Differential Diagnosis of Malignant Lymphadenopathy Using Flow Cytometry on Fine Needle Aspirate: Report on 269 Cases
Abstract
1. Introduction
2. Material and Methods
2.1. Patient Selection
2.2. Flow Cytometry Sample Preparation
2.3. Immunophenotyping
2.4. Histology
2.5. Data Analysis
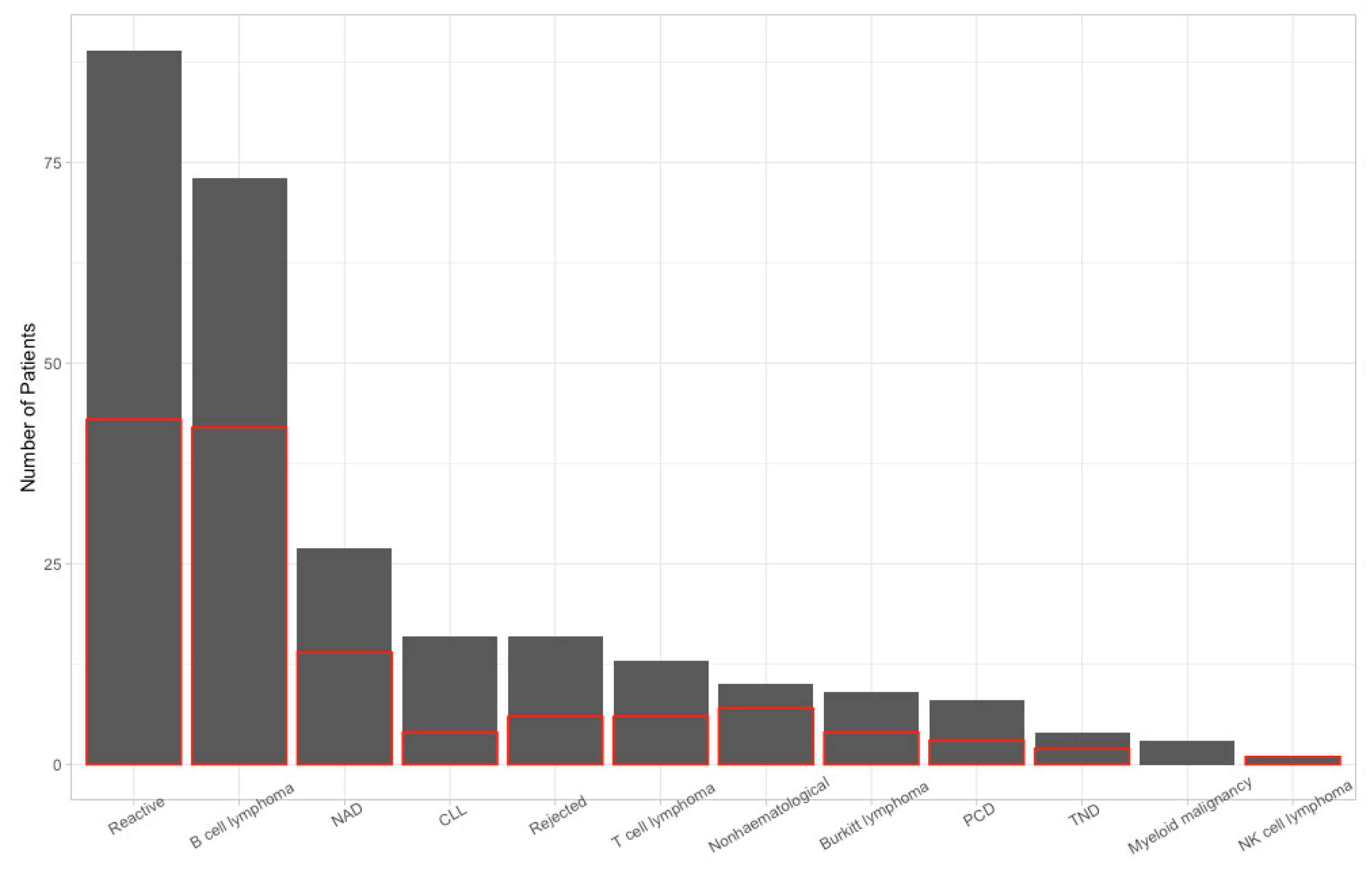
3. Results
4. Discussion
5. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Demurtas, A.; Accinelli, G.; Pacchioni, D.; Godio, L.; Novero, D.; Bussolati, G.; Palestro, G.; Papotti, M.; Stacchini, A. Utility of Flow Cytometry Immunophenotyping in Fine-needle Aspirate Cytologic Diagnosis of Non-Hodgkin Lymphoma: A Series of 252 Cases and Review of the Literature. Appl. Immunohistochem. Mol. Morphol. 2010, 18, 311–322. [Google Scholar] [CrossRef] [PubMed]
- Barrena, S.; Almeida, J.; Del Carmen García-Macias, M.; López, A.; Rasillo, A.; Sayagués, J.M.; Rivas, R.A.; Gutiérrez, M.L.; Ciudad, J.; Flores, T.; et al. Flow cytometry immunophenotyping of fine-needle aspiration specimens: Utility in the diagnosis and classification of non-Hodgkin lymphomas: Utility of FCM analysis on FNA specimens. Histopathology 2011, 58, 906–918. [Google Scholar] [CrossRef] [PubMed]
- Senjug, P.; Trutin Ostović, K.; Miletić, Z.; Tomasović Lončarić, Č.; Štoos-Veić, T.; Gizdić, B.; Kaić, G.; Aralica, G.; Pejša, V.; Jakšić, O. The accuracy of fine needle aspiration cytology and flow cytometry in evaluation of nodal and extranodal sites in patients with suspicion of lymphoma. Coll. Antropol. 2010, 34, 131–137. [Google Scholar] [PubMed]
- Colorado, M.; Cuadrado, M.A.; Insunza, A.; Mazorra, F.; Acinas, O.; Iriondo, A. Simultaneous Cytomorphologic and Multiparametric Flow Cytometric Analysis on Lymph Node Samples Is Faster Than and as Valid as Histopathologic Study to Diagnose Most Non-Hodgkin Lymphomas. Am. J. Clin. Pathol. 2010, 133, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Gunduz, E.; Celebioglu, M.; Meltem Akay, O.; Uskudar Teke, H.; Sahin Mutlu, F.; Gulbas, Z. The role of flow cytometry in the diagnosis of non- Hodgkin’s lymphoma, Hodgkin’s lymphoma, granulomatous inflammation and reactive lymph node specimens. J. BUON 2013, 18, 739–745. [Google Scholar]
- Cozzolino, I.; Rocco, M.; Villani, G.; Picardi, M. Lymph Node Fine-Needle Cytology of Non-Hodgkin Lymphoma: Diagnosis and Classification by Flow Cytometry. Acta Cytol. 2016, 60, 302–314. [Google Scholar] [CrossRef]
- Zeppa, P.; Marino, G.; Troncone, G.; Fulciniti, F.; De Renzo, A.; Picardi, M.; Benincasa, G.; Rotoli, B.; Vetrani, A. Fine-needle cytology and flow cytometry immunophenotyping and subclassification of non-hodgkin lymphoma: A critical review of 307 cases with technical suggestions. Cancer 2003, 102, 55–65. [Google Scholar] [CrossRef]
- Silowash, R.; Pantanowitz, L.; Craig, F.E.; Simons, J.P.; Monaco, S.E. Utilization of Flow Cytometry in Pediatric Fine-Needle Aspiration Biopsy Specimens. Acta Cytol. 2016, 60, 344–353. [Google Scholar] [CrossRef]
- Reddy, D.L.; Venter, W.D.F.; Pather, S. Patterns of Lymph Node Pathology; Fine Needle Aspiration Biopsy as an Evaluation Tool for Lymphadenopathy: A Retrospective Descriptive Study Conducted at the Largest Hospital in Africa. PLoS ONE 2015, 10, e0130148. [Google Scholar] [CrossRef]
- Lumachi, F.; Fassina, A.; Tozzoli, R.; Tregnaghi, A.; Basso, S.M.; Ermani, M. Image-guided fine-needle aspiration cytology and flow cytometry phenotyping of neck lymphadenopathy for the diagnosis of recurrent lymphoma. Clin. Otolaryngol. 2017, 42, 668–672. [Google Scholar] [CrossRef]
- Swart, G.J.; Wright, C.; Brundyn, K.; Mansvelt, E.; du Plessis, M.; ten Oever, D.; Jacobs, P. Fine needle aspiration biopsy and flow cytometry in the diagnosis of lymphoma. Transfus. Apher. Sci. 2007, 37, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Stacchini, A.; Demurtas, A.; Aliberti, S. Extranodal Lymphoproliferative Processes and Flow Cytometry. Acta Cytol. 2016, 60, 315–325. [Google Scholar] [CrossRef] [PubMed]
- Jin, M.; Wakely, P.E. FNA diagnosis of deep-seated lymphoma: An institutional experience. J. Am. Soc. Cytopathol. 2017, 6, 114–119. [Google Scholar] [CrossRef] [PubMed]
- Jin, M.; Wakely, P.E., Jr. Endoscopic/Endobronchial Ultrasound-Guided Fine Needle Aspiration and Ancillary Techniques, Particularly Flow Cytometry, in Diagnosing Deep-Seated Lymphomas. Acta Cytol. 2016, 60, 326–335. [Google Scholar] [CrossRef]
- Choy, B.; Venkataraman, G.; Biernacka, A.; Lastra, R.R.; Mueller, J.; Setia, N.; Reeves, W.; Antic, T. Correlation of cytopathology with flow cytometry and histopathology for the diagnosis of hematologic malignancies in young adults presenting with cervical lymphadenopathy. Diagn. Cytopathol. 2019, 47, 579–583. [Google Scholar] [CrossRef]
- McCroskey, Z.; Khoury, J.D.; Stewart, J.M.; Caraway, N.P. Sensitivity of fine-needle aspiration biopsy for diagnosing and grading follicular lymphomas using a multiparameter approach in a cancer center. J. Am. Soc. Cytopathol. 2017, 6, 80–88. [Google Scholar] [CrossRef]
- Crous, H.; Gillam, A.; Kalokerinos, M.A.; Knezevic, S.; Hobson, P.; Papadimos, D.J.; Shield, P.W. Investigation of lymphoid lesions of the head and neck using combined fine needle aspiration cytology and flow cytometry: Accuracy and pitfalls. Cytopathology 2019, 30, 370–377. [Google Scholar] [CrossRef]
- Grewal, R.; Chetty, M.; Abayomi, E.; Tomuleasa, C.; Fromm, J.R. Use of flow cytometry in the phenotypic diagnosis of Hodgkin’s lymphoma. Cytom. B Clin. Cytom. 2019, 96, 116–127. [Google Scholar] [CrossRef]
- Tarantino, D.R.; McHenry, C.R.; Strickland, T.; Khiyami, A. The role of fine-needle aspiration biopsy and flow cytometry in the evaluation of persistent neck adenopathy. Am. J. Surg. 1998, 176, 413–417. [Google Scholar] [CrossRef]
- Eberle, F.C.; Mani, H.; Jaffe, E.S. Histopathology of Hodgkin’s Lymphoma. Cancer J. 2009, 15, 129–137. [Google Scholar] [CrossRef]
- Scott, G.D.; Lau, H.D.; Kurzer, J.H.; Kong, C.S.; Gratzinger, D.A. Flow immunophenotyping of benign lymph nodes sampled by FNA: Representative with diagnostic pitfalls. Cancer Cytopathol. 2018, 126, 797–808. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.; Chute, D.; Milas, M.; Mitchell, J.; Siperstein, A.; Berber, E. A rare case of chronic lymphocytic leukemia/small lymphocytic lymphoma presenting in the thyroid gland. Thyroid 2010, 20, 1019–1023. [Google Scholar] [CrossRef] [PubMed]
- Bangerter, M.; Brudler, O.; Heinrich, B.; Griesshamnuer, M. Fine needle aspiration cytology and flow cytometry in the diagnosis and subclassification of non-Hodgkin’s lymphoma based on the World Health Organization classification. Acta Cytol. 2007, 51, 390–398. [Google Scholar] [CrossRef] [PubMed]
- Afify, A.; Das, S.; Mingyi, C. Two smalls in one: Coincident small cell carcinoma and small lymphocytic lymphoma in a lymph node diagnosed by fine-needle aspiration biopsy. Cytojournal 2012, 9, 5. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, A.; Vale, S.; Nobre, E.; Barbosa, A.P.; Piloto, E.; Wessling, A.; Mascarenhas, M. Thyroid nodule: First manifestation of chronic lymphocytic leukaemia. Arch Endocrinol. Metab. 2015, 59, 190–194. [Google Scholar] [CrossRef]
- Dunning, K.K.; Wudhikarn, K.; Safo, A.O.; Holman, C.J.; McKenna, R.W.; Pambuccian, S.E. Adrenal extranodal NK/T-cell lymphoma diagnosed by fine-needle aspiration and cerebrospinal fluid cytology and immunophenotyping: A case report. Diagn. Cytopathol. 2009, 37, 686–695. [Google Scholar] [CrossRef]
- Wu, H.H.-J.; Ren, R.; Roepke, J.E. Fine-needle aspiration cytology of blastic natural killer-cell lymphoma (CD4+ CD56+ hematodermic neoplasm). Diagn. Cytopathol. 2004, 30, 268–270. [Google Scholar] [CrossRef]
- Brahimi, M.; Arabi, A.; Soltan, B.E.; Osmani, S.; Benradouane, H.; Bey, M.; Yafour, N.; Benzineb, B.; Attaf, F.; Seddiki, I.; et al. How we assess adequacy of fine-needle aspiration materials intended for flow cytometric analysis. Hematol. Oncol. Stem Cell Ther. 2011, 4, 37–40. [Google Scholar] [CrossRef]
- Eszlinger, M.; Lau, L.; Ghaznavi, S.; Symonds, C.; Chandarana, S.P.; Khalil, M.; Paschke, R. Molecular profiling of thyroid nodule fine-needle aspiration cytology. Nat. Rev. Endocrinol. 2017, 13, 415–424. [Google Scholar] [CrossRef]
- Kitagawa, Y.; Tsunoda, R.; Nanno, M.; Arai, S.; Takai, S. Complementary use of magnetic resonance imaging and fine needle aspiration cytology in the diagnosis of soft tissue tumors. J. Nippon Med. Sch. 2019. [Google Scholar] [CrossRef]
- Capitoli, G.; Piga, I.; Galimberti, S.; Leni, D.; Pincelli, A.I.; Garancini, M.; Clerici, F.; Mahajneh, A.; Brambilla, V.; Smith, A.; et al. MALDI-MSI as a Complementary Diagnostic Tool in Cytopathology: A Pilot Study for the Characterization of Thyroid Nodules. Cancers 2019, 11, 1377. [Google Scholar] [CrossRef]
- Zaharie, F.; Pop, L.A.; Petrushev, B.; Jurj, A.; Muresan, M.S.; Eniu, D.; Fetica, B.; Petkov, B.; Pasca, S.; Piciu, D.; et al. Next-generation sequencing-based characterization of the invasion by anatomical contiguity in a primary osseous diffuse large B-cell lymphoma. Correlation between the genetic profile of the malignancy and the clinical outcome of the patient. Histol. Histopathol. 2019, 34, 663–670. [Google Scholar] [PubMed]
- Colita, A.; Colita, A.; Bumbea, H.; Croitoru, A.; Orban, C.; Lipan, L.E.; Craciun, O.G.; Soare, D.; Ghimici, C.; Manolache, R.; et al. LEAM vs. BEAM vs. CLV Conditioning Regimen for Autologous Stem Cell Transplantation in Malignant Lymphomas. Retrospective Comparison of Toxicity and Efficacy on 222 Patients in the First 100 Days After Transplant, On Behalf of the Romanian Society for Bone Marrow Transplantation. Front. Oncol. 2019, 9, 892. [Google Scholar] [PubMed]
- Grewal, R.; Irimie, A.; Naidoo, N.; Mohamed, N.; Petrushev, B.; Chetty, M.; Tomuleasa, C.; Abayomi, E.A. Hodgkin’s lymphoma and its association with EBV and HIV infection. Crit. Rev. Clin. Lab. Sci. 2018, 55, 102–114. [Google Scholar] [CrossRef] [PubMed]
- Qian, L.; Tomuleasa, C.; Florian, I.A.; Shen, J.; Florian, I.S.; Zdrenghea, M.; Dima, D. Advances in the treatment of newly diagnosed primary central nervous system lymphomas. Blood Res. 2017, 52, 159–166. [Google Scholar] [CrossRef]
- Gafencu, G.A.; Selicean, S.E.; Petrushev, B.; Cucuianu, A.; Dima, D.; Frinc, I.; Irimie, A.; Pileczki, V.; Berindan-Neagoe, I.; Berce, C.; et al. Clinicopathological analysis of a case series of peripheral T-cell lymphomas, not otherwise specified, of lymphoepithelioid variant (Lennert’s lymphoma). A Central European single-center study. Hum. Pathol. 2016, 53, 192–194. [Google Scholar] [CrossRef]
- Brammer, J.E.; Khouri, I.; Gaballa, S.; Anderlini, P.; Tomuleasa, C.; Ahmed, S.; Ledesma, C.; Hosing, C.; Champlin, R.E.; Ciurea, S.O. Outcomes of Haploidentical Stem Cell Transplantation for Lymphoma with Melphalan-Based Conditioning. Biol. Blood Marrow Transplant. 2016, 22, 493–498. [Google Scholar] [CrossRef]
- Grewal, R.; Cucuianu, A.; Swanepoel, C.; Dima, D.; Petrushev, B.; Pop, B.; Berindan-Neagoe, I.; Abayomi, E.A.; Tomuleasa, C. The role of microRNAs in the pathogenesis of HIV-related lymphomas. Crit. Rev. Clin. Lab. Sci. 2015, 52, 232–241. [Google Scholar] [CrossRef]
- Dima, D.; Tomuleasa, C.; Irimie, A.; Florian, I.S.; Petrushev, B.; Berindan-Neagoe, I.; Cucuianu, A. Magnetic resonance imaging-based diagnosis of progressive multifocal leukoencephalopathy in a patient with non-Hodgkin lymphoma after therapy with cyclophosphamide, doxorubicin, vincristine, prednisone, and rituximab. Cancer 2014, 120, 4005–4006. [Google Scholar] [CrossRef] [PubMed]
- Desmirean, M.; Deak, D.; Rus, I.; Dima, D.; Iluta, S.; Preda, A.; Moldovan, T.; Roman, A.; Tomuleasa, C.; Petrushev, B. Paraneoplastic hypereosinophilia in a patient with peripheral T cell lymphoma, not otherwise specified. Med. Pharm. Rep. 2019, 92, 421–426. [Google Scholar] [CrossRef]
- Pratap, S.; Scordino, T.S. Molecular and cellular genetics of non-Hodgkin lymphoma: Diagnostic and prognostic implications. Exp. Mol. Pathol. 2019, 106, 44–51. [Google Scholar] [CrossRef] [PubMed]
- Pasqualucci, L.; Dalla-Favera, R. The Genetic Landscape of Diffuse Large B-Cell Lymphoma. Semin. Hematol. 2015, 52, 67–76. [Google Scholar] [CrossRef] [PubMed]
- Vega, F.; Medeiros, L.J. Chromosomal translocations involved in non-Hodgkin lymphomas. Arch Pathol. Lab. Med. 2003, 127, 1148–1160. [Google Scholar] [PubMed]





| 1. | 2. FITC | 3. PE | 4. PerCP | 5. APC |
|---|---|---|---|---|
| Chronic panel | ||||
| 1 | CD8 | CD4 | CD45 | CD3 |
| 2 | CD5 | CD23 | CD45 | CD19 |
| 3 | CD20 | CD10 | CD45 | CD38 |
| 4 | FMC-7 | CD22 | CD45 | CD200 |
| 5 | Lambda | Kappa | CD45 | CD19 |
| 6 | CD10 | CD34 | CD45 | CD19 |
| Plasma cell panel | ||||
| 1 | CD8 | CD4 | CD45 | CD3 |
| 2 | CD20 | CD79a | CD45 | CD38 |
| 3 | CD56 | CD138 | CD45 | CD38 |
| 4 | CD56 | CD10 | CD45 | CD38 |
| 5 | cLambda | cKappa | CD45 | CD38 |
| Chronic T-cell panel | ||||
| 1 | CD8 | CD4 | CD45 | CD3 |
| 2 | CD5 | CD23 | CD45 | CD19 |
| 3 | CD20 | CD10 | CD45 | CD38 |
| 4 | Lambda | Kappa | CD45 | CD19 |
| 5 | CD7 | CD1a | CD45 | CD2 |
| 6 | CD25 | CD4 | CD45 | CD2 |
| 7 | CD16 | CD30 | CD45 | |
| 8 | CD56 | CD10 | CD45 | CD38 |
| 9 | CD57 | CD8 | CD45 | CD3 |
| Cytoplasmic Markers | ||||
| 10 | cCD79a | CD45 | cCD3 | |
| Acute Leukemia panel | ||||
| 1 | CD8 | CD4 | CD45 | CD3 |
| 2 | CD10 | CD34 | CD45 | CD19 |
| 3 | HLADR | CD33 | CD45 | CD11b |
| 4 | CD7 | CD34 | CD45 | CD2 |
| 5 | CD56 | CD13 | CD45 | CD11b |
| 6 | CD15 | CD117 | CD45 | |
| 7 | CD14 | CD64 | CD45 | |
| Cytoplasmic Markers | ||||
| 1 | cMPO | cCD79a | mCD45 | cCD3 |
| 2 | cIgM | mCD45 | mCD19 | |
| 3 | cTdT control | mCD45 | mCD19 | |
| 4 | cTdT Test | cCD22 | mCD45 | mCD19 |
| Flow Cytometry Diagnosis | Agreement with Histology |
|---|---|
| B-cell lymphoma | 73% |
| Burkitt lymphoma | 66.7% |
| CLL | 100% |
| NAD | 14.3% |
| NK cell lymphoma | 100% |
| Non-hematological | 71.4% |
| PCD | 33.3% |
| Reactive | 29.3% |
| T-cell lymphoma | 66.7% |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Griesel, C.; Desmirean, M.; Esterhuizen, T.; Pasca, S.; Petrushev, B.; Selicean, C.; Roman, A.; Fetica, B.; Teodorescu, P.; Swanepoel, C.; et al. Differential Diagnosis of Malignant Lymphadenopathy Using Flow Cytometry on Fine Needle Aspirate: Report on 269 Cases. J. Clin. Med. 2020, 9, 283. https://doi.org/10.3390/jcm9010283
Griesel C, Desmirean M, Esterhuizen T, Pasca S, Petrushev B, Selicean C, Roman A, Fetica B, Teodorescu P, Swanepoel C, et al. Differential Diagnosis of Malignant Lymphadenopathy Using Flow Cytometry on Fine Needle Aspirate: Report on 269 Cases. Journal of Clinical Medicine. 2020; 9(1):283. https://doi.org/10.3390/jcm9010283
Chicago/Turabian StyleGriesel, Carla, Minodora Desmirean, Tonya Esterhuizen, Sergiu Pasca, Bobe Petrushev, Cristina Selicean, Andrei Roman, Bogdan Fetica, Patric Teodorescu, Carmen Swanepoel, and et al. 2020. "Differential Diagnosis of Malignant Lymphadenopathy Using Flow Cytometry on Fine Needle Aspirate: Report on 269 Cases" Journal of Clinical Medicine 9, no. 1: 283. https://doi.org/10.3390/jcm9010283
APA StyleGriesel, C., Desmirean, M., Esterhuizen, T., Pasca, S., Petrushev, B., Selicean, C., Roman, A., Fetica, B., Teodorescu, P., Swanepoel, C., Tomuleasa, C., & Grewal, R. (2020). Differential Diagnosis of Malignant Lymphadenopathy Using Flow Cytometry on Fine Needle Aspirate: Report on 269 Cases. Journal of Clinical Medicine, 9(1), 283. https://doi.org/10.3390/jcm9010283






