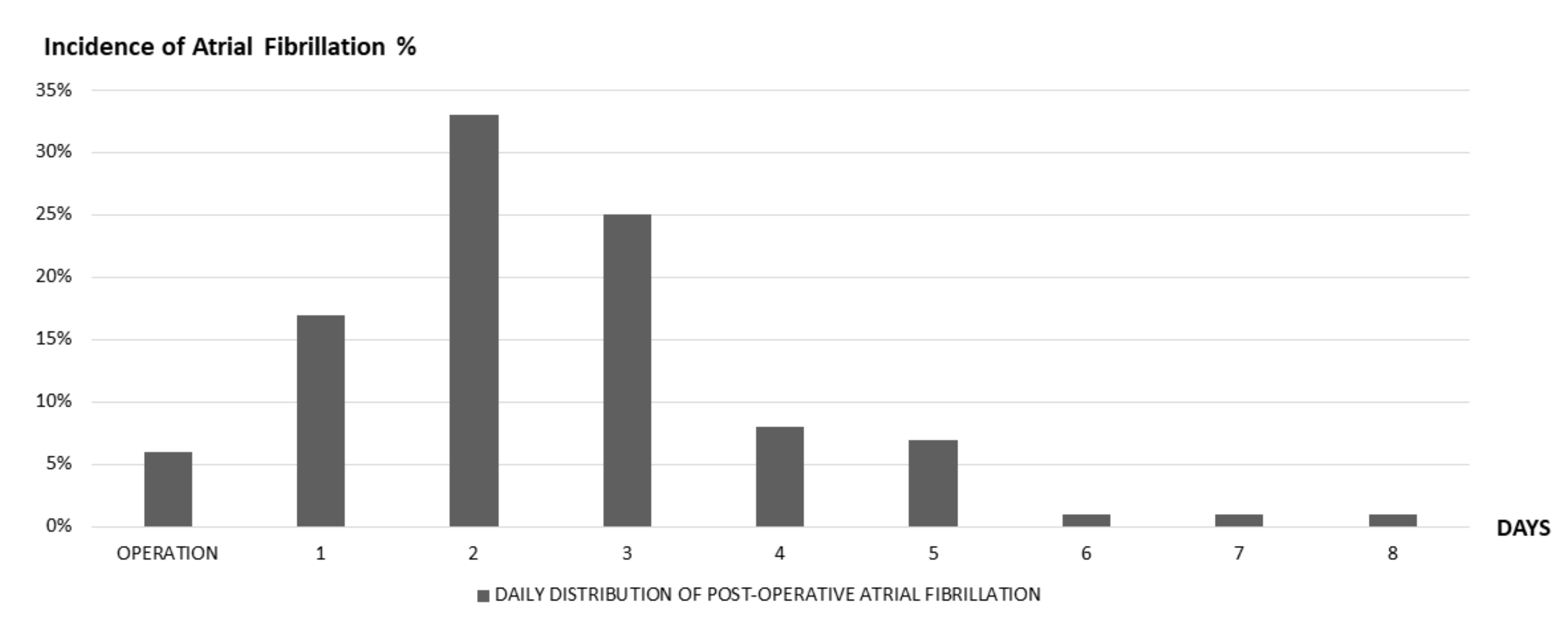
2.1. PAF in Thoracic Surgery Setting: Epidemiology
2.2. PAF and Thromboembolic Risk
2.3. Risk Factors, Triggers, and Pathophysiology of PAF
2.4. Implications of Chemotherapy
2.5. Prediction of PAF
2.6. The Potential Role of Natriuretic Peptides
3. Acute Treatment
3.1. Atrial Fibrillation Prophylaxis
3.2. Anti-Thrombotic Profilaxys
3.3. Long Term Implications of PAF
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| ACEI | angiotensin-converting enzyme inhibitor |
| AF | atrial fibrillation |
| ARB | angiotensin receptor blocker |
| BNP | brain natriuretic peptide |
| CCB | calcium channel blockers |
| COPD | chronic obstructive pulmonary disease |
| ECG | electrocardiogram |
| FEV1 | forced expiratory volume in the 1st second |
| HF | heart failure |
| LOE | level of evidence |
| NP | natriuretic peptide |
| Nt | N-terminal |
| PAF | post-operative atrial fibrillation |
| POISE | perioperative-ischemic-evaluation |
| PV | pulmonary veins |
| RCT | randomized controlled trials |
References
- Kirchhof, P.; Benussi, S.; Kotecha, D.; Ahlsson, A.; Atar, D.; Casadei, B.; Castella, M.; Diener, H.C.; Heidbuchel, H.; Hendriks, J.; et al. 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur. Heart J. 2016, 37, 2893–2962. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, C.; Providência, R.; Ferreira, M.J.; Gonçalves, L.M. Atrial Fibrillation and Non-cardiovascular Diseases: A Systematic Review. Arq. Bras. Cardiol. 2015, 105, 519–526. [Google Scholar] [CrossRef] [PubMed]
- Conen, D.; Wong, J.A.; Sandhu, R.K.; Cook, N.R.; Lee, I.M.; Buring, J.E.; Albert, C.M. Risk of Malignant Cancer Among Women With New-Onset Atrial Fibrillation. JAMA Cardiol. 2016, 1, 389–396. [Google Scholar] [CrossRef] [PubMed]
- Guzzetti, S.; Costantino, G.; Sada, S.; Fundarò, C. Colorectal cancer and atrial fibrillation: A case-control study. Am. J. Med. 2002, 112, 587–588. [Google Scholar] [CrossRef]
- Erichsen, R.; Christiansen, C.F.; Mehnert, F.; Weiss, N.S.; Baron, J.A.; Sørensen, H.T. Colorectal cancer and risk of atrial fibrillation and flutter: A population-based case-control study. Intern. Emerg. Med. 2012, 7, 431–438. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.F.; Liu, C.J.; Chang, P.M.H.; Tsao, H.M.; Lin, Y.J.; Chang, S.L.; Lo, L.W.; Tuan, T.C.; Li, C.H.; Chao, T.F.; et al. Incident thromboembolism and heart failure associated with new-onset atrial fibrillation in cancer patients. Int. J. Cardiol. 2013, 165, 355–357. [Google Scholar] [CrossRef] [PubMed]
- Cheng, W.L.; Kao, Y.H.; Chen, S.A.; Chen, Y.J. Pathophysiology of cancer therapy-provoked atrial fibrillation. Int. J. Cardiol. 2016, 219, 186–194. [Google Scholar] [CrossRef]
- Zamorano, J.L.; Lancellotti, P.; Rodriguez Muñoz, D.; Aboyans, V.; Asteggiano, R.; Galderisi, M.; Habib, G.; Lenihan, D.J.; Lip, G.Y.; Lyon, A.R.; et al. 2016 ESC Position Paper on cancer treatments and cardiovascular toxicity developed under the auspices of the ESC Committee for Practice Guidelines: The Task Force for cancer treatments and cardiovascular toxicity of the European Society of Cardiology (ESC). Eur. Heart J. 2016, 37, 2768–2801. [Google Scholar] [CrossRef]
- Farmakis, D.; Parissis, J.; Filippatos, G. Insights into onco-cardiology: Atrial fibrillation in cancer. J. Am. Coll. Cardiol. 2014, 63, 945–953. [Google Scholar] [CrossRef]
- Chelazzi, C.; Villa, G.; de Gaudio, A.R. Postoperative atrial fibrillation. ISRN Cardiol. 2011, 2011, 203179. [Google Scholar] [CrossRef]
- Gialdini, G.; Nearing, K.; Bhave, P.D.; Bonuccelli, U.; Iadecola, C.; Healey, J.S.; Kamel, H. Perioperative atrial fibrillation and the long-term risk of ischemic stroke. JAMA 2014, 312, 616–622. [Google Scholar] [CrossRef] [PubMed]
- Dunning, J.; Treasure, T.; Versteegh, M.; Nashef, S.A. Guidelines on the prevention and management of de novo atrial fibrillation after cardiac and thoracic surgery. Eur. J. Cardiothorac. Surg. 2006, 30, 852–872. [Google Scholar] [CrossRef] [PubMed]
- Frendl, G.; Sodickson, A.C.; Chung, M.K.; Waldo, A.L.; Gersh, B.J.; Tisdale, J.E.; Calkins, H.; Aranki, S.; Kaneko, T.; Cassivi, S.; et al. 2014 AATS guidelines for the prevention and management of perioperative atrial fibrillation and flutter for thoracic surgical procedures. Executive summary. J. Thorac. Cardiovasc. Surg. 2014, 148, 772–791. [Google Scholar] [CrossRef] [PubMed]
- Frendl, G.; Sodickson, A.C.; Chung, M.K.; Waldo, A.L.; Gersh, B.J.; Tisdale, J.E.; Calkins, H.; Aranki, S.; Kaneko, T.; Cassivi, S.; et al. 2014 AATS guidelines for the prevention and management of perioperative atrial fibrillation and flutter for thoracic surgical procedures. J. Thorac. Cardiovasc. Surg. 2014, 148, e153–e193. [Google Scholar] [CrossRef]
- Roselli, E.E.; Murthy, S.C.; Rice, T.W.; Houghtaling, P.L.; Pierce, C.D.; Karchmer, D.P.; Blackstone, E.H. Atrial fibrillation complicating lung cancer resection. J. Thorac. Cardiovasc. Surg. 2005, 130, 438–444. [Google Scholar] [CrossRef]
- Passman, R.S.; Gingold, D.S.; Amar, D.; Lloyd-Jones, D.; Bennett, C.L.; Zhang, H.; Rusch, V.W. Prediction rule for atrial fibrillation after major noncardiac thoracic surgery. Ann. Thorac. Surg. 2005, 79, 1698–1703. [Google Scholar] [CrossRef]
- Onaitis, M.; D’Amico, T.; Zhao, Y.; O’Brien, S.; Harpole, D. Risk factors for atrial fibrillation after lung cancer surgery: Analysis of the Society of Thoracic Surgeons general thoracic surgery database. Ann. Thorac. Surg. 2010, 90, 368–374. [Google Scholar] [CrossRef]
- Ivanovic, J.; Maziak, D.E.; Ramzan, S.; McGuire, A.L.; Villeneuve, P.J.; Gilbert, S.; Sundaresan, R.S.; Shamji, F.M.; Seely, A.J. Incidence, severity and perioperative risk factors for atrial fibrillation following pulmonary resection. Interact. Cardiovasc. Thorac. Surg. 2014, 18, 340–346. [Google Scholar] [CrossRef]
- Shrivastava, V.; Nyawo, B.; Dunning, J.; Morritt, G. Is there a role for prophylaxis against atrial fibrillation for patients undergoing lung surgery? Interact. Cardiovasc. Thorac. Surg. 2004, 3, 656–662. [Google Scholar] [CrossRef]
- Siu, C.W.; Tung, H.M.; Chu, K.W.; Jim, M.H.; Lau, C.P.; Tse, H.F. Prevalence and predictors of new-onset atrial fibrillation after elective surgery for colorectal cancer. Pacing Clin. Electrophysiol. 2005, 28, S120–S123. [Google Scholar] [CrossRef]
- Ojima, T.; Iwahashi, M.; Nakamori, M.; Nakamura, M.; Katsuda, M.; Iida, T.; Hayata, K.; Yamaue, H. Atrial fibrillation after esophageal cancer surgery: An analysis of 207 consecutive patients. Surg. Today 2014, 44, 839–847. [Google Scholar] [CrossRef] [PubMed]
- Cardinale, D.; Colombo, A.; Sandri, M.T.; Lamantia, G.; Colombo, N.; Civelli, M.; Salvatici, M.; Veronesi, G.; Veglia, F.; Fiorentini, C.; et al. Increased perioperative N-terminal pro-B-type natriuretic peptide levels predict atrial fibrillation after thoracic surgery for lung cancer. Circulation 2007, 115, 1339–1344. [Google Scholar] [CrossRef] [PubMed]
- Nojiri, T.; Inoue, M.; Takeuchi, Y.; Maeda, H.; Shintani, Y.; Sawabata, N.; Hamasaki, T.; Okumura, M. Impact of cardiopulmonary complications of lung cancer surgery on long-term outcomes. Surg. Today 2015, 45, 740–745. [Google Scholar] [CrossRef] [PubMed]
- Imperatori, A.; Mariscalco, G.; Riganti, G.; Rotolo, N.; Conti, V.; Dominioni, L. Atrial fibrillation after pulmonary lobectomy for lung cancer affects long-term survival in a prospective single-center study. J. Cardiothorac. Surg. 2012, 7, 4. [Google Scholar] [CrossRef] [PubMed]
- Henri, C.; Giraldeau, G.; Dorais, M.; Cloutier, A.S.; Girard, F.; Noiseux, N.; Ferraro, P.; Rinfret, S. Atrial fibrillation after pulmonary transplantation: Incidence, impact on mortality, treatment effectiveness, and risk factors. Circ. Arrhythm. Electrophysiol. 2012, 5, 61–67. [Google Scholar] [CrossRef]
- Bhave, P.D.; Goldman, L.E.; Vittinghoff, E.; Maselli, J.; Auerbach, A. Incidence, predictors, and outcomes associated with postoperative atrial fibrillation after major noncardiac surgery. Am. Heart J. 2012, 164, 918–924. [Google Scholar] [CrossRef]
- Vaporciyan, A.A.; Correa, A.M.; Rice, D.C.; Roth, J.A.; Smythe, W.R.; Swisher, S.G.; Walsh, G.L.; Putnam, J.B., Jr. Risk factors associated with atrial fibrillation after noncardiac thoracic surgery: Analysis of 2588 patients. J. Thorac. Cardiovasc. Surg. 2004, 127, 779–786. [Google Scholar] [CrossRef]
- Cardinale, D.; Martinoni, A.; Cipolla, C.M.; Civelli, M.; Lamantia, G.; Fiorentini, C.; Mezzetti, M. Atrial fibrillation after operation for lung cancer: Clinical and prognostic significance. Ann. Thorac. Surg. 1999, 68, 1827–1831. [Google Scholar] [CrossRef]
- Riber, L.P.; Larsen, T.B.; Christensen, T.D. Postoperative atrial fibrillation prophylaxis after lung surgery: Systematic review and meta-analysis. Ann. Thorac. Surg. 2014, 98, 1989–1997. [Google Scholar] [CrossRef]
- Rena, O.; Papalia, E.; Oliaro, A.; Casadio, C.; Ruffini, E.; Filosso, P.; Sacerdote, C.; Maggi, G. Supraventricular arrhythmias after resection surgery of the lung. Eur. J. Cardiothorac. Surg. 2001, 20, 688–693. [Google Scholar] [CrossRef]
- Polanczyk, C.A.; Goldman, L.; Marcantonio, E.R.; Orav, E.J.; Lee, T.H. Supraventricular arrhythmia in patients having noncardiac surgery: Clinical correlates and effect on length of stay. Ann. Intern. Med. 1998, 129, 279–285. [Google Scholar] [CrossRef] [PubMed]
- Fitzpatrick, T.; Carrier, M.; le Gal, G. Cancer, atrial fibrillation, and stroke. Thromb. Res. 2017, 155, 101–105. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.C.; Muo, C.H.; Lee, Y.T.; Yu, Y.H.; Sung, F.C. Lung cancer and incidence of stroke: A population-based cohort study. Stroke 2011, 42, 3034–3039. [Google Scholar] [CrossRef] [PubMed]
- Cestari, D.M.; Weine, D.M.; Panageas, K.S.; Segal, A.Z.; DeAngelis, L.M. Stroke in patients with cancer: Incidence and etiology. Neurology 2004, 62, 2025–2030. [Google Scholar] [CrossRef]
- Schwarzbach, C.J.; Schaefer, A.; Ebert, A.; Held, V.; Bolognese, M.; Kablau, M.; Hennerici, M.G.; Fatar, M. Stroke and cancer: The importance of cancer-associated hypercoagulation as a possible stroke etiology. Stroke 2012, 43, 3029–3034. [Google Scholar] [CrossRef]
- Wu, D.H.; Xu, M.Y.; Mao, T.; Cao, H.; Wu, D.J.; Shen, Y.F. Risk factors for intraoperative atrial fibrillation: A retrospective analysis of 10,563 lung operations in a single center. Ann. Thorac. Surg. 2012, 94, 193–197. [Google Scholar] [CrossRef]
- Muranishi, Y.; Sonobe, M.; Menju, T.; Aoyama, A.; Chen-Yoshikawa, T.F.; Sato, T.; Date, H. Atrial fibrillation after lung cancer surgery: Incidence, severity, and risk factors. Surg. Today 2017, 47, 252–258. [Google Scholar] [CrossRef]
- Iwata, T.; Nagato, K.; Nakajima, T.; Suzuki, H.; Yoshida, S.; Yoshino, I. Risk factors predictive of atrial fibrillation after lung cancer surgery. Surg. Today 2016, 46, 877–886. [Google Scholar] [CrossRef]
- Kavurmaci, O.; Akcam, T.I.; Ergonul, A.G.; Turhan, K.; Cakan, A.; Cagirici, U. Is the Risk of Postoperative Atrial Fibrillation Predictable in Patients Undergoing Surgery Due to Primary Lung Cancer? Heart Lung. Circ. 2018, 27, 835–841. [Google Scholar] [CrossRef]
- Finkel, T.; Serrano, M.; Blasco, M.A. The common biology of cancer and ageing. Nature 2007, 448, 767. [Google Scholar] [CrossRef]
- Maesen, B.; Nijs, J.; Maessen, J.; Allessie, M.; Schotten, U. Post-operative atrial fibrillation: A maze of mechanisms. Europace 2012, 14, 159–174. [Google Scholar] [CrossRef] [PubMed]
- Tisdale, J.E.; Wroblewski, H.A.; Kesler, K.A. Prophylaxis of atrial fibrillation after noncardiac thoracic surgery. Semin. Thorac. Cardiovasc. Surg. 2010, 22, 310–320. [Google Scholar] [CrossRef] [PubMed]
- Terzi, A.; Furlan, G.; Chiavacci, P.; Dal Corso, B.; Luzzani, A.; Dalla Volta, S. Prevention of atrial tachyarrhythmias after non-cardiac thoracic surgery by infusion of magnesium sulfate. Thorac. Cardiovasc. Surg. 1996, 44, 300–303. [Google Scholar] [CrossRef] [PubMed]
- Amar, D.; Roistacher, N.; Burt, M.; Reinsel, R.A.; Ginsberg, R.J.; Wilson, R.S. Clinical and echocardiographic correlates of symptomatic tachydysrhythmias after noncardiac thoracic surgery. Chest 1995, 108, 349–354. [Google Scholar] [CrossRef]
- Schotten, U.; Verheule, S.; Kirchhof, P.; Goette, A. Pathophysiological mechanisms of atrial fibrillation: A translational appraisal. Physiol. Rev. 2011, 91, 265–325. [Google Scholar] [CrossRef]
- Dixit, S. Atrial fibrillation after major thoracic surgery: New insights into underlying mechanisms. J. Am. Coll. Cardiol. 2009, 54, 2049–2051. [Google Scholar] [CrossRef]
- Merritt, R.E.; Shrager, J.B. Prophylaxis and management of atrial fibrillation after general thoracic surgery. Thorac. Surg. Clin. 2012, 22, 13–23. [Google Scholar] [CrossRef]
- Amar, D.; Roistacher, N.; Burt, M.E.; Rusch, V.W.; Bains, M.S.; Leung, D.H.; Downey, R.J.; Ginsberg, R.J. Effects of diltiazem versus digoxin on dysrhythmias and cardiac function after pneumonectomy. Ann. Thorac. Surg. 1997, 63, 1374–1381. [Google Scholar] [CrossRef]
- Van Mieghem, W.; Tits, G.; Demuynck, K.; Lacquet, L.; Deneffe, G.; Tjandra-Maga, T.; Demedts, M. Verapamil as prophylactic treatment for atrial fibrillation after lung operations. Ann. Thorac. Surg. 1996, 61, 1083–1085. [Google Scholar] [CrossRef]
- Akoum, N.; Daccarett, M.; McGann, C.; Segerson, N.; Vergara, G.; Kuppahally, S.; Badger, T.; Burgon, N.; Haslam, T.; Kholmovski, E.; et al. Atrial fibrosis helps select the appropriate patient and strategy in catheter ablation of atrial fibrillation: A DE-MRI guided approach. J. Cardiovasc. Electrophysiol. 2011, 22, 16–22. [Google Scholar] [CrossRef]
- Kanmanthareddy, A.; Vallakati, A.; Reddy Yeruva, M.A.D.H.U.; Dixit, S.; Di Biase, L.; Mansour, M.; Boolani, H.; Gunda, S.; Bunch, T.J.; Day, J.D.; et al. Pulmonary vein isolation for atrial fibrillation in the postpneumonectomy population: A feasibility, safety, and outcomes study. J. Cardiovasc. Electrophysiol. 2015, 26, 385–389. [Google Scholar] [PubMed]
- Haissaguerre, M.; Jaïs, P.; Shah, D.C.; Takahashi, A.; Hocini, M.; Quiniou, G.; Garrigue, S.; Le Mouroux, A.; Le Métayer, P.; Clémenty, J. Spontaneous initiation of atrial fibrillation by ectopic beats originating in the pulmonary veins. N. Engl. J. Med. 1998, 339, 659–666. [Google Scholar] [PubMed]
- Jais, P.; Hocini, M.; Macle, L.; Choi, K.J.; Deisenhofer, I.; Weerasooriya, R.; Shah, D.C.; Garrigue, S.; Raybaud, F.; Scavee, C.; et al. Distinctive electrophysiological properties of pulmonary veins in patients with atrial fibrillation. Circulation 2002, 106, 2479–2485. [Google Scholar] [CrossRef] [PubMed]
- Chung, M.K.; Martin, D.O.; Sprecher, D.; Wazni, O.; Kanderian, A.; Carnes, C.A.; Bauer, J.A.; Tchou, P.J.; Niebauer, M.J.; Natale, A.; et al. C-reactive protein elevation in patients with atrial arrhythmias: Inflammatory mechanisms and persistence of atrial fibrillation. Circulation 2001, 104, 2886–2891. [Google Scholar] [CrossRef]
- Aviles, R.J.; Martin, D.O.; Apperson-Hansen, C.; Houghtaling, P.L.; Rautaharju, P.; Kronmal, R.A.; Tracy, R.P.; Van Wagoner, D.R.; Psaty, B.M.; Lauer, M.S.; et al. Inflammation as a risk factor for atrial fibrillation. Circulation 2003, 108, 3006–3010. [Google Scholar] [CrossRef]
- Hu, Y.-F.; Chen, Y.J.; Lin, Y.J.; Chen, S.A. Inflammation and the pathogenesis of atrial fibrillation. Nat. Rev. Cardiol. 2015, 12, 230. [Google Scholar] [CrossRef]
- Lohani, K.R.; Nandipati, K.C.; Rollins, S.E.; Fetten, K.; Lee, T.H.; Pallati, P.K.; Yamamoto, S.R.; Mittal, S.K. Transthoracic approach is associated with increased incidence of atrial fibrillation after esophageal resection. Surg. Endosc. 2015, 29, 2039–2045. [Google Scholar] [CrossRef]
- Ma, J.Y.; Wang, Y.; Zhao, Y.F.; Wu, Z.; Liu, L.X.; Kou, Y.L.; Yang, J.J. Atrial fibrillation after surgery for esophageal carcinoma: Clinical and prognostic significance. World J. Gastroenterol. 2006, 12, 449–452. [Google Scholar] [CrossRef]
- De Decker, K.; Jorens, P.G.; van Schil, P. Cardiac complications after noncardiac thoracic surgery: An evidence-based current review. Ann. Thorac. Surg. 2003, 75, 1340–1348. [Google Scholar] [CrossRef]
- Ueda, T.; Suzuki, K.; Matsunaga, T.; Takamochi, K.; Oh, S. Postoperative atrial fibrillation is less frequent in pulmonary segmentectomy compared with lobectomy. Gen. Thorac. Cardiovasc. Surg. 2018, 66, 95–100. [Google Scholar] [CrossRef]
- Mariscalco, G.; Biancari, F.; Zanobini, M.; Cottini, M.; Piffaretti, G.; Saccocci, M.; Banach, M.; Beghi, C.; Angelini, G.D. Bedside tool for predicting the risk of postoperative atrial fibrillation after cardiac surgery: The POAF score. J. Am. Heart Assoc. 2014, 3, e000752. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.T.; Strauss, D.M.; Stone, L.E.; Stoltzfus, J.C.; Puc, M.M.; Burfeind, W.R. Preoperative CHA2DS2-VASc Score Predicts Postoperative Atrial Fibrillation after Lobectomy. Thorac. Cardiovasc. Surg. 2019, 67, 125–130. [Google Scholar] [CrossRef] [PubMed]
- Guglin, M.; Aljayeh, M.; Saiyad, S.; Ali, R.; Curtis, A.B. Introducing a new entity: Chemotherapy-induced arrhythmia. Europace 2009, 11, 1579–1586. [Google Scholar] [CrossRef] [PubMed]
- Rice, D.C.; Correa, A.M.; Vaporciyan, A.A.; Sodhi, N.; Smythe, W.R.; Swisher, S.G.; Walsh, G.L.; Putnam, J.B., Jr.; Komaki, R.; Ajani, J.A.; et al. Preoperative chemoradiotherapy prior to esophagectomy in elderly patients is not associated with increased morbidity. Ann. Thorac. Surg. 2005, 79, 391–397. [Google Scholar] [CrossRef] [PubMed]
- Peer, M.; Stav, D.; Cyjon, A.; Sandbank, J.; Vasserman, M.; Haitov, Z.; Sasson, L.; Schreiber, L.; Ezri, T.; Priel, I.E.; et al. Morbidity and mortality after major pulmonary resections in patients with locally advanced stage IIIA non-small cell lung carcinoma who underwent induction therapy. Heart Lung Circ. 2015, 24, 69–76. [Google Scholar] [CrossRef]
- Tsujimoto, H.; Ono, S.; Chochi, K.; Sugasawa, H.; Ichikura, T.; Mochizuki, H. Preoperative chemoradiotherapy for esophageal cancer enhances the postoperative systemic inflammatory response. Jpn. J. Clin. Oncol. 2006, 36, 632–637. [Google Scholar] [CrossRef]
- Cai, G.L.; Chen, J.; Hu, C.B.; Yan, M.L.; Xu, Q.H.; Yan, J. Value of plasma brain natriuretic peptide levels for predicting postoperative atrial fibrillation: A systemic review and meta-analysis. World J. Surg. 2014, 38, 51–59. [Google Scholar] [CrossRef]
- Amar, D. Postoperative atrial fibrillation: Is there a need for prevention? J. Thorac. Cardiovasc. Surg. 2016, 151, 913–935. [Google Scholar] [CrossRef]
- Turagam, M.K.; Mirza, M.; Werner, P.H.; Sra, J.; Kress, D.C.; Tajik, A.J.; Jahangir, A. Circulating Biomarkers Predictive of Postoperative Atrial Fibrillation. Cardiol. Rev. 2016, 24, 76–87. [Google Scholar] [CrossRef]
- Struthers, A.; Lang, C. The potential to improve primary prevention in the future by using BNP/N-BNP as an indicator of silent ‘pancardiac’ target organ damage: BNP/N-BNP could become for the heart what microalbuminuria is for the kidney. Eur. Heart J. 2007, 28, 1678–16782. [Google Scholar] [CrossRef]
- Clerico, A.; Giannoni, A.; Vittorini, S.; Passino, C. Thirty years of the heart as an endocrine organ: Physiological role and clinical utility of cardiac natriuretic hormones. Am. J. Physiol. Heart Circ. Physiol. 2011, 301, H12–H20. [Google Scholar] [CrossRef] [PubMed]
- Rodseth, R.N.; Buse, G.A.L.; Bolliger, D.; Burkhart, C.S.; Cuthbertson, B.H.; Gibson, S.C.; Mahla, E.; Leibowitz, D.W.; Biccard, B.M. The predictive ability of pre-operative B-type natriuretic peptide in vascular patients for major adverse cardiac events: An individual patient data meta-analysis. J. Am. Coll. Cardiol. 2011, 58, 522–529. [Google Scholar] [CrossRef] [PubMed]
- Karthikeyan, G.; Moncur, R.A.; Levine, O.; Heels-Ansdell, D.; Chan, M.T.; Alonso-Coello, P.; Yusuf, S.; Sessler, D.; Villar, J.C.; Berwanger, O.; et al. Is a pre-operative brain natriuretic peptide or N-terminal pro-B-type natriuretic peptide measurement an independent predictor of adverse cardiovascular outcomes within 30 days of noncardiac surgery? A systematic review and meta-analysis of observational studies. J. Am. Coll. Cardiol. 2009, 54, 1599–1606. [Google Scholar]
- Cardinale, D.; Cosentino, N.; Moltrasio, M.; Sandri, M.T.; Petrella, F.; Colombo, A.; Bacchiani, G.; Tessitore, A.; Bonomi, A.; Veglia, F.; et al. Acute kidney injury after lung cancer surgery: Incidence and clinical relevance, predictors, and role of N-terminal pro B-type natriuretic peptide. Lung Cancer 2018, 123, 155–159. [Google Scholar] [CrossRef] [PubMed]
- Biccard, B.M.; Lurati Buse, G.A.; Burkhart, C.; Cuthbertson, B.H.; Filipovic, M.; Gibson, S.C.; Mahla, E.; Leibowitz, D.W.; Rodseth, R.N. The influence of clinical risk factors on pre-operative B-type natriuretic peptide risk stratification of vascular surgical patients. Anaesthesia 2012, 67, 55–59. [Google Scholar] [CrossRef] [PubMed]
- Poldermans, D.; Hoeks, S.E.; Feringa, H.H. Pre-operative risk assessment and risk reduction before surgery. J. Am. Coll. Cardiol. 2008, 51, 1913–1924. [Google Scholar] [CrossRef] [PubMed]
- Rodseth, R.N.; Biccard, B.M.; Le Manach, Y.; Sessler, D.I.; Buse, G.A.L.; Thabane, L.; Schutt, R.C.; Bolliger, D.; Cagini, L.; Cardinale, D.; et al. The prognostic value of pre-operative and post-operative B-type natriuretic peptides in patients undergoing noncardiac surgery: B-type natriuretic peptide and N-terminal fragment of pro-B-type natriuretic peptide: A systematic review and individual patient data meta-analysis. J. Am. Coll. Cardiol. 2014, 63, 170–180. [Google Scholar]
- Rodseth, R.N.; Biccard, B.M.; Chu, R.; Buse, G.A.L.; Thabane, L.; Bakhai, A.; Bolliger, D.; Cagini, L.; Cahill, T.J.; Cardinale, D.; et al. Postoperative B-type natriuretic peptide for prediction of major cardiac events in patients undergoing noncardiac surgery: Systematic review and individual patient meta-analysis. Anesthesiology 2013, 119, 270–283. [Google Scholar] [CrossRef]
- Cardinale, D.; Sandri, M.T.; Colombo, A.; Salvatici, M.; Tedeschi, I.; Bacchiani, G.; Beggiato, M.; Meroni, C.A.; Civelli, M.; Lamantia, G.; et al. Prevention of Atrial Fibrillation in High-risk Patients Undergoing Lung Cancer Surgery: The PRESAGE Trial. Ann. Surg. 2016, 264, 244–251. [Google Scholar] [CrossRef]
- Amar, D.; Zhang, H.; Shi, W.; Downey, R.J.; Bains, M.S.; Park, B.J.; Flores, R.; Rizk, N.; Thaler, H.T.; Rusch, V.W. Brain natriuretic peptide and risk of atrial fibrillation after thoracic surgery. J. Thorac. Cardiovasc. Surg. 2012, 144, 1249–1253. [Google Scholar] [CrossRef][Green Version]
- Wazni, O.M.; Martin, D.O.; Marrouche, N.F.; Latif, A.A.; Ziada, K.; Shaaraoui, M.; Almahameed, S.; Schweikert, R.A.; Saliba, W.I.; Gillinov, A.M.; et al. Plasma B-type natriuretic peptide levels predict postoperative atrial fibrillation in patients undergoing cardiac surgery. Circulation 2004, 110, 124–127. [Google Scholar] [PubMed]
- Nojiri, T.; Maeda, H.; Takeuchi, Y.; Funakoshi, Y.; Kimura, T.; Maekura, R.; Yamamoto, K.; Okumura, M. Predictive value of B-type natriuretic peptide for postoperative atrial fibrillation following pulmonary resection for lung cancer. Eur. J. Cardiothorac. Surg. 2010, 37, 787–791. [Google Scholar] [CrossRef] [PubMed]
- Yoshimura, M.; Mizuno, Y.; Nakayama, M.; Sakamoto, T.; Sugiyama, S.; Kawano, H.; Soejima, H.; Hirai, N.; Saito, Y.; Nakao, K.; et al. B-type natriuretic peptide as a marker of the effects of enalapril in patients with heart failure. Am. J. Med. 2002, 112, 716–720. [Google Scholar] [CrossRef]
- Johnson, W.; Omland, T.; Hall, C.; Lucas, C.; Myking, O.L.; Collins, C.; Pfeffer, M.; Rouleau, J.L.; Stevenson, L.W. Neurohormonal activation rapidly decreases after intravenous therapy with diuretics and vasodilators for class IV heart failure. J. Am. Coll. Cardiol. 2002, 39, 1623–1629. [Google Scholar] [CrossRef]
- Salvatici, M.; Cardinale, D.; Spaggiari, L.; Veglia, F.; Tedesco, C.C.; Solli, P.; Cipolla, C.M.; Zorzino, L.; Passerini, R.; Riggio, D.; et al. Atrial fibrillation after thoracic surgery for lung cancer: Use of a single cut-off value of N-terminal pro-B type natriuretic peptide to identify patients at risk. Biomarkers 2010, 15, 259–265. [Google Scholar] [CrossRef]
- Bobbio, A.; Caporale, D.; Internullo, E.; Ampollini, L.; Bettati, S.; Rossini, E.; Carbognani, P.; Rusca, M. Postoperative outcome of patients undergoing lung resection presenting with new-onset atrial fibrillation managed by amiodarone or diltiazem. Eur. J. Cardiothorac. Surg. 2007, 31, 70–74. [Google Scholar] [CrossRef]
- Danelich, I.M.; Lose, J.M.; Wright, S.S.; Asirvatham, S.J.; Ballinger, B.A.; Larson, D.W.; Lovely, J.K. Practical management of postoperative atrial fibrillation after noncardiac surgery. J. Am. Coll. Surg. 2014, 219, 831–841. [Google Scholar] [CrossRef]
- Boriani, G.; Ferruzzi, L.; Corti, B.; Ruffato, A.; Gavelli, G.; Mattioli, S. Short-term onset of fatal pulmonary toxicity in a patient treated with intravenous amiodarone for post-operative atrial fibrillation. Int. J. Cardiol. 2012, 159, e1–e4. [Google Scholar] [CrossRef]
- Riber, L.P.; Christensen, T.D.; Jensen, H.K.; Hoejsgaard, A.; Pilegaard, H.K. Amiodarone significantly decreases atrial fibrillation in patients undergoing surgery for lung cancer. Ann. Thorac. Surg. 2012, 94, 339–344. [Google Scholar] [CrossRef]
- Nojiri, T.; Yamamoto, K.; Maeda, H.; Takeuchi, Y.; Funakoshi, Y.; Inoue, M.; Okumura, M. Effect of low-dose human atrial natriuretic peptide on postoperative atrial fibrillation in patients undergoing pulmonary resection for lung cancer: A double-blind, placebo-controlled study. J. Thorac. Cardiovasc. Surg. 2012, 143, 488–494. [Google Scholar] [CrossRef]
- Ciszewski, P.; Tyczka, J.; Nadolski, J.; Roszak, M.; Dyszkiewicz, W. Comparative efficacy and usefulness of acebutolol and diltiazem for the prevention of atrial fibrillation during perioperative time in patients undergoing pulmonary resection. Thorac. Cardiovasc. Surg. 2013, 61, 365–372. [Google Scholar] [PubMed]
- Sedrakyan, A.; Treasure, T.; Browne, J.; Krumholz, H.; Sharpin, C.; van der Meulen, J. Pharmacologic prophylaxis for postoperative atrial tachyarrhythmia in general thoracic surgery: Evidence from randomized clinical trials. J. Thorac. Cardiovasc. Surg. 2005, 129, 997–1005. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Gao, S. Systematic Review and Meta-analysis of Atrial Fibrillation Prophylaxis After Lung Surgery. J. Cardiovasc. Pharmacol. 2016, 67, 351–357. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.C.; Huang, T.Y.; Deng, Q.W.; Liu, W.F.; Liu, J.; Deng, W.T.; Liu, K.X.; Li, C. Prophylaxis Against Atrial Fibrillation After General Thoracic Surgery: Trial Sequential Analysis and Network Meta-Analysis. Chest 2017, 151, 149–159. [Google Scholar] [CrossRef] [PubMed]
- POISE Study Group. Effects of extended-release metoprolol succinate in patients undergoing non-cardiac surgery (POISE trial): A randomised controlled trial. Lancet 2008, 371, 1839–1847. [Google Scholar] [CrossRef]
- Jakobsen, C.J.; Bille, S.; Ahlburg, P.; Rybro, L.; Hjortholm, K.; Andresen, E.B. Perioperative metoprolol reduces the frequency of atrial fibrillation after thoracotomy for lung resection. J. Cardiothorac. Vasc. Anesth. 1997, 11, 746–751. [Google Scholar] [CrossRef]
- Bayliff, C.D.; Massel, D.R.; Inculet, R.I.; Malthaner, R.A.; Quinton, S.D.; Powell, F.S.; Kennedy, R.S. Propranolol for the prevention of postoperative arrhythmias in general thoracic surgery. Ann. Thorac. Surg. 1999, 67, 182–186. [Google Scholar] [CrossRef]
- Reinhart, K.; Baker, W.L.; Siv, M.L. Beyond the guidelines: New and novel agents for the prevention of atrial fibrillation after cardiothoracic surgery. J. Cardiovasc. Pharmacol. Ther. 2011, 16, 5–13. [Google Scholar] [CrossRef]
- Jibrini, M.B.; Molnar, J.; Arora, R.R. Prevention of atrial fibrillation by way of abrogation of the renin-angiotensin system: A systematic review and meta-analysis. Am. J. Ther. 2008, 15, 36–43. [Google Scholar] [CrossRef]
- Ozaydin, M.; Dede, O.; Varol, E.; Kapan, S.; Turker, Y.; Peker, O.; Duver, H.; Ibrisim, E. Effect of renin-angiotensin aldosteron system blockers on postoperative atrial fibrillation. Int. J. Cardiol. 2008, 127, 362–367. [Google Scholar] [CrossRef]
- DiNicolantonio, J.J.; Beavers, C.J.; Menezes, A.R.; Lavie, C.J.; O’Keefe, J.H.; Meier, P.; Vorobcsuk, A.; Aradi, D.; Komócsi, A.; Chatterjee, S. Meta-analysis comparing carvedilol versus metoprolol for the prevention of postoperative atrial fibrillation following coronary artery bypass grafting. Am. J. Cardiol. 2014, 113, 565–569. [Google Scholar] [CrossRef] [PubMed]
- Amar, D.; Zhang, H.; Heerdt, P.M.; Park, B.; Fleisher, M.; Thaler, H.T. Statin use is associated with a reduction in atrial fibrillation after noncardiac thoracic surgery independent of C-reactive protein. Chest 2005, 128, 3421–3427. [Google Scholar] [CrossRef] [PubMed]
- Blanchard, L.; Collard, C.D. Non-antiarrhythmic agents for prevention of postoperative atrial fibrillation: Role of statins. Curr. Opin. Anaesthesiol. 2007, 20, 53–56. [Google Scholar] [CrossRef] [PubMed]
- De Waal, B.A.; Buise, M.P.; van Zundert, A.A. Perioperative statin therapy in patients at high risk for cardiovascular morbidity undergoing surgery: A review. Br. J. Anaesth. 2015, 114, 44–52. [Google Scholar] [CrossRef]
- Bang, C.N.; Greve, A.M.; Abdulla, J.; Køber, L.; Gislason, G.H.; Wachtell, K. The preventive effect of statin therapy on new-onset and recurrent atrial fibrillation in patients not undergoing invasive cardiac interventions: A systematic review and meta-analysis. Int. J. Cardiol. 2013, 167, 624–630. [Google Scholar] [CrossRef]
- Imazio, M.; Belli, R.; Brucato, A.; Ferrazzi, P.; Patrini, D.; Martinelli, L.; Polizzi, V.; Cemin, R.; Leggieri, A.; Caforio, A.L.; et al. Rationale and design of the COlchicine for Prevention of the Post-pericardiotomy Syndrome and Post-operative Atrial Fibrillation (COPPS-2 trial): A randomized, placebo-controlled, multicenter study on the use of colchicine for the primary prevention of the postpericardiotomy syndrome, postoperative effusions, and postoperative atrial fibrillation. Am. Heart J. 2013, 166, 13–19. [Google Scholar]
- Imazio, M.; Brucato, A.; Ferrazzi, P.; Pullara, A.; Adler, Y.; Barosi, A.; Caforio, A.L.; Cemin, R.; Chirillo, F.; Comoglio, C.; et al. Colchicine for prevention of postpericardiotomy syndrome and postoperative atrial fibrillation: The COPPS-2 randomized clinical trial. JAMA 2014, 312, 1016–1023. [Google Scholar] [CrossRef]
- Imazio, M.; Brucato, A.; Ferrazzi, P.; Rovere, M.E.; Gandino, A.; Cemin, R.; Ferrua, S.; Belli, R.; Maestroni, S.; Simon, C.; et al. Colchicine reduces postoperative atrial fibrillation: Results of the Colchicine for the Prevention of the Postpericardiotomy Syndrome (COPPS) atrial fibrillation substudy. Circulation 2011, 124, 2290–2295. [Google Scholar] [CrossRef]
- Worden, J.C.; Asare, K. Postoperative atrial fibrillation: Role of inflammatory biomarkers and use of colchicine for its prevention. Pharmacotherapy 2014, 34, 1167–1173. [Google Scholar] [CrossRef]
- Nojiri, T.; Yamamoto, K.; Maeda, H.; Takeuchi, Y.; Ose, N.; Susaki, Y.; Inoue, M.; Okumura, M. A Double-Blind Placebo-Controlled Study of the Effects of Olprinone, a Specific Phosphodiesterase III Inhibitor, for Preventing Postoperative Atrial Fibrillation in Patients Undergoing Pulmonary Resection for Lung Cancer. Chest 2015, 148, 1285–1292. [Google Scholar] [CrossRef]
- Masson, S.; Wu, J.H.; Simon, C.; Barlera, S.; Marchioli, R.; Mariani, J.; Macchia, A.; Lombardi, F.; Vago, T.; Aleksova, A.; et al. Circulating cardiac biomarkers and postoperative atrial fibrillation in the OPERA trial. Eur. J. Clin. Invest. 2015, 45, 170–178. [Google Scholar] [CrossRef] [PubMed]
- Mozaffarian, D.; Marchioli, R.; Macchia, A.; Silletta, M.G.; Ferrazzi, P.; Gardner, T.J.; Latini, R.; Libby, P.; Lombardi, F.; O’Gara, P.T.; et al. Fish oil and postoperative atrial fibrillation: The Omega-3 Fatty Acids for Prevention of Post-operative Atrial Fibrillation (OPERA) randomized trial. JAMA 2012, 308, 2001–2011. [Google Scholar] [CrossRef] [PubMed]
- Mozaffarian, D.; Marchioli, R.; Macchia, A.; Silletta, M.G.; Ferrazzi, P.; Gardner, T.J.; Latini, R.; Libby, P.; Lombardi, F.; O’Gara, P.T.; et al. Fish oil and post-operative atrial fibrillation: A meta-analysis of randomized controlled trials. J. Am. Coll. Cardiol. 2013, 61, 2194–2196. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.H.; Marchioli, R.; Silletta, M.G.; Masson, S.; Sellke, F.W.; Libby, P.; Milne, G.L.; Brown, N.J.; Lombardi, F.; Damiano, R.J., Jr.; et al. Oxidative Stress Biomarkers and Incidence of Postoperative Atrial Fibrillation in the Omega-3 Fatty Acids for Prevention of Postoperative Atrial Fibrillation (OPERA) Trial. J. Am. Heart Assoc. 2015, 4, e001886. [Google Scholar] [CrossRef]
- Butt, J.H.; Olesen, J.B.; Havers-Borgersen, E.; Gundlund, A.; Andersson, C.; Gislason, G.H.; Torp-Pedersen, C.; Køber, L.; Fosbøl, E.L. Risk of Thromboembolism Associated With Atrial Fibrillation Following Noncardiac Surgery. J. Am. Coll. Cardiol. 2018, 72, 2027–2036. [Google Scholar] [CrossRef]
- Boriani, G.; Glotzer, T.V.; Santini, M.; West, T.M.; De Melis, M.; Sepsi, M.; Gasparini, M.; Lewalter, T.; Camm, J.A.; Singer, D.E. Device-detected atrial fibrillation and risk for stroke: An analysis of >10,000 patients from the SOS AF project (Stroke preventiOn Strategies based on Atrial Fibrillation information from implanted devices). Eur. Heart J. 2014, 35, 508–516. [Google Scholar] [CrossRef]
- Verma, A.; Bhatt, D.L.; Verma, S. Long-Term Outcomes of Post-Operative Atrial Fibrillation: Guilty as Charged. J. Am. Coll. Cardiol. 2018, 71, 749–751. [Google Scholar] [CrossRef]
- Chin, J.H.; Moon, Y.J.; Jo, J.Y.; Han, Y.A.; Kim, H.R.; Lee, E.H.; Choi, I.C. Association between Postoperatively Developed Atrial Fibrillation and Long-Term Mortality after Esophagectomy in Esophageal Cancer Patients: An Observational Study. PLoS ONE 2016, 11, e0154931. [Google Scholar] [CrossRef]
- Amar, D. Postoperative atrial fibrillation. Heart Dis. 2002, 4, 117–123. [Google Scholar] [CrossRef]
- Garner, M.; Routledge, T.; King, J.E.; Pilling, J.E.; Veres, L.; Harrison-Phipps, K.; Bille, A.; Harling, L. New-onset atrial fibrillation after anatomic lung resection: Predictive factors, treatment and follow-up in a UK thoracic centre. Interact. Cardiovasc. Thorac. Surg. 2017, 24, 260–264. [Google Scholar] [CrossRef][Green Version]
- Higuchi, S.; Kabeya, Y.; Matsushita, K.; Arai, N.; Tachibana, K.; Tanaka, R.; Kawachi, R.; Takei, H.; Suzuki, Y.; Kogure, M.; et al. Perioperative Atrial Fibrillation in Noncardiac Surgeries for Malignancies and One-Year Recurrence. Can. J. Cardiol. 2019, 35, 1449–1456. [Google Scholar] [CrossRef] [PubMed]
- Ahlsson, A.; Fengsrud, E.; Bodin, L.; Englund, A. Postoperative atrial fibrillation in patients undergoing aortocoronary bypass surgery carries an eightfold risk of future atrial fibrillation and a doubled cardiovascular mortality. Eur. J. Cardiothorac. Surg. 2010, 37, 1353–1359. [Google Scholar] [CrossRef] [PubMed]
- Lowres, N.; Mulcahy, G.; Gallagher, R.; Ben Freedman, S.; Marshman, D.; Kirkness, A.; Orchard, J.; Neubeck, L. Self-monitoring for atrial fibrillation recurrence in the discharge period post-cardiac surgery using an iPhone electrocardiogram. Eur. J. Cardiothorac. Surg. 2016, 50, 44–51. [Google Scholar] [CrossRef] [PubMed]
- Higuchi, S.; Kabeya, Y.; Matsushita, K.; Tachibana, K.; Kawachi, R.; Takei, H.; Suzuki, Y.; Abe, N.; Imanishi, Y.; Moriyama, K. The study protocol for PREDICT AF RECURRENCE: A PRospEctive cohort stuDy of surveIllanCe for perioperaTive Atrial Fibrillation RECURRENCE in major non-cardiac surgery for malignancy. BMC Cardiovasc. Disord. 2018, 18, 127. [Google Scholar] [CrossRef]
- El-Chami, M.F.; Merchant, F.M.; Smith, P.; Levy, M.; Nelms, A.G.; Merlino, J.; Puskas, J.; Leon, A.R. Management of New-Onset Postoperative Atrial Fibrillation Utilizing Insertable Cardiac Monitor Technology to Observe Recurrence of AF (MONITOR-AF). Pacing Clin. Electrophysiol. 2016, 39, 1083–1089. [Google Scholar] [CrossRef]
- Landymore, R.W.; Howell, F. Recurrent atrial arrhythmias following treatment for postoperative atrial fibrillation after coronary bypass operations. Eur. J. Cardiothorac. Surg. 1991, 5, 436–439. [Google Scholar] [CrossRef]
- Yilmaz, A.T.; Demírkiliç, U.; Arslan, M.; Kurulay, E.; Özal, E.; Tatar, H.; Öztürk, Ö.Y. Long-term prevention of atrial fibrillation after coronary artery bypass surgery: Comparison of quinidine, verapamil, and amiodarone in maintaining sinus rhythm. J. Card. Surg. 1996, 11, 61–64. [Google Scholar] [CrossRef]

| Procedure Type/Surgical Risk | Low | Intermediate | High |
|---|---|---|---|
| Minor Procedures | Bronchoscopy +/− Biopsy | ||
| Tracheal Stenting | |||
| Thoracostomy Tube Placement | |||
| Pleurodesis | |||
| Moderate Procedures | Tracheostomy | Simpaticectomy | |
| Rigid Bronchoscopy | |||
| Mediastinoscopy | |||
| Toracoscopic Wedge Resection | |||
| Major Procedures | Segmentectomy | Pleurectomy | |
| Lobectomy | |||
| Transplant | |||
| Fistula Repair | |||
| Bullectomy | |||
| Pneumonectomy | |||
| Tracheal Resection | |||
| Anterior Mediastinal Resection |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fabiani, I.; Colombo, A.; Bacchiani, G.; Cipolla, C.M.; Cardinale, D.M. Incidence, Management, Prevention and Outcome of Post-Operative Atrial Fibrillation in Thoracic Surgical Oncology. J. Clin. Med. 2020, 9, 37. https://doi.org/10.3390/jcm9010037
Fabiani I, Colombo A, Bacchiani G, Cipolla CM, Cardinale DM. Incidence, Management, Prevention and Outcome of Post-Operative Atrial Fibrillation in Thoracic Surgical Oncology. Journal of Clinical Medicine. 2020; 9(1):37. https://doi.org/10.3390/jcm9010037
Chicago/Turabian StyleFabiani, Iacopo, Alessandro Colombo, Giulia Bacchiani, Carlo Maria Cipolla, and Daniela Maria Cardinale. 2020. "Incidence, Management, Prevention and Outcome of Post-Operative Atrial Fibrillation in Thoracic Surgical Oncology" Journal of Clinical Medicine 9, no. 1: 37. https://doi.org/10.3390/jcm9010037
APA StyleFabiani, I., Colombo, A., Bacchiani, G., Cipolla, C. M., & Cardinale, D. M. (2020). Incidence, Management, Prevention and Outcome of Post-Operative Atrial Fibrillation in Thoracic Surgical Oncology. Journal of Clinical Medicine, 9(1), 37. https://doi.org/10.3390/jcm9010037





