Prognostic Abilities of Serial Neuron-Specific Enolase and Lactate and their Combination in Cardiac Arrest Survivors During Targeted Temperature Management
Abstract
1. Introduction
2. Methods
2.1. Setting and Study Population
2.2. TTM Protocol
2.3. Data Collection and Outcomes
2.4. Statistical Analyses
3. Results and Discussion
4. Conclusions
Author Contributions
Conflicts of Interest
References
- Hawkes, M.A.; Rabinstein, A.A. Neurological prognostication after cardiac arrest in the era of target temperature management. Curr. Neurol. Neurosci. Rep. 2019, 19, 10. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Kim, M.J.; You, J.S.; Lee, H.S.; Park, Y.S.; Park, I.; Chung, S.P. Multimodal approach for neurologic prognostication of out-of-hospital cardiac arrest patients undergoing targeted temperature management. Resuscitation 2019, 134, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Wihersaari, L.; Tiainen, M.; Skrifvars, M.B.; Bendel, S.; Kaukonen, K.M.; Vaahersalo, J.; Romppanen, J.; Pettila, V.; Reinikainen, M.; Finnresusci study group. Usefulness of neuron specific enolase in prognostication after cardiac arrest: Impact of age and time to ROSC. Resuscitation 2019, 139, 214–221. [Google Scholar] [CrossRef]
- Jung, C.; Bueter, S.; Wernly, B.; Masyuk, M.; Saeed, D.; Albert, A.; Fuernau, G.; Kelm, M.; Westenfeld, R. Lactate clearance predicts good neurological outcomes in cardiac arrest patients treated with extracorporeal cardiopulmonary resuscitation. J. Clin. Med. 2019, 8, 374. [Google Scholar] [CrossRef] [PubMed]
- Callaway, C.W.; Donnino, M.W.; Fink, E.L.; Geocadin, R.G.; Golan, E.; Kern, K.B.; Leary, M.; Meurer, W.J.; Peberdy, M.A.; Thompson, T.M.; et al. Part 8: Post-cardiac arrest care: 2015 American Heart Association Guidelines Update for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation 2015, 132, S465–S482. [Google Scholar] [CrossRef]
- Hassager, C.; Nagao, K.; Hildick-Smith, D. Out-of-hospital cardiac arrest: In-hospital intervention strategies. Lancet 2018, 391, 989–998. [Google Scholar] [CrossRef]
- Park, J.H.; Wee, J.H.; Choi, S.P.; Oh, J.H.; Cheol, S. Assessment of serum biomarkers and coagulation/fibrinolysis markers for prediction of neurological outcomes of out of cardiac arrest patients treated with therapeutic hypothermia. Clin. Exp. Emerg. Med. 2019, 6, 9–18. [Google Scholar] [CrossRef]
- Martinez-Losas, P.; Lopez de Sa, E.; Armada, E.; Rosillo, S.; Monedero, M.C.; Rey, J.R.; Caro-Codon, J.; Buno Soto, A.; Lopez Sendon, J.L. Neuron-specific enolase kinetics: An additional tool for neurological prognostication after cardiac arrest. Rev. Esp. Cardiol. 2019. [Google Scholar] [CrossRef]
- Rossetti, A.O.; Rabinstein, A.A.; Oddo, M. Neurological prognostication of outcome in patients in coma after cardiac arrest. Lancet Neurol. 2016, 15, 597–609. [Google Scholar] [CrossRef]
- Stammet, P.; Collignon, O.; Hassager, C.; Wise, M.P.; Hovdenes, J.; Aneman, A.; Horn, J.; Devaux, Y.; Erlinge, D.; Kjaergaard, J.; et al. Neuron-specific enolase as a predictor of death or poor neurological outcome after out-of-hospital cardiac arrest and targeted temperature management at 33 degrees C and 36 degrees C. J. Am. Coll. Cardiol. 2015, 65, 2104–2114. [Google Scholar] [CrossRef]
- Zandbergen, E.G.; Hijdra, A.; Koelman, J.H.; Hart, A.A.; Vos, P.E.; Verbeek, M.M.; de Haan, R.J.; PROPAC Study Group. Prediction of poor outcome within the first 3 days of postanoxic coma. Neurology 2006, 66, 62–68. [Google Scholar] [CrossRef] [PubMed]
- Sandroni, C.; Cariou, A.; Cavallaro, F.; Cronberg, T.; Friberg, H.; Hoedemaekers, C.; Horn, J.; Nolan, J.P.; Rossetti, A.O.; Soar, J. Prognostication in comatose survivors of cardiac arrest: An advisory statement from the European Resuscitation Council and the European Society of Intensive Care Medicine. Resuscitation 2014, 85, 1779–1789. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B.C.; Zhang, Z.; Zhu, J.J.; Liu, L.J.; Liu, C.F. Blood lactate or lactate clearance: Which is robust to predict the neurological outcomes after cardiac arrest? A systematic review and meta-analysis. BioMed Res. Int. 2018, 2018, 8014213. [Google Scholar] [CrossRef] [PubMed]
- Donnino, M.W.; Andersen, L.W.; Giberson, T.; Gaieski, D.F.; Abella, B.S.; Peberdy, M.A.; Rittenberger, J.C.; Callaway, C.W.; Ornato, J.; Clore, J.; et al. Initial lactate and lactate change in post-cardiac arrest: A multicenter validation study. Crit. Care Med. 2014, 42, 1804–1811. [Google Scholar] [CrossRef]
- Donnino, M.W.; Miller, J.; Goyal, N.; Loomba, M.; Sankey, S.S.; Dolcourt, B.; Sherwin, R.; Otero, R.; Wira, C. Effective lactate clearance is associated with improved outcome in post-cardiac arrest patients. Resuscitation 2007, 75, 229–234. [Google Scholar] [CrossRef]
- Kliegel, A.; Losert, H.; Sterz, F.; Holzer, M.; Zeiner, A.; Havel, C.; Laggner, A.N. Serial lactate determinations for prediction of outcome after cardiac arrest. Medicine (Baltim.) 2004, 83, 274–279. [Google Scholar] [CrossRef]
- Booth, C.M.; Boone, R.H.; Tomlinson, G.; Detsky, A.S. Is this patient dead, vegetative, or severely neurologically impaired? Assessing outcome for comatose survivors of cardiac arrest. JAMA 2004, 291, 870–879. [Google Scholar] [CrossRef]
- Shinozaki, K.; Oda, S.; Sadahiro, T.; Nakamura, M.; Hirayama, Y.; Abe, R.; Tateishi, Y.; Hattori, N.; Shimada, T.; Hirasawa, H. S-100B and neuron-specific enolase as predictors of neurological outcome in patients after cardiac arrest and return of spontaneous circulation: A systematic review. Crit. Care 2009, 13, R121. [Google Scholar] [CrossRef]
- Marangos, P.J.; Schmechel, D.E. Neuron specific enolase, a clinically useful marker for neurons and neuroendocrine cells. Annu. Rev. Neurosci. 1987, 10, 269–295. [Google Scholar] [CrossRef]
- Grubb, N.R.; Simpson, C.; Sherwood, R.A.; Abraha, H.D.; Cobbe, S.M.; O’Carroll, R.E.; Deary, I.; Fox, K.A. Prediction of cognitive dysfunction after resuscitation from out-of-hospital cardiac arrest using serum neuron-specific enolase and protein S-100. Heart 2007, 93, 1268–1273. [Google Scholar] [CrossRef][Green Version]
- Schrage, B.; Rubsamen, N.; Becher, P.M.; Roedl, K.; Soffker, G.; Schwarzl, M.; Dreher, A.; Schewel, J.; Ghanem, A.; Grahn, H.; et al. Neuron-specific-enolase as a predictor of the neurologic outcome after cardiopulmonary resuscitation in patients on ECMO. Resuscitation 2019, 136, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Rafecas, A.; Baneras, J.; Sans-Rosello, J.; Ortiz-Perez, J.T.; Rueda-Sobella, F.; Santamarina, E.; Mila, L.; Sionis, A.; Gaig, C.; Garcia-Garcia, C.; et al. Change in neuron specific enolase levels in out-of-hospital cardiopulmonary arrest survivors as a simple and useful tool to predict neurological prognosis. Rev. Esp. Cardiol. 2019. [Google Scholar] [CrossRef] [PubMed]
- Ryoo, S.M.; Kim, W.Y. Clinical applications of lactate testing in patients with sepsis and septic shock. J. Emerg. Crit. Care Med. 2018, 2, 14. [Google Scholar] [CrossRef]
- Kim, J.C.; Lee, B.K.; Lee, D.H.; Jung, Y.H.; Cho, Y.S.; Lee, S.M.; Lee, S.J.; Park, C.H.; Jeung, K.W. Association between lactate clearance during post-resuscitation care and neurologic outcome in cardiac arrest survivors treated with targeted temperature management. Clin. Exp. Emerg. Med. 2017, 4, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Cocchi, M.N.; Salciccioli, J.; Yankama, T.; Chase, M.; Patel, P.V.; Liu, X.; Mader, T.J.; Donnino, M.W. Predicting outcome after out-of-hospital cardiac arrest: Lactate, need for vasopressors, and cytochrome c. J. Intensive Care Med. 2019, 0885066619873315. [Google Scholar] [CrossRef]
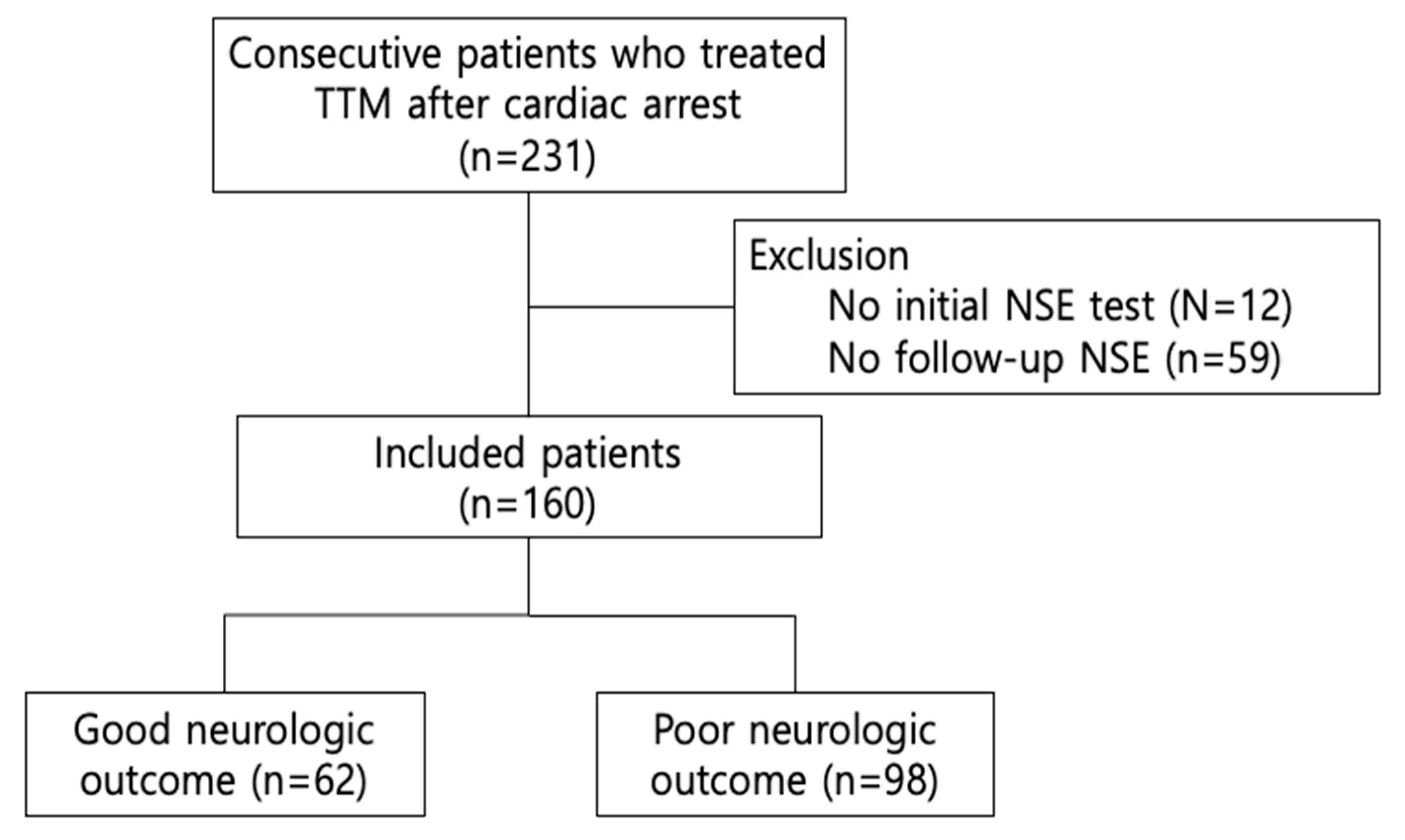
| Characteristics | Total (n = 160) | Poor Neurological Outcome (n = 98) | Good Neurological Outcome (n = 62) | p Value |
|---|---|---|---|---|
| Age, years | 58.8 ± 16.9 | 62.8 ± 15.9 | 52.6 ± 16.5 | <0.05 |
| Male | 108 (67.5) | 61 (62.2) | 47 (75.8) | 0.074 |
| Past medical history | ||||
| Acute coronary syndrome | 26 (16.3) | 14 (14.3) | 12 (19.4) | 0.397 |
| Arrhythmia | 17 (10.6) | 8 (8.2) | 9 (14.5) | 0.204 |
| Hypertension | 59 (36.9) | 40 (40.8) | 19 (30.6) | 0.194 |
| Diabetes | 41 (25.6) | 31 (31.6) | 10 (16.1) | <0.05 |
| Malignancy | 14 (8.8) | 11 (11.2) | 3 (4.8) | 0.164 |
| Vital signs | ||||
| Systolic pressure, mmHg | 137.8 ± 54.1 | 140.0 ± 54.6 | 134.1 ± 53.6 | 0.562 |
| Diastolic pressure, mmHg | 76.7 ± 28.7 | 73.0 ± 25.1 | 82.8 ± 33.4 | 0.071 |
| Pulse rate, beats/min | 112.6 ± 32.7 | 116.8 ± 33.6 | 105.5 ± 30.2 | 0.067 |
| Laboratory findings, initial | ||||
| White blood cell, 103/μL | 12.4 [9.0–16.9] | 12.1 [8.4–16.8] | 13.0 [10.0–17.9] | 0.355 |
| Hemoglobin, g/dL | 12.5 [10.7–14.6] | 11.7 [9.8–14.0] | 14.1 [12.2–15.5] | <0.05 |
| BUN, mg/mL | 17.0 [13.0–26.0] | 20.0 [14.0–31.5] | 15.0 [12.0–19.0] | <0.05 |
| Creatinine, ng/dL | 1.2 [0.9–1.7] | 1.3 [0.9–1.9] | 1.2 [0.9–1.4] | 0.081 |
| AST, mg/dL | 114.0 [52.0–225.3] | 114.0 [47.0–236.3] | 114.0 [52.8–189.5] | 0.603 |
| ALT, mg/dL | 76.5 [33.0–140.8] | 79.5 [26.8–150.8] | 74.5 [40.3–154.5] | 0.517 |
| Albumin, g/dL | 3.1 ± 0.7 | 2.8 ± 0.6 | 3.4 ± 0.5 | <0.05 |
| C-reactive protein, mg/dL | 0.2 [0.1–3.5] | 0.4 [0.1–2.1] | 0.1 [0.1–0.5] | <0.05 |
| Witnessed | 121 (75.6) | 68 (69.4) | 53 (85.5) | <0.05 |
| Bystander CPR | 117 (73.1) | 68 (69.4) | 49 (79.0) | 0.180 |
| Arrest cause | <0.05 | |||
| Presumed cardiac cause | 88 (55.0) | 41 (41.8) | 47 (75.8) | |
| Other medical cause | 37 (23.1) | 26 (26.5) | 11(17.7) | |
| Hanging | 12 (7.5) | 10 (10.2) | 2 (3.2) | |
| Asphyxia | 16 (10.0) | 16 (16.3) | 0 (0.0) | |
| Others | 7 (4.4) | 5 (5.1) | 2 (3.2) | |
| Prehospital initial rhythm | <0.05 | |||
| Shockable | 49 (30.6) | 18 (18.4) | 31 (50.0) | |
| Non-shockable | 71 (44.4) | 64 (65.3) | 7 (11.3) | |
| Unknown | 40 (25.0) | 16 (16.3) | 24 (38.7) | |
| TTM protocol | 0.223 | |||
| Hypothermia | 145 (90.6) | 91 (92.9) | 54 (87.1) | |
| Normothermia | 15 (9.4) | 7 (7.1) | 8 (12.9) |
| Characteristics | Poor Neurological Outcome (n = 9 8) | Good Neurological Outcome (n = 62) | p Value | AUC |
|---|---|---|---|---|
| Neuron specific enolase (NSE) | ||||
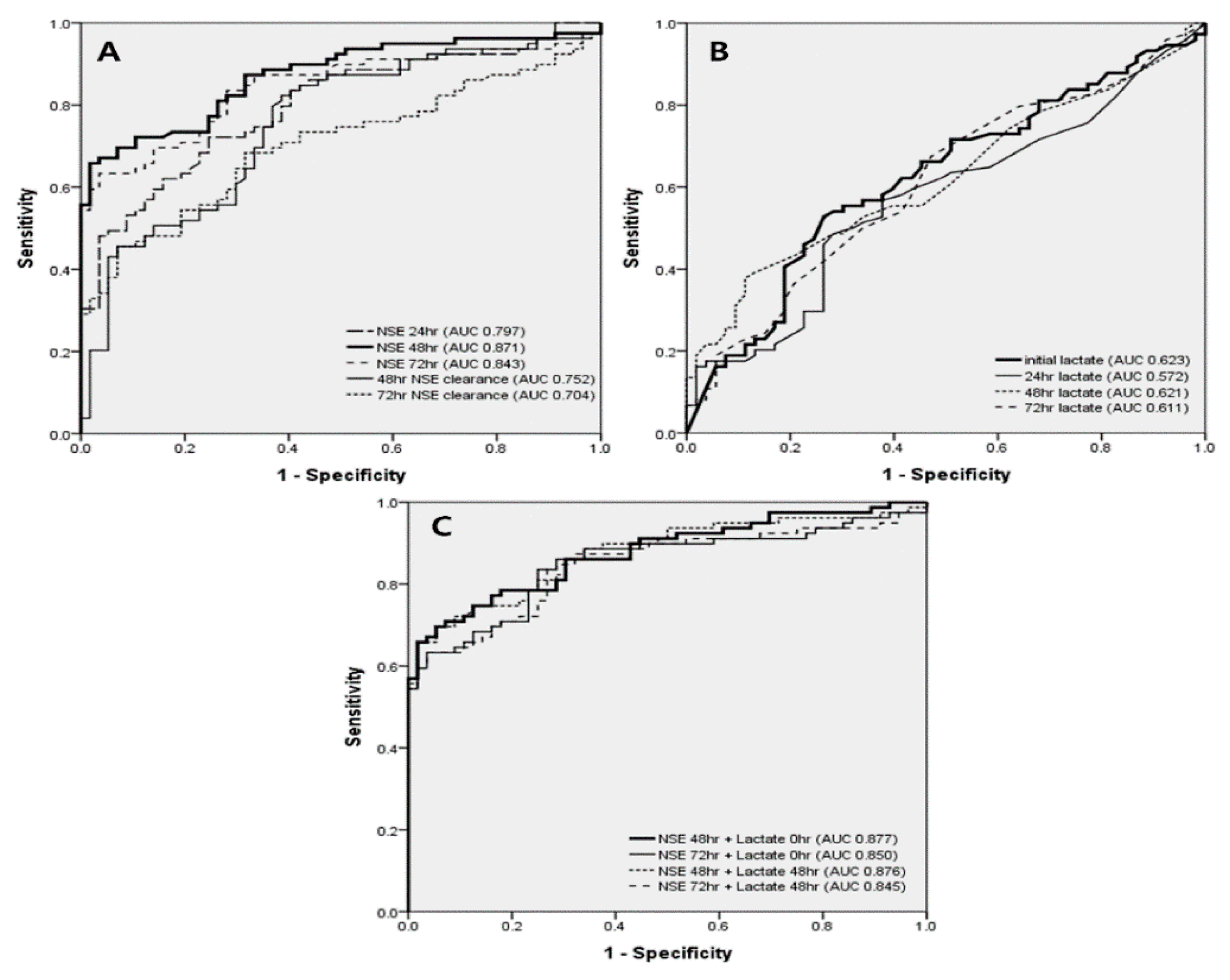
| NSE 24 h, ng/mL | 88.8 [46.7–193.5] | 35.6 [26.6–51.8] | <0.05 | 0.797 |
| NSE 48 h, ng/mL | 158.5 [48.2–325.9] | 29.1 [20.0–42.8] | <0.05 | 0.871 |
| NSE 72 h, ng/mL | 155.5 [45.6–340.0] | 25.7 [19.8–44.8] | <0.05 | 0.843 |
| NSE 48 h clearance, % | −42.0 [−123.5–11.2] | 28.1 [17.5–42.0] | <0.05 | 0.752 |
| NSE 72 h clearance, % | −38.6 [−200.4–26.3] | 26.0 [12.0–50.1] | <0.05 | 0.704 |
| Lactate | ||||
| Initial lactate, mmol/L | 10.3 [7.1–13.5] | 7.5 [4.1–10.2] | <0.05 | 0.623 |
| Lactate 24 h, mmol/L | 2.5 [1.3–4.2] | 1.7 [1.3–3.4] | <0.05 | 0.572 |
| Lactate 48 h, mmol/L | 1.5 [0.9–2.7] | 1.2 [0.9–1.9] | <0.05 | 0.621 |
| Lactate 72 h, mmol/L | 1.4 [1.0–2.0] | 1.0 [0.9–1.6] | <0.05 | 0.611 |
| Lactate 24 h clearance (%) | 71.3 [44.9–84.4] | 73.3 [55.8–83.4] | 0.581 | 0.515 |
| Lactate 48 h clearance (%) | 80.0 [68.1–89.4] | 83.8 [71.3–80.3] | 0.295 | 0.546 |
| Lactate 72 h clearance (%) | 82.9 [75.6–88.9] | 84.9 [78.0–89.7] | 0.462 | 0.524 |
| Combination NSE and lactate | ||||
| NSE 48 h + initial lactate | 169.3 [63.1–340.9] | 37.4 [29.3–52.7] | <0.05 | 0.877 |
| NSE 72 h + initial lactate | 165.3 [56.3–353.0] | 36.7 [27.5–51.6] | <0.05 | 0.850 |
| NSE 48 h + 48 h lactate | 160.9 [52.5–326.7] | 28.8 [21.0–41.2] | <0.05 | 0.876 |
| NSE 72 h + 48 h lactate | 156.8 [46.9–341.8] | 26.5 [20.7–46.7] | <0.05 | 0.845 |
| Characteristics | Poor Neurological Outcome (n = 98) | Good Neurological Outcome (n = 62) | Univariate Analysis OR (95% CI) | p Value | Multivariate Analysis OR (95% CI) | p Value |
|---|---|---|---|---|---|---|
| Age | 62.8 ± 15.9 | 52.6 ± 16.5 | OR 1.04 (95% CI: 1.02, 1.06) | <0.05 | ||
| Male | 61 (62.2) | 47 (75.8) | OR 0.53 (95% CI: 0.26, 1.07) | 0.076 | ||
| Past medical history | ||||||
| Diabetes | 31 (31.6) | 10 (16.1) | OR 2.41 (95% CI: 1.08, 5.35) | <0.05 | ||
| Initial vital signs | ||||||
| Diastolic pressure, mmHg | 73.0 ± 25.1 | 82.8 ± 33.4 | OR 0.99 (95% CI: 0.98, 1.00) | 0.076 | ||
| Pulse rate, beats/min | 116.8 ± 33.6 | 105.5 ± 30.2 | OR 1.01 (95% CI: 1.00, 1.02) | 0.071 | ||
| Witnessed cardiac arrest | 68 (69.4) | 53 (85.5) | OR 0.39 (95% CI: 0.17, 0.88) | <0.05 | ||
| Presumed cardiac cause arrest | 41 (41.8) | 47 (75.8) | OR 0.23 (95% CI: 0.11, 0.47) | <0.05 | ||
| Initial shockable rhythm | 18 (22.0) | 31 (81.6) | OR 0.06 (95% CI: 0.02, 0.17) | <0.05 | OR 0.01 (95% CI: 0.00, 0.32) | <0.05 |
| Laboratory findings, initial | ||||||
| Hemoglobin, mg/dL | 11.7 [9.8–14.0] | 14.1 [12.2–15.5] | OR 1.00 (95% CI: 0.99, 1.00) | 0.667 | ||
| BUN, mg/mL | 20.0 [14.0–31.5] | 15.0 [12.0–19.0] | OR 1.04 (95% CI: 1.01, 1.06) | <0.05 | ||
| Creatinine, ng/dL | 1.3 [0.9–1.9] | 1.2 [0.9–1.4] | OR 1.29 (95% CI: 1.00, 1.66) | 0.050 | ||
| Albumin | 2.8 ± 0.6 | 3.4 ± 0.5 | OR 0.13 (95% CI: 0.06, 0.28) | <0.05 | ||
| C-reactive protein | 0.4 [0.1–2.1] | 0.1 [0.1–0.5] | OR 1.24 (95% CI: 1.03, 1.50) | <0.05 | ||
| NSE 48 h + initial lactate | 166.9 [59.0–340.2] | 37.4 [29.3–52.7] | OR 1.04 (95% CI: 1.02, 1.06) | <0.05 | OR 1.07 (95% CI: 1.02, 1.12) | <0.05 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ryoo, S.M.; Kim, Y.-J.; Sohn, C.H.; Ahn, S.; Seo, D.W.; Kim, W.Y. Prognostic Abilities of Serial Neuron-Specific Enolase and Lactate and their Combination in Cardiac Arrest Survivors During Targeted Temperature Management. J. Clin. Med. 2020, 9, 159. https://doi.org/10.3390/jcm9010159
Ryoo SM, Kim Y-J, Sohn CH, Ahn S, Seo DW, Kim WY. Prognostic Abilities of Serial Neuron-Specific Enolase and Lactate and their Combination in Cardiac Arrest Survivors During Targeted Temperature Management. Journal of Clinical Medicine. 2020; 9(1):159. https://doi.org/10.3390/jcm9010159
Chicago/Turabian StyleRyoo, Seung Mok, Youn-Jung Kim, Chang Hwan Sohn, Shin Ahn, Dong Woo Seo, and Won Young Kim. 2020. "Prognostic Abilities of Serial Neuron-Specific Enolase and Lactate and their Combination in Cardiac Arrest Survivors During Targeted Temperature Management" Journal of Clinical Medicine 9, no. 1: 159. https://doi.org/10.3390/jcm9010159
APA StyleRyoo, S. M., Kim, Y.-J., Sohn, C. H., Ahn, S., Seo, D. W., & Kim, W. Y. (2020). Prognostic Abilities of Serial Neuron-Specific Enolase and Lactate and their Combination in Cardiac Arrest Survivors During Targeted Temperature Management. Journal of Clinical Medicine, 9(1), 159. https://doi.org/10.3390/jcm9010159





