The Immunomodulary Effects of Systematic Exercise in Older Adults and People with Parkinson’s Disease
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Participants
2.2. Assessment Procedure
2.3. Ethical Approval
2.4. Anthropometric Measurements and Body Composition Assessment
2.5. Balance Training with Moderate-Intensity Exercise
Heart Rate and Estimation of Maximal Heart Rate
2.6. Postural Control Measurement
2.7. Blood Sampling
2.8. Measurements of Biochemical Parameters
2.9. Statistical Analysis
3. Results
3.1. Characteristics of the Participants
3.2. Baseline Characteristics of Postural Control
3.3. Training Effects on Postural Control
3.4. Baseline Characteristics of Biochemical Indicators
3.5. Training Effects on Biochemical Indicators
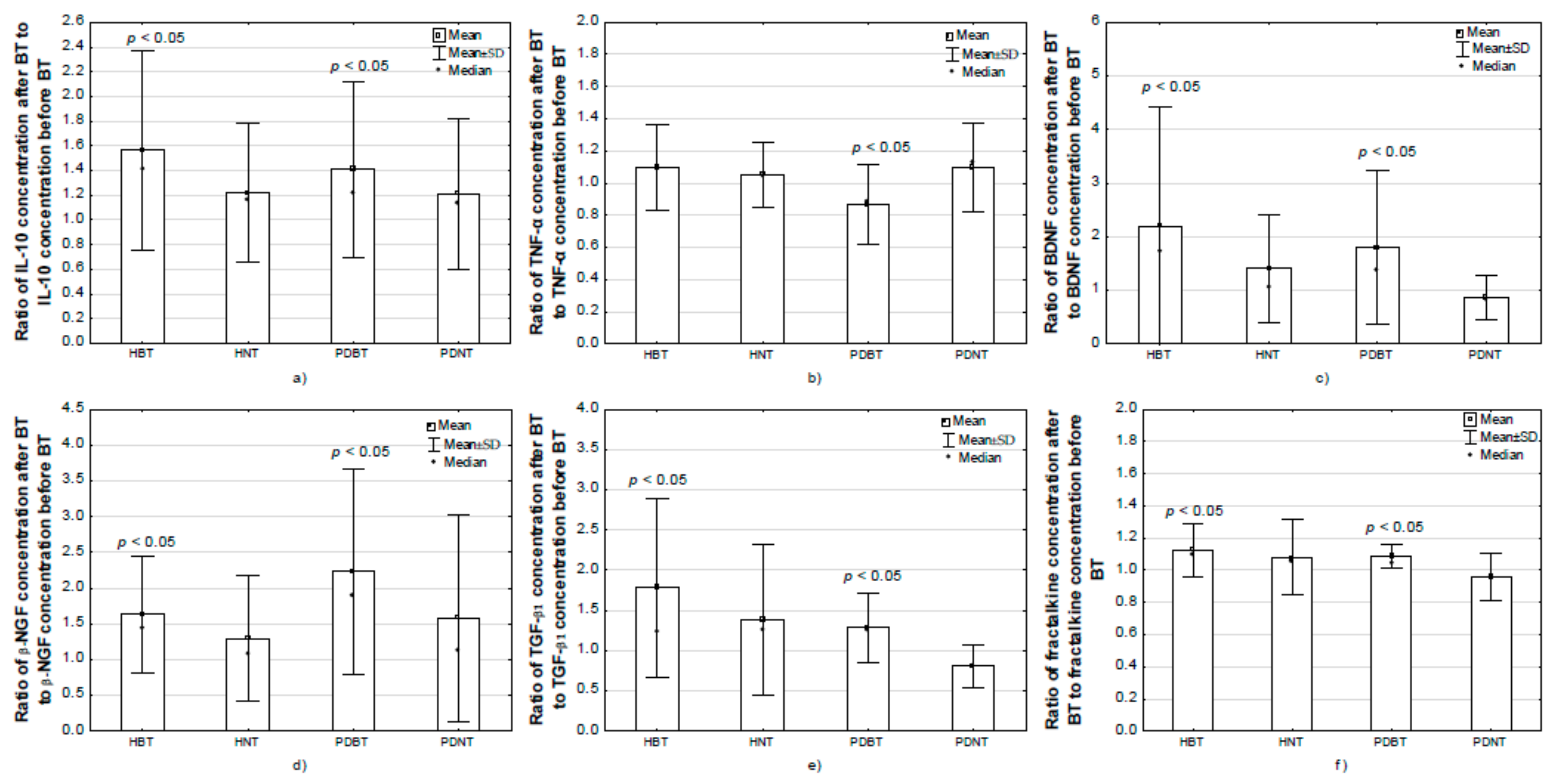
3.5.1. Training Effects on Plasma Interleukin-6 Concentration
3.5.2. Training Effects on Plasma Interleukin-10 Concentration
3.5.3. Training Effects on Plasma TNF-α Concentration
3.5.4. Training Effects on Serum BDNF Concentration
3.5.5. Training Effects on Serum β-NGF Concentration
3.5.6. Training Effects on Serum TGF-β1 Concentration
3.5.7. Training Effects on Serum IGF-1 Concentration
3.5.8. Training Effects on Serum CD200 Concentration
3.5.9. Training Effects on Serum Fractalkine Concentration
3.6. Correlations
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Abd El-Kader, S.M.; Al-Shreef, F.M. Inflammatory cytokines and immune system modulation by aerobic versus resisted exercise training for elderly. Afr. Health Sci. 2018, 18, 120–131. [Google Scholar] [CrossRef] [PubMed]
- Franceschi, C.; Garagnani, P.; Parini, P.; Giuliani, C.; Santoro, A. Inflammaging: A new immune—Metabolic viewpoint for age-related diseases. Nat. Rev. Endocrinol. 2018, 14, 576–590. [Google Scholar] [CrossRef] [PubMed]
- Maier, S.F. Bi-directional immune-brain communication: Implications for understanding stress, pain, and cognition. Brain Behav. Immun. 2003, 17, 69–85. [Google Scholar] [CrossRef]
- Perry, V.H.; Teeling, J. Microglia and macrophages of the central nervous system: The contribution of microglia priming and systemic inflammation to chronic neurodegeneration. Semin. Immunopathol. 2013, 35, 601–612. [Google Scholar] [CrossRef] [PubMed]
- Mee-inta, O.; Zhao, Z.W.; Kuo, Y.M. Physical Exercise Inhibits Inflammation and Microglial Activation. Cells 2019, 8, 691. [Google Scholar] [CrossRef] [PubMed]
- Moehle, M.S.; West, A.B. M1 and M2 immune activation in Parkinson’s Disease: Foe and ally? Neuroscience 2015, 302, 59–73. [Google Scholar] [CrossRef]
- Łabuzek, K.; Skrudlik, E.; Gabryel, B.; Okopień, B. Anti-inflammatory microglial cell function in the light of the latest scientific research. Ann. Acad. Medicae Silesiensis 2015, 69, 99–110. [Google Scholar] [CrossRef]
- Cardona, A.E.; Pioro, E.P.; Sasse, M.E.; Kostenko, V.; Cardona, S.M.; Dijkstra, I.M.; Huang, D.R.; Kidd, G.; Dombrowski, S.; Dutta, R.; et al. Control of microglial neurotoxicity by the fractalkine receptor. Nat. Neurosci. 2006, 9, 917–924. [Google Scholar] [CrossRef]
- Lauro, C.; Catalano, M.; Trettel, F.; Limatola, C. Fractalkine in the nervous system: Neuroprotective or neurotoxic molecule? Ann. N. Y. Acad. Sci. 2015, 1351, 141–148. [Google Scholar] [CrossRef]
- Mosley, R.L.; Hutter-Saunders, J.A.; Stone, D.K.; Gendelman, H.E. Inflammation and adaptive immunity in Parkinson’s disease. Cold Spring Harb. Perspect. Med. 2012, 2. [Google Scholar] [CrossRef]
- Mogi, M.; Harada, M.; Riederer, P.; Narabayashi, H.; Fujita, K.; Nagatsu, T. Tumor necrosis factor-α (TNF-α) increases both in the brain and in the cerebrospinal fluid from parkinsonian patients. Neurosci. Lett. 1994, 165, 208–210. [Google Scholar] [CrossRef]
- Lange-Asschenfeldt, C.; Kojda, G. Alzheimer’s disease, cerebrovascular dysfunction and the benefits of exercise: From vessels to neurons. Exp. Gerontol. 2008, 43, 499–504. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Cabrera, M.C.; Domenech, E.; Viña, J. Moderate exercise is an antioxidant: Upregulation of antioxidant genes by training. Free Radic. Biol. Med. 2008, 44, 126–131. [Google Scholar] [CrossRef] [PubMed]
- Simioni, C.; Zauli, G.; Martelli, A.M.; Vitale, M.; Sacchetti, G.; Gonelli, A.; Neri, L.M. Oxidative stress: Role of physical exercise and antioxidant nutraceuticals in adulthood and aging. Oncotarget 2018, 9, 17181–17198. [Google Scholar] [CrossRef] [PubMed]
- Febbraio, M.A.; Pedersen, B.K. Muscle-derived interleukin-6: Mechanisms for activation and possible biological roles. FASEB J. 2002, 16, 1335–1347. [Google Scholar] [CrossRef] [PubMed]
- Gruol, D.L. IL-6 regulation of synaptic function in the CNS. Neuropharmacology 2015, 96, 42–54. [Google Scholar] [CrossRef]
- Pedersen, B.K.; Steensberg, A.; Fischer, C.; Keller, C.; Ostrowski, K.; Schjerling, P. Exercise and cytokines with particular focus on muscle-derived il-6. Exerc. Immunol. Rev. 2001, 7, 18–31. [Google Scholar]
- Steinbacher, P.; Eckl, P. Impact of oxidative stress on exercising skeletal muscle. Biomolecules 2015, 5, 356–377. [Google Scholar] [CrossRef]
- Spielman, L.J.; Little, J.P.; Klegeris, A. Physical activity and exercise attenuate neuroinflammation in neurological diseases. Brain Res. Bull. 2016, 125, 19–29. [Google Scholar] [CrossRef]
- Rall, L.C.; Roubenoff, R.; Cannon, J.G.; Abad, L.W.; Dinarello, C.A.; Meydani, S.N. Effects of progressive resistance training on immune response in aging and chronic inflammation. Med. Sci. Sports Exerc. 1996, 28, 1356–1365. [Google Scholar] [CrossRef]
- Bermon, S.; Philip, P.; Candito, M.; Ferrari, P.; Dolisi, C. Effects of strength exercise and training on the natural killer cell counts in elderly humans. J. Sports Med. Phys. Fit. 2001, 41, 196–202. [Google Scholar]
- El-Kader, S.M.A.; Al-Jiffri, O.H. Aerobic exercise modulates cytokine profile and sleep quality in elderly. Afr. Health Sci. 2019, 19, 2198–2207. [Google Scholar] [CrossRef] [PubMed]
- Libardi, C.A.; De Souza, G.V.; Cavaglieri, C.R.; Madruga, V.A.; Chacon-Mikahil, M.P.T. Effect of resistance, endurance, and concurrent training on TNF-α, IL-6, and CRP. Med. Sci. Sports Exerc. 2012, 44, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Zoladz, J.A.; Pilc, A. The effect of physical activity on the brain derived neurotrophic factor: From animal to human studies. J. Physiol. Pharmacol. 2010, 61, 533–541. [Google Scholar] [PubMed]
- Seifert, T.; Brassard, P.; Wissenberg, M.; Rasmussen, P.; Nordby, P.; Stallknecht, B.; Adser, H.; Jakobsen, A.H.; Pilegaard, H.; Nielsen, H.B.; et al. Endurance training enhances BDNF release from the human brain. AJP Regul. Integr. Comp. Physiol. 2010, 298, R372–R377. [Google Scholar] [CrossRef]
- Radak, Z.; Toldy, A.; Szabo, Z.; Siamilis, S.; Nyakas, C.; Silye, G.; Jakus, J.; Goto, S. The effects of training and detraining on memory, neurotrophins and oxidative stress markers in rat brain. Neurochem. Int. 2006, 49, 387–392. [Google Scholar] [CrossRef]
- Seidler, R.D.; Bernard, J.A.; Burutolu, T.B.; Fling, B.W.; Gordon, M.T.; Gwin, J.T.; Kwak, Y.; Lipps, D.B. Motor control and Aging: Links to age-related brain structural, functional and biomechanical effects. Neurosci. Biobehav. Rev. 2010, 34, 721–733. [Google Scholar] [CrossRef]
- Yitayeh, A.; Teshome, A. The effectiveness of physiotherapy treatment on balance dysfunction and postural instability in persons with Parkinson’s disease: A systematic review and meta-analysis. BMC Sports Sci. Med. Rehabil. 2016, 8, 17. [Google Scholar] [CrossRef]
- Lacroix, A.; Hortobágyi, T.; Beurskens, R.; Granacher, U. Effects of Supervised vs. Unsupervised Training Programs on Balance and Muscle Strength in Older Adults: A Systematic Review and Meta-Analysis. Sports Med. 2017, 47, 2341–2361. [Google Scholar] [CrossRef]
- Thomas, E.; Battaglia, G.; Patti, A.; Brusa, J.; Leonardi, V.; Palma, A.; Bellafiore, M. Physical activity programs for balance and fall prevention in elderly: A systematic review. Medicine 2019, 98. [Google Scholar] [CrossRef]
- Kubica, J.; Szymura, J.; Domagalik, A.; Golda, S.; Wiecek, M.; Fafrowicz, M.; Marek, T.; Pera, J. Systematic Balance Exercises Influence Cortical Activation and Serum BDNF Levels in Older Adults. J. Clin. Med. 2019, 8, 1910. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, H.; Monahan, K.D.; Seals, D.R. Age-predicted maximal heart rate revisited. J. Am. Coll. Cardiol. 2001, 37, 153–156. [Google Scholar] [CrossRef]
- Tinetti, M.E. Performance-Oriented Assessment of Mobility Problems in Elderly Patients. J. Am. Geriatr. Soc. 1986, 34, 119–126. [Google Scholar] [CrossRef] [PubMed]
- Di Liegro, C.M.; Schiera, G.; Proia, P.; Di Liegro, I. Physical activity and brain health. Genes 2019, 10, 720. [Google Scholar] [CrossRef]
- Valdiglesias, V.; Sánchez-Flores, M.; Maseda, A.; Lorenzo-López, L.; Marcos-Pérez, D.; López-Cortón, A.; Strasser, B.; Fuchs, D.; Laffon, B.; Millán-Calenti, J.C.; et al. Immune Biomarkers in Older Adults: Role of Physical Activity. J. Toxicol. Environ. Health A 2017, 80, 605–620. [Google Scholar] [CrossRef]
- Simpson, R.J.; Kunz, H.; Agha, N.; Graff, R. Exercise and the Regulation of Immune Functions. In Progress in Molecular Biology and Translational Science; Academic Press: Cambridge, MA, USA, 2015; Volume 135, pp. 355–380. [Google Scholar]
- Nieman, D.C.; Henson, D.A. Role of endurance exercise in immune senescence. Med. Sci. Sports Exerc. 1994, 26, 172–181. [Google Scholar] [CrossRef]
- Nieman, D.C.; Wentz, L.M. The compelling link between physical activity and the body’s defense system. J. Sport Health Sci. 2019, 8, 201–217. [Google Scholar] [CrossRef]
- Krüger, K.; Mooren, F.C.; Eder, K.; Ringseis, R. Immune and Inflammatory Signaling Pathways in Exercise and Obesity. Am. J. Lifestyle Med. 2016, 10, 268–279. [Google Scholar] [CrossRef]
- Kelly, N.A.; Wood, K.H.; Allendorfer, J.B.; Ford, M.P.; Bickel, C.S.; Marstrander, J.E.; Amara, A.W.; Anthony, T.; Bamman, M.M.; Skidmore, F.M. High-Intensity Exercise Acutely Increases Substantia Nigra and Prefrontal Brain Activity in Parkinson’s Disease. Med. Sci. Monit. 2017, 23, 6064–6071. [Google Scholar] [CrossRef][Green Version]
- Villoslada, P.; Genain, C.P. Role of nerve growth factor and other trophic factors in brain inflammation. Prog. Brain Res. 2004, 146, 403–414. [Google Scholar]
- Ding, Q.; Vaynman, S.; Akhavan, M.; Ying, Z.; Gomez-Pinilla, F. Insulin-like growth factor I interfaces with brain-derived neurotrophic factor-mediated synaptic plasticity to modulate aspects of exercise-induced cognitive function. Neuroscience 2006, 140, 823–833. [Google Scholar] [CrossRef] [PubMed]
- Berg, U.; Bang, P. Exercise and circulating insulin-like growth factor I. Horm. Res. Paediatr. 2004, 62, 50–58. [Google Scholar] [CrossRef] [PubMed]
- Zoladz, J.A.; Majerczak, J.; Zeligowska, E.; Mencel, J.; Jaskolski, A.; Jaskolska, A.; Marusiak, J. Moderate-intensity interval training increases serum brain-derived neurotrophic factor level and decreases inflammation in parkinson’s disease patients. J. Physiol. Pharmacol. 2014, 65, 441–448. [Google Scholar] [PubMed]
- De Coelho, F.G.M.; Gobbi, S.; Andreatto, C.A.A.; Corazza, D.I.; Pedroso, R.V.; Santos-Galduróz, R.F. Physical exercise modulates peripheral levels of brain-derived neurotrophic factor (BDNF): A systematic review of experimental studies in the elderly. Arch. Gerontol. Geriatr. 2013, 56, 10–15. [Google Scholar] [CrossRef] [PubMed]
- Nofuji, Y.; Suwa, M.; Sasaki, H.; Ichimiya, A.; Nishichi, R.; Kumagai, S. Different Circulating Brain-Derived Neurotrophic Factor Responses to Acute Exercise between Physically Active and Sedentary Subjects. J. Sports Sci. Med. 2012, 11, 83–88. [Google Scholar]
- Zhang, J.; Yao, W.; Hashimoto, K. Brain-derived Neurotrophic Factor (BDNF)-TrkB Signaling in Inflammation-related Depression and Potential Therapeutic Targets. Curr. Neuropharmacol. 2016, 14, 721–731. [Google Scholar] [CrossRef]
- Sellami, M.; Gasmi, M.; Denham, J.; Hayes, L.D.; Stratton, D.; Padulo, J.; Bragazzi, N. Effects of acute and chronic exercise on immunological parameters in the elderly aged: Can physical activity counteract the effects of aging? Front. Immunol. 2018, 9. [Google Scholar] [CrossRef]
- Dextera, D.T.; Jenner, P. Parkinson disease: From pathology to molecular disease mechanisms. Free Radic. Biol. Med. 2013, 62, 132–144. [Google Scholar] [CrossRef]
- Bachiller, S.; Jiménez-Ferrer, I.; Paulus, A.; Yang, Y.; Swanberg, M.; Deierborg, T.; Boza-Serrano, A. Microglia in neurological diseases: A road map to brain-disease dependent-inflammatory response. Front. Cell. Neurosci. 2018, 12, 488. [Google Scholar] [CrossRef]
- Butovsky, O.; Jedrychowski, M.P.; Moore, C.S.; Cialic, R.; Lanser, A.J.; Gabriely, G.; Koeglsperger, T.; Dake, B.; Wu, P.M.; Doykan, C.E.; et al. Identification of a unique TGF-β-dependent molecular and functional signature in microglia. Nat. Neurosci. 2014, 17, 131–143. [Google Scholar] [CrossRef]
- Zhou, X.; Spittau, B.; Krieglstein, K. TGFβ signalling plays an important role in IL4-induced alternative activation of microglia. J. Neuroinflamm. 2012, 9, 210. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Liu, Z.; Cao, B.B.; Qiu, Y.H.; Peng, Y.P. TGF-β1 Neuroprotection via Inhibition of Microglial Activation in a Rat Model of Parkinson’s Disease. J. Neuroimmune Pharmacol. 2017, 12, 433–446. [Google Scholar] [CrossRef] [PubMed]
- Antunes, B.M.; Campos, E.Z.; dos Santos, R.V.T.; Rosa-Neto, J.C.; Franchini, E.; Bishop, N.C.; Lira, F.S. Anti-inflammatory response to acute exercise is related with intensity and physical fitness. J. Cell. Biochem. 2019, 120, 5333–5342. [Google Scholar] [CrossRef]
- Peake, J.M.; Gatta, P.D.; Suzuki, K.; Nieman, D.C. Cytokine expression and secretion by skeletal muscle cells: Regulatory mechanisms and exercise effects. Exerc. Immunol. Rev. 2015, 21, 8–25. [Google Scholar] [PubMed]
- Steensberg, A.; Fischer, C.P.; Keller, C.; Møller, K.; Klarlund Pedersen, B. IL-10, and cortisol in humans. Am. J. Physiol. Endocrinol. Metab. 2003, 285, 433–437. [Google Scholar] [CrossRef]
- Chen, T.C.C.; Chen, H.L.; Pearce, A.J.; Nosaka, K. Attenuation of eccentric exercise-induced muscle damage by preconditioning exercises. Med. Sci. Sports Exerc. 2012, 44, 2090–2098. [Google Scholar] [CrossRef]
- Lauro, C.; Chece, G.; Monaco, L.; Antonangeli, F.; Peruzzi, G.; Rinaldo, S.; Paone, A.; Cutruzzolà, F.; Limatola, C. Fractalkine Modulates Microglia Metabolism in Brain Ischemia. Front. Cell. Neurosci. 2019, 13, 414. [Google Scholar] [CrossRef]
- Pabon, M.M.; Bachstetter, A.D.; Hudson, C.E.; Gemma, C.; Bickford, P.C. CX3CL1 reduces neurotoxicity and microglial activation in a rat model of Parkinson’s disease. J. Neuroinflamm. 2011, 8. [Google Scholar] [CrossRef]
- Lauro, C.; Catalano, M.; Di Paolo, E.; Chece, G.; de Costanzo, I.; Trettel, F.; Limatola, C. Fractalkine/CX3CL1 engages different neuroprotective responses upon selective glutamate receptor overactivation. Front. Cell. Neurosci. 2015, 8. [Google Scholar] [CrossRef]
- Walker, D.G.; Dalsing-Hernandez, J.E.; Campbell, N.A.; Lue, L.F. Decreased expression of CD200 and CD200 receptor in Alzheimer’s disease: A potential mechanism leading to chronic inflammation. Exp. Neurol. 2009, 215, 5–19. [Google Scholar] [CrossRef]
- Van Henriette, P. Exercise and the brain: Something to chew on. Rev. Bras. Oncol. Clínica 2013, 32, 141–147. [Google Scholar]
- Protas, E.J.; Stanley, R.K.; Jankovic, J.; MacNeill, B. Cardiovascular and metabolic responses to upper- and lower-extremity exercise in men with idiopathic Parkinson’s disease. Phys. Ther. 1996, 76, 34–40. [Google Scholar] [CrossRef] [PubMed][Green Version]
| Variables | PDBT (n = 16) | PDNT (n = 13) | HBT (n = 16) | HNT (n = 16) | All PD (n = 29) | All H (n = 32) | All BT (n = 32) | All NT (n = 29) |
|---|---|---|---|---|---|---|---|---|
| H&Y scale (stage: 2/3) | 9/7 | 9/4 | 18/11 | |||||
| Gender (M/F) | 11/5 | 8/5 | 10/6 | 10/6 | 19/10 | 20/12 | 21/11 | 18/11 |
| Age (years) | 66.00 ± 2.59 | 65.23 ± 7.40 | 67.25 ± 2.52 | 65.69 ± 3.70 | 65.66 ± 7.48 | 66.47 ± 3.21 | 66.63 ± 7.44 | 65.48 ± 5.55 |
| Body height (cm) | 166.55 ± 7.84 | 164.88 ± 8.28 | 165.17 ± 8.99 | 169.78 ± 9.38 | 165.80 ± 7.94 | 167.09 ± 9.24 | 165.86 ± 8.32 | 167.16 ± 8.99 |
| Body mass (kg) | 81.76 ± 12.29 | 77.18 ± 14.17 | 75.09 ± 12.61 | 80.08 ± 15.01 | 79.71 ± 13.13 | 77.58 ± 13.87 | 78.43 ± 12.71 | 78.78 ± 14.46 |
| BMI (kg/m2) | 29.49 ± 4.02 | 28.20 ± 3.72 | 27.33 ± 2.16 | 29.10 (25.29–30.35) | 28.91 ± 3.87 | 27.45 (26.10–29.30) | 28.41 ± 3.36 | 29.00 (25.90–30.60) |
| Baseline Characteristic | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Variables | Training | PDBT (n = 16) | PDNT (n = 13) | HBT (n = 16) | HNT (n = 16) | GROUP | F (p) BT | GROUP × BT | All PD (n = 29) | All Healthy (n = 32) |
| POMA (points) | pre BT | 21.38 ± 2.25 ‡§ | 21.31 ± 2.59 #† | 25.94 ± 1.57 | 27.31 ± 1.14 | 58.05 | 29.05 | 12.72 | 21.34 ± 2.36 a | 26.63 ± 1.52 |
| after BT | 23.13 ± 2.16 *‡§& | 20.62 ± 2.66 #† | 28.00 ± 0.00 * | 27.88 ± 0.62 | (<0.01) | (<0.01) | (<0.01) | |||
| Baseline Characteristic | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Variables | Training | PDBT (n = 16) | PDNT (n = 13) | HBT (n = 16) | HNT (n = 16) | GROUP | F (p) BT | GROUP × BT | All PD (n = 29) | All Healthy (n = 32) |
| IL-6 (pg/mL) | pre BT | 2.08 ± 1.31 | 2.13 ± 0.81 | 2.51 ± 1.20 | 2.56 ± 1.27 | 1.36 | 0.84 | 0.55 | 2.10 ± 1.10 | 2.54 ± 1.21 |
| after BT | 2.27 ± 1.14 | 2.21 ± 1.51 | 2.39 ± 0.95 | 3.06 ± 1.44 | (0.26) | (0.36) | (0.64) | |||
| IL-10 (pg/mL) | pre BT | 2.75 ± 1.09 | 2.59 ± 1.52 | 2.82 ± 1.67 | 2.71 ± 1.15 | 1.08 | 10.05 | 2.41 | 2.68 ± 1.28 | 2.77 ± 1.41 |
| after BT | 3.76 ± 2.29 * | 2.69 ± 1.36 † | 4.42 ± 3.42 * | 2.92 ± 0.95 $ | (0.36) | (<0.01) | (0.07) | |||
| TNF-α (pg/mL) | pre BT | 1.06 ± 0.28 | 0.90 ± 0.33 | 0.97 ± 0.19 | 1.23 ± 0.39 | 4.09 | 0.17 | 2.84 | 0.99 ± 0.31 | 1.10 ± 0.33 |
| after BT | 0.89 ± 0.30 *‡ | 0.92 ± 0.19 # | 1.06 ± 0.29 $ | 1.27 ± 0.45 | (0.01) | (0.89) | (0.04) | |||
| BDNF (ng/mL) | pre BT | 21.19 ± 8.36 | 30.08 ± 8.04 | 20.21 ± 13.33 | 28.10 ± 12.33 | 0.51 | 11.52 | 4.56 | 25.17 ± 9.24 | 24.28 ± 13.23 |
| after BT | 30.37 ± 6.33 * | 25.78 ± 11.72 | 34.98 ± 20.62 * | 33.13 ± 17.74 | (0.67) | (<0.01) | (0.01) | |||
| β-NGF (pg/mL) | pre BT | 109.42 ± 87.14 ‡§ | 114.73 ± 66.06 †# | 310.42 ± 156.88 | 345.00 ± 162.32 | 12.92 | 10.14 | 1.58 | 111.80 ± 77.11 a | 327.71 ± 158.01 |
| after BT | 224.83 ± 194.20 *‡ § | 131.36 ± 104.00 †# | 459.36 ± 212.27 * | 385.05 ± 219.94 | (<0.01) | (<0.01) | (0.20) | |||
| TGF-β1 (ng/mL) | pre BT | 21.54 ± 5.27 | 27.04 ± 7.10 | 19.21 ± 8.22 | 23.46 ± 14.17 | 0.14 | 3.36 | 4.65 | 24.01 ± 6.65 | 21.34 ± 11.59 |
| after BT | 27.88 ± 11.02 * | 21.45 ± 7.60 | 26.97 ± 7.25 * | 25.22 ± 7.30 | (0.93) | (0.06) | (<0.01) | |||
| IGF-I (ng/mL) | pre BT | 180.62 ± 51.60 | 183.20 ± 65.44 | 143.68 ± 37.78 | 163.78 ± 46.03 | 1.80 | 1.86 | 2.00 | 181.78 ± 57.13 a | 153.73 ± 42.67 |
| after BT | 211.36 ± 117.61 | 180.04 ± 69.46 | 165.21 ± 40.90 | 151.94 ± 37.98 | (0.15) | (0.17) | (0.12) | |||
| CD200 (pg/mL) | pre BT | 102.86 ± 77.20 | 128.89 ± 84.31 | 91.39 ± 78.69 | 89.43 ± 69.63 | 0.59 | 0.00 | 0.45 | 114.53 ± 80.08 | 90.41 ± 73.01 |
| after BT | 101.75 ± 44.22 | 115.25 ± 84.21 | 105 ± 73 ± 97.18 | 87.69 ± 67.58 | (0.62) | (0.99) | (0.72) | |||
| Fractalkine (ng/mL) | pre BT | 0.44 ± 0.06 | 0.47 ± 0.11 | 0.42 ± 0.11 | 0.42 ± 0.11 | 0.50 | 5.20 | 3.04 | 0.46 ± 0.09 | 0.42 ± 0.11 |
| after BT | 0.48 ± 0.07 * | 0.45 ± 0.13 | 0.48 ± 0.15 * | 0.43 ± 0.08 | (0.68) | (0.02) | (0.03) | |||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szymura, J.; Kubica, J.; Wiecek, M.; Pera, J. The Immunomodulary Effects of Systematic Exercise in Older Adults and People with Parkinson’s Disease. J. Clin. Med. 2020, 9, 184. https://doi.org/10.3390/jcm9010184
Szymura J, Kubica J, Wiecek M, Pera J. The Immunomodulary Effects of Systematic Exercise in Older Adults and People with Parkinson’s Disease. Journal of Clinical Medicine. 2020; 9(1):184. https://doi.org/10.3390/jcm9010184
Chicago/Turabian StyleSzymura, Jadwiga, Jadwiga Kubica, Magdalena Wiecek, and Joanna Pera. 2020. "The Immunomodulary Effects of Systematic Exercise in Older Adults and People with Parkinson’s Disease" Journal of Clinical Medicine 9, no. 1: 184. https://doi.org/10.3390/jcm9010184
APA StyleSzymura, J., Kubica, J., Wiecek, M., & Pera, J. (2020). The Immunomodulary Effects of Systematic Exercise in Older Adults and People with Parkinson’s Disease. Journal of Clinical Medicine, 9(1), 184. https://doi.org/10.3390/jcm9010184





