Ipragliflozin Additively Ameliorates Non-Alcoholic Fatty Liver Disease in Patients with Type 2 Diabetes Controlled with Metformin and Pioglitazone: A 24-Week Randomized Controlled Trial
Abstract
1. Introduction
2. Methods
2.1. Study Design
2.2. Laboratory and Imaging Studies
2.3. Outcomes
2.4. Statistical Analysis
3. Results
3.1. Baseline Characteristics of the Study Population
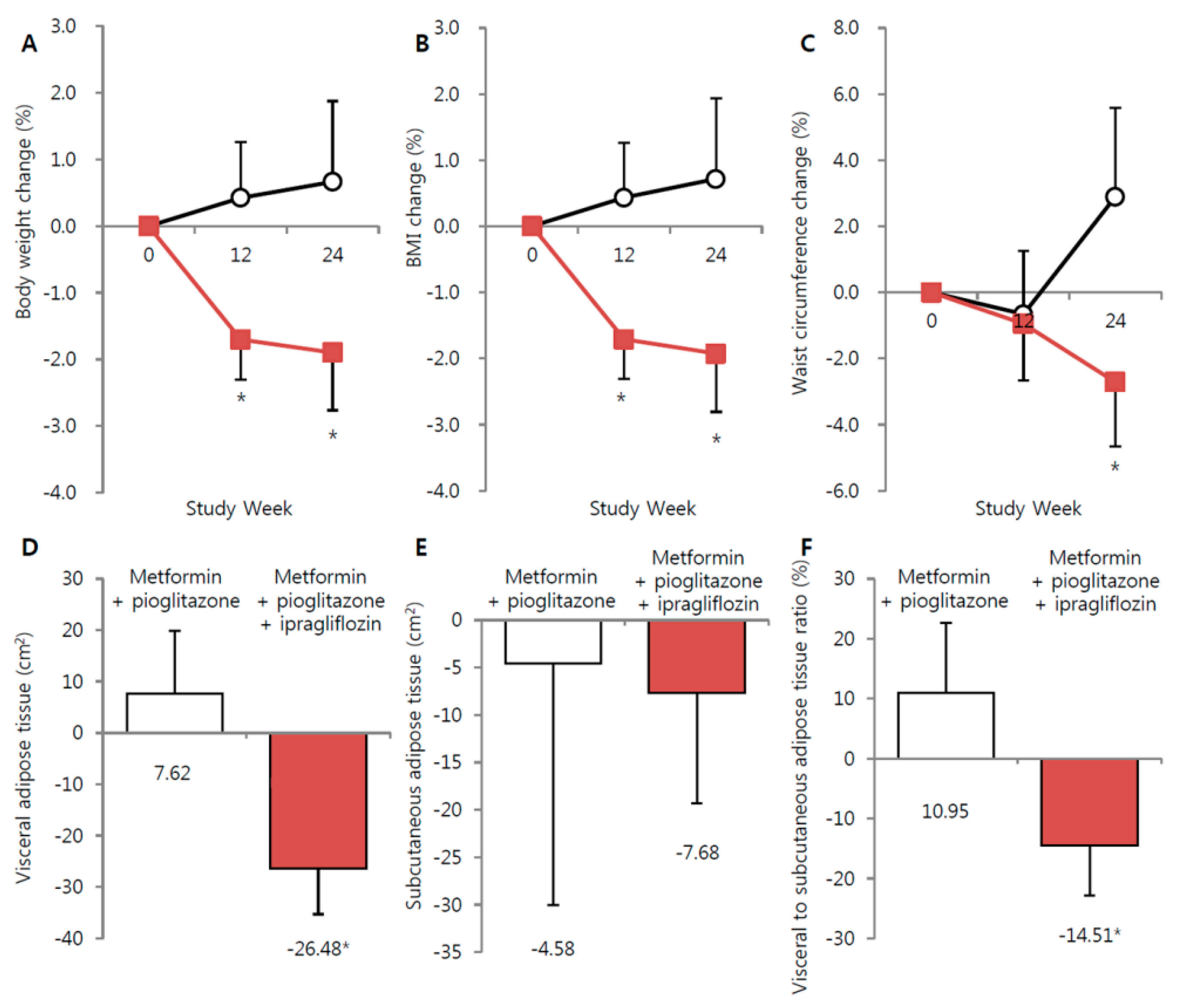
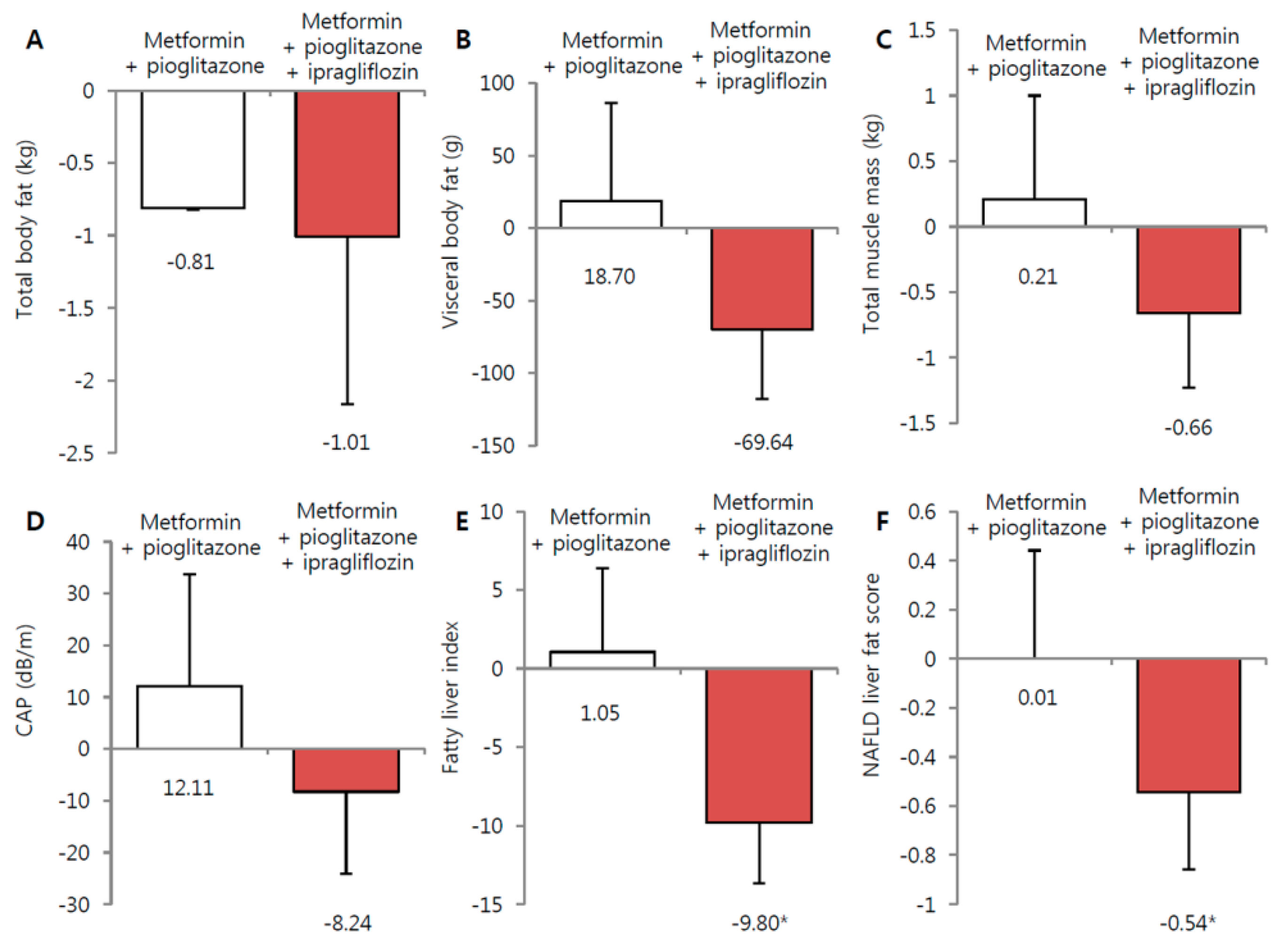
3.2. Effect of Ipragliflozin Add-On Treatment on Body Weight and Fat Depots
3.3. Effect of Ipragliflozin Treatment on NAFLD, Glycemic, and Lipid Parameters
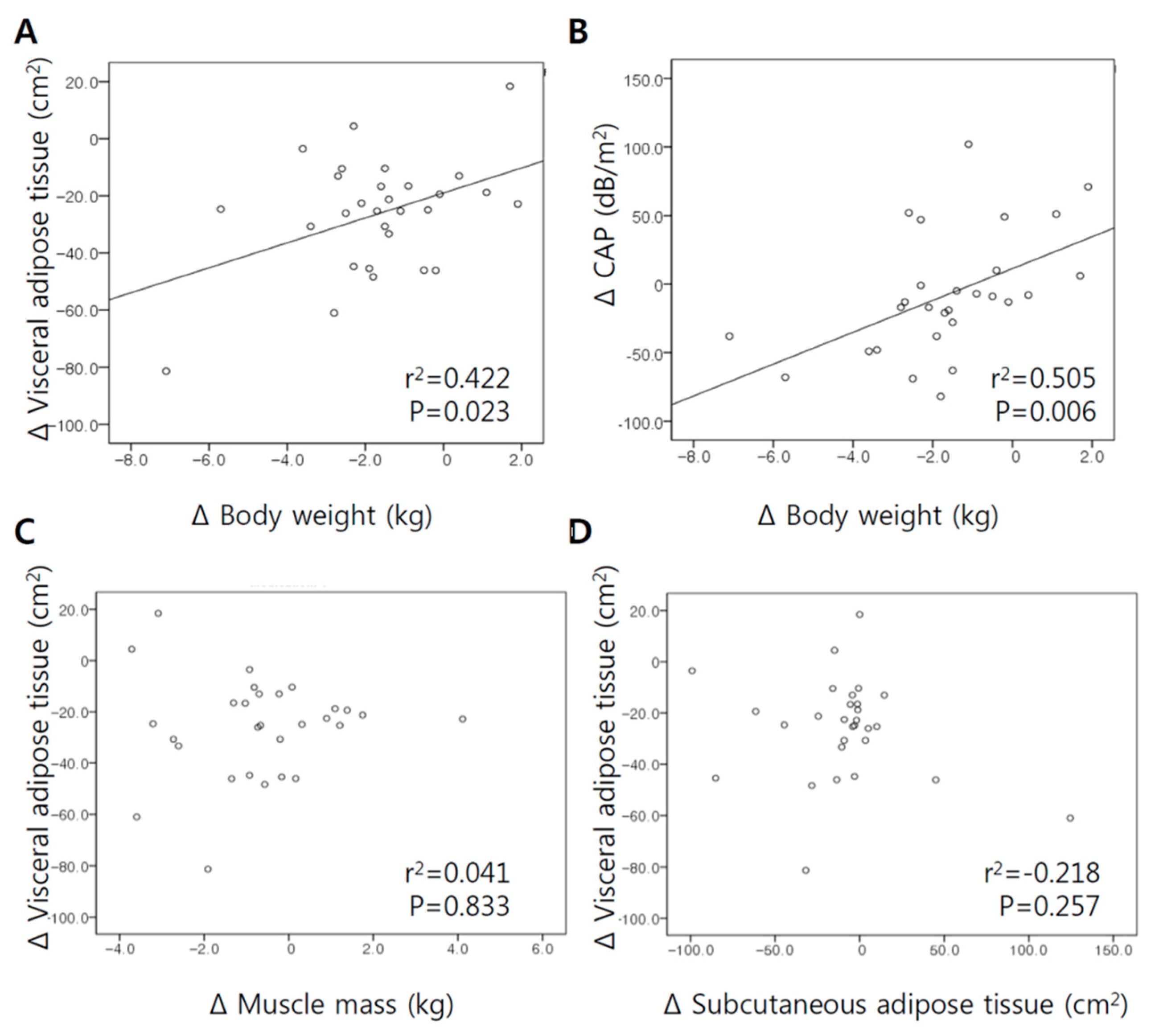
3.4. Correlation between Changes in Body Weight, VFA, and Muscle Mass Following Ipragliflozin Add-On Treatment
3.5. Safety≈
4. Discussion
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| DXA | dual-energy x-ray absorptiometry |
| eGFR | estimated glomerular filtration rate |
| FPG | fasting plasma glucose |
| HbA1c | glycated hemoglobin |
| HDL-C | high-density lipoprotein cholesterol |
| HOMA-IR | homeostasis model assessment of insulin resistance |
| LDL-C | low-density lipoprotein cholesterol |
| NAFLD | non-alcoholic fatty liver disease; |
| SGLT2 | sodium/glucose cotransporter 2; |
| SFA | subcutaneous fat area |
| TZD | thiazolidinedione |
| VFA | visceral fat area |
References
- Tilg, H.; Moschen, A.R.; Roden, M. NAFLD and diabetes mellitus. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 32–42. [Google Scholar] [CrossRef] [PubMed]
- Yoon, H.J.; Lee, Y.-H.; Cha, B.-S. Causal relationship of non-alcoholic fatty liver disease with obesity and insulin resistance. J. Korean Diabetes 2014, 15, 76–81. [Google Scholar] [CrossRef][Green Version]
- Han, E.; Lee, Y.H. Non-Alcoholic Fatty Liver Disease: The Emerging Burden in Cardiometabolic and Renal Diseases. Diabetes Metab. J. 2017, 41, 430–437. [Google Scholar] [CrossRef] [PubMed]
- European Association for the Study of the Liver and European Association for the Study of Diabetes. EASL-EASD-EASO Clinical Practice Guidelines for the management of non-alcoholic fatty liver disease. Obes. Facts 2016, 9, 65–90. [Google Scholar] [CrossRef]
- Lee, Y.H.; Kim, J.H.; Kim, S.R.; Jin, H.Y.; Rhee, E.J.; Cho, Y.M.; Lee, B.W. Lobeglitazone, a Novel Thiazolidinedione, Improves Non-Alcoholic Fatty Liver Disease in Type 2 Diabetes: Its Efficacy and Predictive Factors Related to Responsiveness. J. Korean Med. Sci. 2017, 32, 60–69. [Google Scholar] [CrossRef]
- Han, E.; Jang, S.Y.; Kim, G.; Lee, Y.H.; Choe, E.Y.; Nam, C.M.; Kang, E.S. Rosiglitazone Use and the Risk of Bladder Cancer in Patients With Type 2 Diabetes. Medicine 2016, 95, e2786. [Google Scholar] [CrossRef]
- DeFronzo, R.A.; Chilton, R.; Norton, L.; Clarke, G.; Ryder, R.E.; Abdul-Ghani, M. Revitalization of pioglitazone: The optimum agent to be combined with a sodium-glucose co-transporter-2 inhibitor. Diabetes Obes. Metab. 2016, 18, 454–462. [Google Scholar] [CrossRef]
- Belfort, R.; Harrison, S.A.; Brown, K.; Darland, C.; Finch, J.; Hardies, J.; Balas, B.; Gastaldelli, A.; Tio, F.; Pulcini, J.; et al. A placebo-controlled trial of pioglitazone in subjects with nonalcoholic steatohepatitis. N. Engl. J. Med. 2006, 355, 2297–2307. [Google Scholar] [CrossRef]
- Basu, A.; Jensen, M.D.; McCann, F.; Mukhopadhyay, D.; Joyner, M.J.; Rizza, R.A. Effects of pioglitazone versus glipizide on body fat distribution, body water content, and hemodynamics in type 2 diabetes. Diabetes Care 2006, 29, 510–514. [Google Scholar] [CrossRef]
- Ratziu, V.; Giral, P.; Jacqueminet, S.; Charlotte, F.; Hartemann-Heurtier, A.; Serfaty, L.; Podevin, P.; Lacorte, J.M.; Bernhardt, C.; Bruckert, E.; et al. Rosiglitazone for nonalcoholic steatohepatitis: One-year results of the randomized placebo-controlled Fatty Liver Improvement with Rosiglitazone Therapy (FLIRT) Trial. Gastroenterology 2008, 135, 100–110. [Google Scholar] [CrossRef]
- Sanyal, A.J.; Chalasani, N.; Kowdley, K.V.; McCullough, A.; Diehl, A.M.; Bass, N.M.; Neuschwander-Tetri, B.A.; Lavine, J.E.; Tonascia, J.; Unalp, A.; et al. Pioglitazone, Vitamin E or placebo for nonalcoholic steatohepatitis. N. Engl. J. Med. 2010, 362, 1675–1685. [Google Scholar] [CrossRef]
- Jung, C.H.; Jang, J.E.; Park, J.Y. A Novel Therapeutic Agent for Type 2 Diabetes Mellitus: SGLT2 Inhibitor. Diabetes Metab. J. 2014, 38, 261–273. [Google Scholar] [CrossRef]
- Zinman, B.; Wanner, C.; Lachin, J.M.; Fitchett, D.; Bluhmki, E.; Hantel, S.; Mattheus, M.; Devins, T.; Johansen, O.E.; Woerle, H.J.; et al. Empagliflozin, Cardiovascular Outcomes, and Mortality in Type 2 Diabetes. N. Engl. J. Med. 2015, 373, 2117–2128. [Google Scholar] [CrossRef]
- Neal, B.; Perkovic, V.; Mahaffey, K.W.; de Zeeuw, D.; Fulcher, G.; Erondu, N.; Shaw, W.; Law, G.; Desai, M.; Matthews, D.R. Canagliflozin and Cardiovascular and Renal Events in Type 2 Diabetes. N. Engl. J. Med. 2017, 377, 644–657. [Google Scholar] [CrossRef]
- Han, E.; Shin, E.; Kim, G.; Lee, J.-Y.; Lee, Y.-H.; Lee, B.-W.; Kang, E.S.; Cha, B.S. Combining SGLT2 inhibition with a thiazolidinedione additively attenuate the very early phase of diabetic nephropathy progression in type 2 diabetes mellitus. Front. Endocrinol. 2018, 9, 412. [Google Scholar] [CrossRef]
- Fioretto, P.; Zambon, A.; Rossato, M.; Busetto, L.; Vettor, R. SGLT2 Inhibitors and the Diabetic Kidney. Diabetes Care 2016, 39 (Suppl. S2), S165–S171. [Google Scholar] [CrossRef]
- Han, E.; Kim, A.; Lee, S.J.; Kim, J.Y.; Kim, J.H.; Lee, W.J.; Lee, B.W. Characteristics of Dapagliflozin Responders: A Longitudinal, Prospective, Nationwide Dapagliflozin Surveillance Study in Korea. Diabetes Ther. 2018, 9, 1689–1701. [Google Scholar] [CrossRef]
- Komiya, C.; Tsuchiya, K.; Shiba, K.; Miyachi, Y.; Furuke, S.; Shimazu, N.; Yamaguchi, S.; Kanno, K.; Ogawa, Y. Ipragliflozin Improves Hepatic Steatosis in Obese Mice and Liver Dysfunction in Type 2 Diabetic Patients Irrespective of Body Weight Reduction. PLoS ONE 2016, 11, e0151511. [Google Scholar] [CrossRef]
- Min, K.W.; Ku, B.J.; Lee, J.H.; Kim, M.S.; Ahn, K.J.; Lee, M.K.; Kokubo, S.; Yoshida, S.; Cho, H.J.; Cha, B.S. Addition of Ipragliflozin to Metformin Treatment in Korean Patients with Type 2 Diabetes Mellitus: Subgroup Analysis of a Phase 3 Trial. Diabetes Metab. J. 2017, 41, 135–145. [Google Scholar] [CrossRef]
- Chon, Y.E.; Jung, K.S.; Kim, S.U.; Park, J.Y.; Park, Y.N.; Kim, D.Y.; Ahn, S.H.; Chon, C.Y.; Lee, H.W.; Park, Y.; et al. Controlled attenuation parameter (CAP) for detection of hepatic steatosis in patients with chronic liver diseases: A prospective study of a native Korean population. Liver Int. 2014, 34, 102–109. [Google Scholar] [CrossRef]
- Bedogni, G.; Bellentani, S.; Miglioli, L.; Masutti, F.; Passalacqua, M.; Castiglione, A.; Tiribelli, C. The Fatty Liver Index: A simple and accurate predictor of hepatic steatosis in the general population. BMC Gastroenterol. 2006, 6, 33. [Google Scholar] [CrossRef]
- Kotronen, A.; Peltonen, M.; Hakkarainen, A.; Sevastianova, K.; Bergholm, R.; Johansson, L.M.; Lundbom, N.; Rissanen, A.; Ridderstråle, M.; Groop, L.; et al. Prediction of non-alcoholic fatty liver disease and liver fat using metabolic and genetic factors. Gastroenterology 2009, 137, 865–872. [Google Scholar] [CrossRef]
- Lee, J.W.; Lee, H.R.; Shim, J.Y.; Im, J.A.; Kim, S.H.; Choi, H.; Lee, D.C. Viscerally obese women with normal body weight have greater brachial-ankle pulse wave velocity than nonviscerally obese women with excessive body weight. Clin. Endocrinol. 2007, 66, 572–578. [Google Scholar] [CrossRef]
- Lee, J.Y.; Lee, H.S.; Lee, D.C.; Chu, S.H.; Jeon, J.Y.; Kim, N.K.; Lee, J.W. Visceral fat accumulation is associated with colorectal cancer in postmenopausal women. PLoS ONE 2014, 9, e110587. [Google Scholar] [CrossRef]
- Radholm, K.; Figtree, G.; Perkovic, V.; Solomon, S.D.; Mahaffey, K.W.; de Zeeuw, D.; Fulcher, G.; Barrett, T.D.; Shaw, W.; Desai, M.; et al. Canagliflozin and Heart Failure in Type 2 Diabetes Mellitus: Results From the CANVAS Program. Circulation 2018, 138, 458–468. [Google Scholar] [CrossRef]
- Forst, T.; Guthrie, R.; Goldenberg, R.; Yee, J.; Vijapurkar, U.; Meininger, G.; Stein, P. Efficacy and safety of canagliflozin over 52 weeks in patients with type 2 diabetes on background metformin and pioglitazone. Diabetes Obes. Metab. 2014, 16, 467–477. [Google Scholar] [CrossRef]
- Kovacs, C.S.; Seshiah, V.; Swallow, R.; Jones, R.; Rattunde, H.; Woerle, H.J.; Broedl, U.C. Empagliflozin improves glycaemic and weight control as add-on therapy to pioglitazone or pioglitazone plus metformin in patients with type 2 diabetes: A 24-week, randomized, placebo-controlled trial. Diabetes Obes. Metab. 2014, 16, 147–158. [Google Scholar] [CrossRef]
- Kovacs, C.S.; Seshiah, V.; Merker, L.; Christiansen, A.V.; Roux, F.; Salsali, A.; Kim, G.; Stella, P.; Woerle, H.J. Empagliflozin as Add-on Therapy to Pioglitazone with or without Metformin in Patients with Type 2 Diabetes Mellitus. Clin. Ther. 2015, 37, 1773–1788. [Google Scholar] [CrossRef]
- Honda, Y.; Imajo, K.; Kato, T.; Kessoku, T.; Ogawa, Y.; Tomeno, W.; Kato, S.; Mawatari, H.; Fujita, K.; Yoneda, M.; et al. The Selective SGLT2 Inhibitor Ipragliflozin Has a Therapeutic Effect on Nonalcoholic Steatohepatitis in Mice. PLoS ONE 2016, 11, e0146337. [Google Scholar] [CrossRef]
- Watanabe, Y.; Nakayama, K.; Taniuchi, N.; Horai, Y.; Kuriyama, C.; Ueta, K.; Arakawa, K.; Senbonmatsu, T.; Shiotani, M. Beneficial effects of canagliflozin in combination with pioglitazone on insulin sensitivity in rodent models of obese type 2 diabetes. PLoS ONE 2015, 10, e0116851. [Google Scholar] [CrossRef]
- Bolinder, J.; Ljunggren, O.; Johansson, L.; Wilding, J.; Langkilde, A.M.; Sjostrom, C.D.; Sugg, J.; Parikh, S. Dapagliflozin maintains glycaemic control while reducing weight and body fat mass over 2 years in patients with type 2 diabetes mellitus inadequately controlled on metformin. Diabetes Obes. Metab. 2014, 16, 159–169. [Google Scholar] [CrossRef] [PubMed]
- Bolinder, J.; Ljunggren, O.; Kullberg, J.; Johansson, L.; Wilding, J.; Langkilde, A.M.; Sugg, J.; Parikh, S. Effects of dapagliflozin on body weight, total fat mass, and regional adipose tissue distribution in patients with type 2 diabetes mellitus with inadequate glycemic control on metformin. J. Clin. Endocrinol. Metab. 2012, 97, 1020–1031. [Google Scholar] [CrossRef] [PubMed]
- Tahara, A.; Kurosaki, E.; Yokono, M.; Yamajuku, D.; Kihara, R.; Hayashizaki, Y.; Takasu, T.; Imamura, M.; Li, Q.; Tomiyama, H.; et al. Effects of sodium-glucose cotransporter 2 selective inhibitor ipragliflozin on hyperglycaemia, oxidative stress, inflammation and liver injury in streptozotocin-induced type 1 diabetic rats. J. Pharm. Pharmacol. 2014, 66, 975–987. [Google Scholar] [CrossRef] [PubMed]
- Seko, Y.; Sumida, Y.; Sasaki, K.; Itoh, Y.; Iijima, H.; Hashimoto, T.; Ishii, S.; Inagaki, N. Effects of canagliflozin, an SGLT2 inhibitor, on hepatic function in Japanese patients with type 2 diabetes mellitus: Pooled and subgroup analyses of clinical trials. J. Gastroenterol. 2018, 53, 140–151. [Google Scholar] [CrossRef]
- Leiter, L.A.; Forst, T.; Polidori, D.; Balis, D.A.; Xie, J.; Sha, S. Effect of canagliflozin on liver function tests in patients with type 2 diabetes. Diabetes Metab. 2016, 42, 25–32. [Google Scholar] [CrossRef]
| Parameters | Metformin + Pioglitazone (n = 15) | Metformin + Pioglitazone + Ipragliflozin (n = 30) | p-Value |
|---|---|---|---|
| Age (years) | 56.7 ± 11.8 | 52.5 ± 10.3 | 0.233 |
| Sex (male), N (%) | 9 (60.0) | 19 (63.3) | >0.999 |
| Diabetes duration (years) | 10.1 ± 5.6 | 9.1 ± 6.0 | 0.563 |
| Waist circumference (cm) | 100.5 ± 7.7 | 102.1 ± 12.2 | 0.606 |
| Weight (kg) | 81.4 ± 8.5 | 84.2 ± 16.9 | 0.471 |
| BMI (kg/m2) | 30.2 ± 2.5 | 30.4 ± 5.4 | 0.871 |
| SBP (mmHg) | 124.9 ±9.2 | 125.3 ± 11.1 | 0.889 |
| DBP (mmHg) | 74.0 ± 9.0 | 75.6 ± 10.0 | 0.605 |
| HTN, N (%) | 11 (73.3) | 18 (60.0) | 0.378 |
| Dyslipidemia, N (%) | 15 (100.0) | 29 (96.7) | >0.999 |
| FPG (mg/dL) | 118.4 ± 19.7 | 120.1 ± 21.8 | 0.790 |
| HbA1c (%) | 6.6 ± 0.6 | 6.7 ± 0.7 | 0.592 |
| HbA1c (mmol/mol) | 48.2 ± 6.4 | 49.4 ± 7.5 | 0.598 |
| HOMA-IR * | 3.4 ± 2.8 | 2.7 ± 1.8 | 0.351 |
| HOMA-β (%) * | 73.5 ± 38.0 | 69.0 ± 59.4 | 0.382 |
| Total cholesterol (mg/dL) | 170.3 ± 21.7 | 186.7 ± 34.9 | 0.110 |
| HDL cholesterol (mg/dL) | 49.9 ± 11.9 | 50.2 ± 13.3 | 0.960 |
| Triglycerides (mg/dL) | 152.5 ± 91.8 | 161.3 ± 66.3 | 0.551 |
| LDL cholesterol (mg/dL) | 89.8 ± 17.3 | 104.2 ± 28.8 | 0.103 |
| WBC (103/μL) | 6.9 ± 1.1 | 7.1 ± 2.0 | 0.661 |
| Hemoglobin (mg/dL) | 13.7 ± 1.5 | 14.1 ± 1.4 | 0.408 |
| Platelet (109/L) * | 239.5 ± 52.4 | 257.3 ± 59.1 | 0.398 |
| Creatinine (mg/dL) | 0.8 ± 0.2 | 0.7 ± 0.2 | 0.381 |
| eGFR, EPI (mL/min/1.73 m2) | 94.8 ± 10.1 | 101.9 ± 11.8 | 0.053 |
| Uric acid (mg/dL) | 5.3 ± 1.3 | 5.5 ± 1.5 | 0.692 |
| Albumin (g/dL) | 4.3 ± 0.3 | 4.4 ± 0.2 | 0.633 |
| γ-GT (IU/L) * | 31.8 ± 15.6 | 40.5 ± 47.0 | 0.677 |
| AST (IU/L) * | 30.4 ± 19.6 | 26.3 ± 12.9 | 0.433 |
| ALT (IU/L) * | 31.1 ± 13.5 | 32.9 ± 24.8 | 0.779 |
| Transient elastography, CAP (dB/m) | 307.7 ± 37.0 | 305.5 ± 39.5 | 0.858 |
| NAFLD score | |||
| Fatty liver index | 66.5 ± 18.7 | 68.2 ± 21.3 | 0.786 |
| NAFLD liver fat score | 0.9 ± 1.4 | 0.6 ± 1.5 | 0.518 |
| Abdominal fat CT scan | |||
| VFA (cm2) | 223.3 ± 90.8 | 203.0 ± 55.0 | 0.528 |
| SFA (cm2) | 230.1 ± 80.6 | 213.3 ± 111.0 | 0.336 |
| Ratio of VFA to SFA (%) | 49.0 ± 11.4 | 46.1 ± 12.2 | 0.422 |
| DXA scan | |||
| Total fat mass (kg) | 25.0 ± 7.7 | 24.7 ± 7.0 | 0.869 |
| Total fat ratio (%) | 29.6 ± 7.7 | 29.6 ± 6.5 | 0.857 |
| Estimated visceral fat (g) | 664.1 ± 190.5 | 690.8 ± 169.1 | 0.600 |
| Total muscle mass (kg) | 55.8 ± 9.9 | 56.6 ± 12.1 | 0.832 |
| Metformin + Pioglitazone (n = 15) | Metformin + Pioglitazone + Ipragliflozin (n = 29) | p-Value | |
|---|---|---|---|
| Total VAT (g) | |||
| Baseline | 664.1 ± 190.5 | 698.0 ± 167.6 | 0.547 |
| Week 24 | 686.4 ± 185.3 | 626.4 ± 198.9 | 0.338 |
| Changes from baseline | 22.3 ± 40.1 | −71.5 ± 21.5 * | 0.029 |
| Total fat (kg) | |||
| Baseline | 25.0 ± 7.6 | 25.8 ± 8.0 | 0.766 |
| Week 24 | 24.3 ± 5.6 | 24.7 ± 8.2 | 0.849 |
| Changes from baseline | 0.7 ± 1.3 | −1.0 ± 0.3 * | 0.774 |
| Total muscle (kg) | |||
| Baseline | 55.8 ± 9.9 | 56.6 ± 12.1 | 0.832 |
| Week 24 | 56.1 ± 1.0 | 55.9 ± 11.4 | 0.970 |
| Changes from baseline | 0.2 ± 0.3 | −0.8 ± 0.3 * | 0.079 |
| VFA (cm2) | |||
| Baseline | 223.3 ± 90.8 | 209.1 ± 63.3 | 0.546 |
| Week 24 | 230.3 ± 87.6 | 182.9 ± 63.7 | 0.046 |
| Changes from baseline | 7.0 ± 7.7 | −26.2 ± 3.7 ** | <0.001 |
| SFA (cm2) | |||
| Baseline | 230.1 ± 80.6 | 267.5 ± 115.4 | 0.269 |
| Week 24 | 228.7 ± 90.0 | 258.2 ± 99.7 | 0.341 |
| Changes from baseline | −1.4 ± 5.0 | −9.3 ± 7.2 | 0.460 |
| CAP (dB/m) | |||
| Baseline | 307.7 ± 37.0 | 306.6 ± 39.8 | 0.928 |
| Week 24 | 319.5 ± 44.8 | 298.6 ± 45.2 | 0.207 |
| Changes from baseline | 11.7 ± 12.1 | −8.0 ± 8.5 | 0.182 |
| Body weight (kg) | |||
| Baseline | 81.4 ± 8.5 | 84.3 ± 17.2 | 0.470 |
| Week 12 | 81.8 ± 8.4 | 82.8 ± 17.1 | 0.780 |
| Week 24 | 81.9 ± 7.6 | 82.6 ± 16.9 | 0.854 |
| Changes from baseline | 0.4 ± 0.6 | −1.6 ± 0.4 ** | 0.003 |
| BMI (kg/m2) | |||
| Baseline | 30.2 ± 2.5 | 30.6 ± 5.3 | 0.734 |
| Week 12 | 30.3 ± 2.5 | 30.1 ± 5.4 | 0.850 |
| Week 24 | 30.4 ± 2.6 | 30.1 ± 5.3 | 0.745 |
| Changes from baseline | 0.2 ± 0.2 | −0.6 ± 0.1 ** | 0.001 |
| Waist circumference (cm) | |||
| Baseline | 100.5 ± 7.7 | 102.4 ± 12.3 | 0.542 |
| Week 12 | 99.9 ± 8.4 | 101.2 ± 11.4 | 0.698 |
| Week 24 | 100.0 ± 8.2 | 99.2 ± 11.6 | 0.815 |
| Changes from baseline | 0.5 ± 0.7 | −3.2 ± 0.8 * | 0.038 |
| SBP (mmHg) | |||
| Baseline | 124.9 ± 9.2 | 125.8 ± 11.1 | 0.790 |
| Week 12 | 121.7 ± 9.6 | 124.6 ± 9.2 | 0.337 |
| Week 24 | 128.1 ± 9.6 | 125.1 ± 10.8 | 0.382 |
| Changes from baseline | 3.2 ± 2.0 | −0.6 ± 2.1 | 0.242 |
| DBP (mmHg) | |||
| Baseline | 74.0 ± 9.0 | 75.5 ± 10.2 | 0.622 |
| Week 12 | 72.1 ± 7.8 | 77.8 ± 9.9 | 0.062 |
| Week 24 | 75.0 ± 8.5 | 77.7 ± 8.5 | 0.449 |
| Changes from baseline | 1.6 ± 2.5 | 2.1 ± 1.6 | 0.868 |
| FPG (mg/dL) | |||
| Baseline | 118.4 ± 19.7 | 121.2 ± 21.3 | 0.674 |
| Week 12 | 140.7 ± 39.2 * | 125.5 ± 20.6 | 0.180 |
| Week 24 | 116.3 ± 20.9 | 117.5 ± 19.8 | 0.846 |
| Changes from baseline | −2.1 ± 8.0 | −3.7 ± 3.4 | 0.835 |
| HbA1c (%) | |||
| Baseline | 6.6 ± 0.6 | 6.7 ± 0.7 | 0.595 |
| Week 12 | 6.9 ± 0.9 | 6.4 ± 0.5 | 0.035 |
| Week 24 | 6.8 ± 0.7 * | 6.5 ± 0.7 | 0.287 |
| Changes from baseline | 0.2 ± 0.1 | −0.1 ± 0.2 * | 0.129 |
| HOMA-IR | |||
| Baseline | 3.4 ± 2.8 | 2.7 ± 1.8 | 0.273 |
| Week 24 | 3.5 ± 2.5 | 2.2 ± 1.4 | 0.050 |
| Changes from baseline | 0.1 ± 0.8 | −0.5 ± 0.2 * | 0.400 |
| HOMA-β | |||
| Baseline | 73.5 ± 38.0 | 66.6 ± 59.0 | 0.182 |
| Week 24 | 112.7 ± 151.1 | 55.7 ± 41.2 | 0.039 |
| Changes from baseline | 39.1 ± 37.4 | −10.9 ± 8.4 | 0.212 |
| AST (IU/L) | |||
| Baseline | 30.4 ± 19.6 | 26.6 ± 13.0 | 0.485 |
| Week 12 | 28.5 ± 14.4 | 27.8 ± 16.8 | 0.781 |
| Week 24 | 24.7 ± 10.0 | 24.3 ± 10.6 | 0.845 |
| Changes from baseline | 5.7 ± 3.2 | 2.3 ± 1.1 | 0.323 |
| ALT (IU/L) | |||
| Baseline | 31.1 ± 13.5 | 33.4 ± 25.1 | 0.839 |
| Week 12 | 27.3 ± 8.8 | 31.0 ± 20.9 | 0.781 |
| Week 24 | 26.5 ± 11.8 | 25.6 ± 16.9 | 0.511 |
| Changes from baseline | −4.7 ± 2.9 | −7.8 ± 2.6 ** | 0.458 |
| γ-GT (IU/L) | |||
| Baseline | 31.8 ± 15.6 | 40.9 ± 47.8 | 0.671 |
| Week 24 | 29.9 ± 13.8 | 29.7 ± 24.6 | 0.510 |
| Changes from baseline | −1.9 ± 2.0 | −11.2 ± 4.9 ** | 0.189 |
| Total cholesterol (mg/dL) | |||
| Baseline | 170.3 ± 21.7 | 187.1 ± 35.4 | 0.109 |
| Week 12 | 170.9 ± 30.1 | 183.4 ± 33.2 | 0.260 |
| Week 24 | 158.4 ± 23.2 | 184.6 ± 35.6 | 0.012 |
| Changes from baseline | −11.9 ± 5.8 | −2.5 ± 3.6 | 0.158 |
| HDL (mg/dL) | |||
| Baseline | 50.0 ± 11.9 | 50.7 ± 13.2 | 0.853 |
| Week 12 | 51.0 ± 16.2 | 51.1 ± 12.2 | 0.742 |
| Week 24 | 49.2 ± 13.0 | 52.4 ± 10.6 | 0.271 |
| Changes from baseline | −0.7 ± 1.0 | 1.7 ± 1.6 | 0.302 |
| Triglyceride (mg/dL) | |||
| Baseline | 152.5 ± 91.8 | 159.7 ± 66.9 | 0.612 |
| Week 12 | 177.0 ± 135.5 | 166.5 ± 99.1 | 0.825 |
| Week 24 | 163.5 ± 106.2 | 149.0 ± 56.4 | 0.744 |
| Changes from baseline | 11.0 ± 13.9 | −10.8 ± 11.6 | 0.258 |
| LDL (mg/dL) | |||
| Baseline | 91.3 ± 16.9 | 102.8 ± 28.9 | 0.106 |
| Week 12 | 92.0 ± 19.4 | 100.8 ± 29.1 | 0.469 |
| Week 24 | 82.0 ± 20.8 | 102.4 ± 29.4 | 0.024 |
| Changes from baseline | −9.3 ± 3.7 ** | 2.1 ± 2.7 | 0.130 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, E.; Lee, Y.-h.; Lee, B.-W.; Kang, E.S.; Cha, B.-S. Ipragliflozin Additively Ameliorates Non-Alcoholic Fatty Liver Disease in Patients with Type 2 Diabetes Controlled with Metformin and Pioglitazone: A 24-Week Randomized Controlled Trial. J. Clin. Med. 2020, 9, 259. https://doi.org/10.3390/jcm9010259
Han E, Lee Y-h, Lee B-W, Kang ES, Cha B-S. Ipragliflozin Additively Ameliorates Non-Alcoholic Fatty Liver Disease in Patients with Type 2 Diabetes Controlled with Metformin and Pioglitazone: A 24-Week Randomized Controlled Trial. Journal of Clinical Medicine. 2020; 9(1):259. https://doi.org/10.3390/jcm9010259
Chicago/Turabian StyleHan, Eugene, Yong-ho Lee, Byung-Wan Lee, Eun Seok Kang, and Bong-Soo Cha. 2020. "Ipragliflozin Additively Ameliorates Non-Alcoholic Fatty Liver Disease in Patients with Type 2 Diabetes Controlled with Metformin and Pioglitazone: A 24-Week Randomized Controlled Trial" Journal of Clinical Medicine 9, no. 1: 259. https://doi.org/10.3390/jcm9010259
APA StyleHan, E., Lee, Y.-h., Lee, B.-W., Kang, E. S., & Cha, B.-S. (2020). Ipragliflozin Additively Ameliorates Non-Alcoholic Fatty Liver Disease in Patients with Type 2 Diabetes Controlled with Metformin and Pioglitazone: A 24-Week Randomized Controlled Trial. Journal of Clinical Medicine, 9(1), 259. https://doi.org/10.3390/jcm9010259





