Impact of Disease-Specific Fears on Pulmonary Rehabilitation Trajectories in Patients with COPD
Abstract
1. Introduction
2. Experimental Section
2.1. Participants
2.2. Pulmonary Rehabilitation Program
2.3. Measurements
2.3.1. Lung Function and Exercise Capacity
2.3.2. Self-Report Measures
2.4. Procedure
2.5. Data Analysis
3. Results
3.1. Patient Characteristics
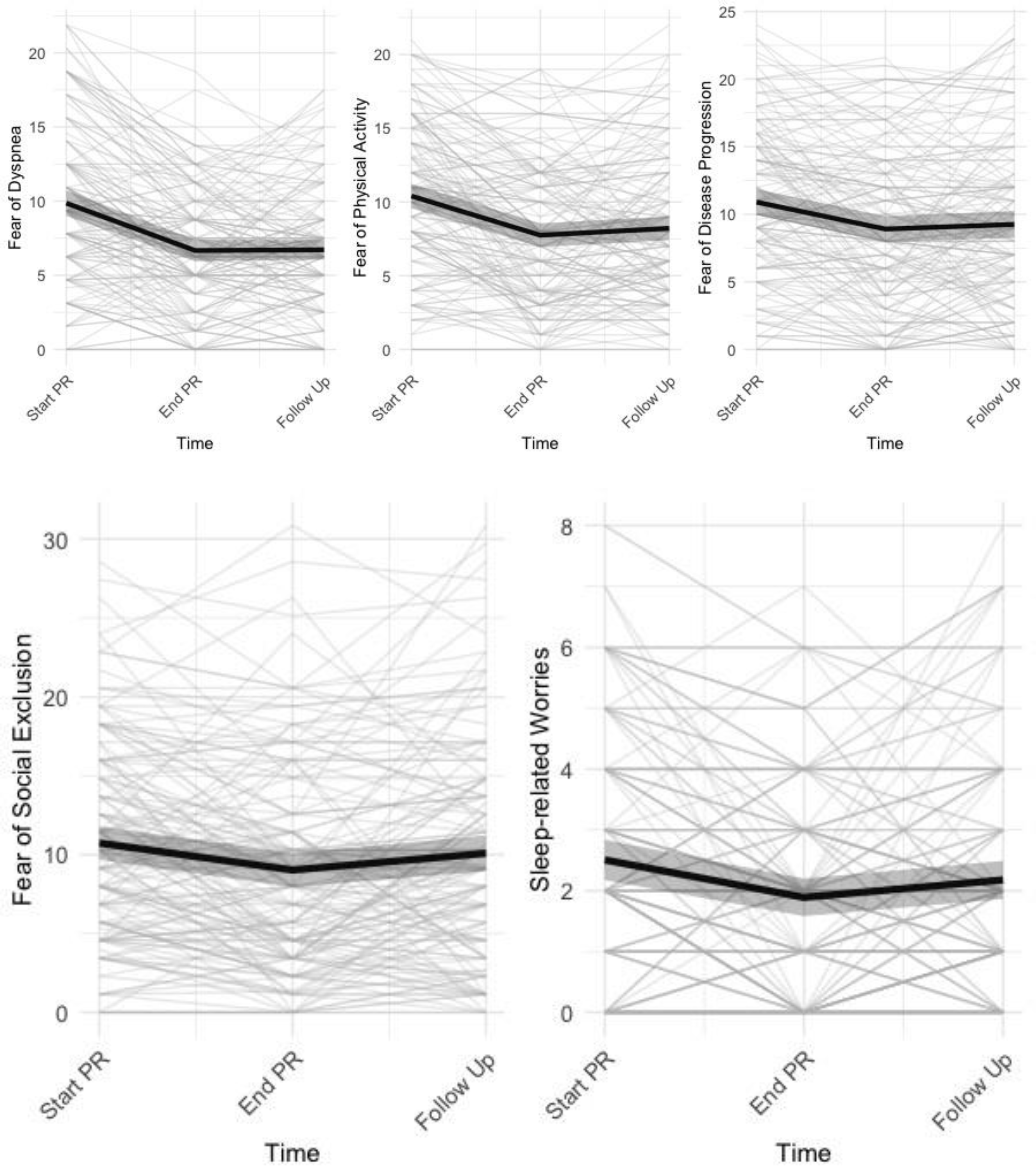
3.2. Change in Disease-Specific Fears and other Pulmonary Rehabilitation Outcomes
3.3. Impact of Disease-Specific Fears on Pulmonary Rehabilitation Outcomes
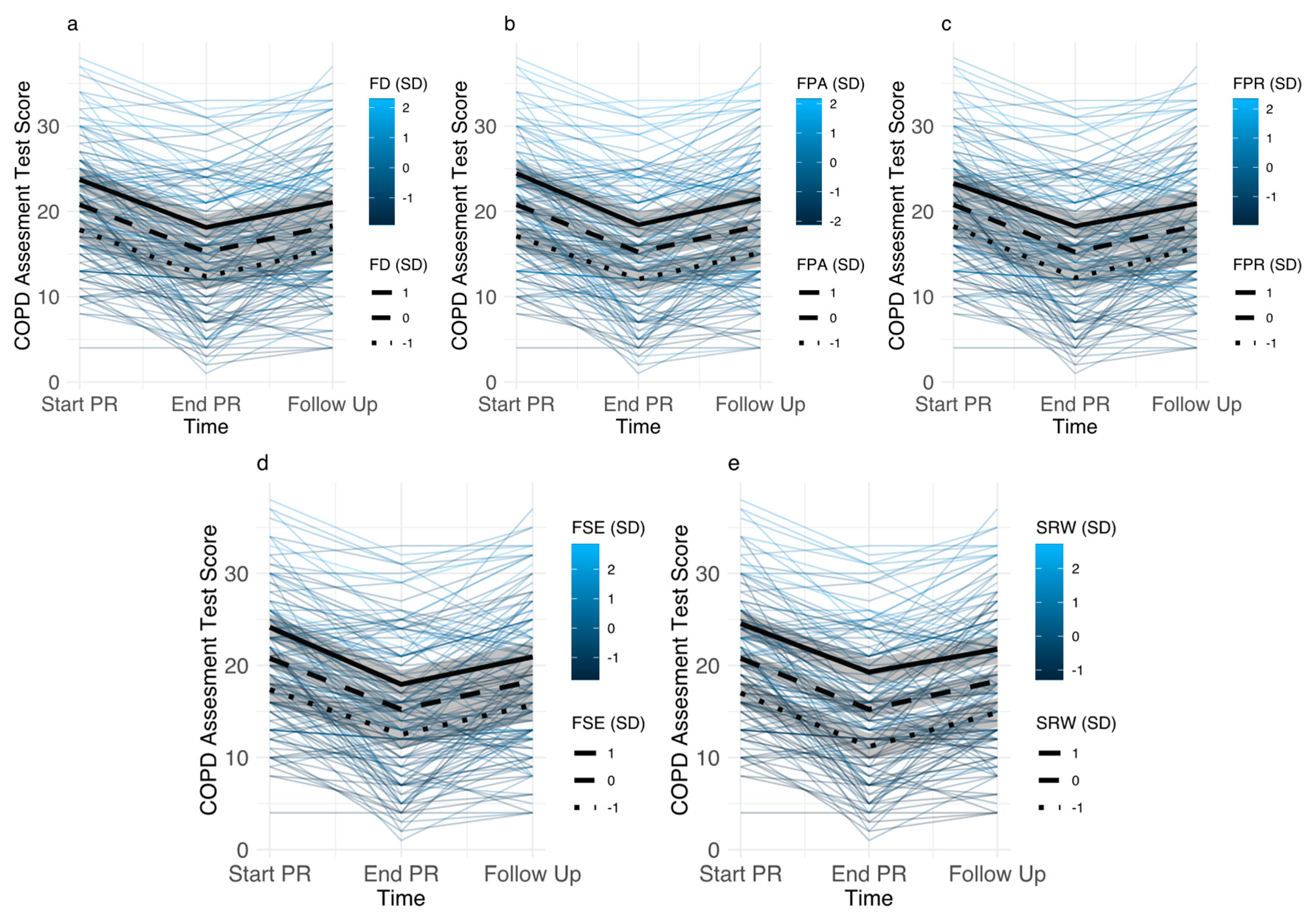
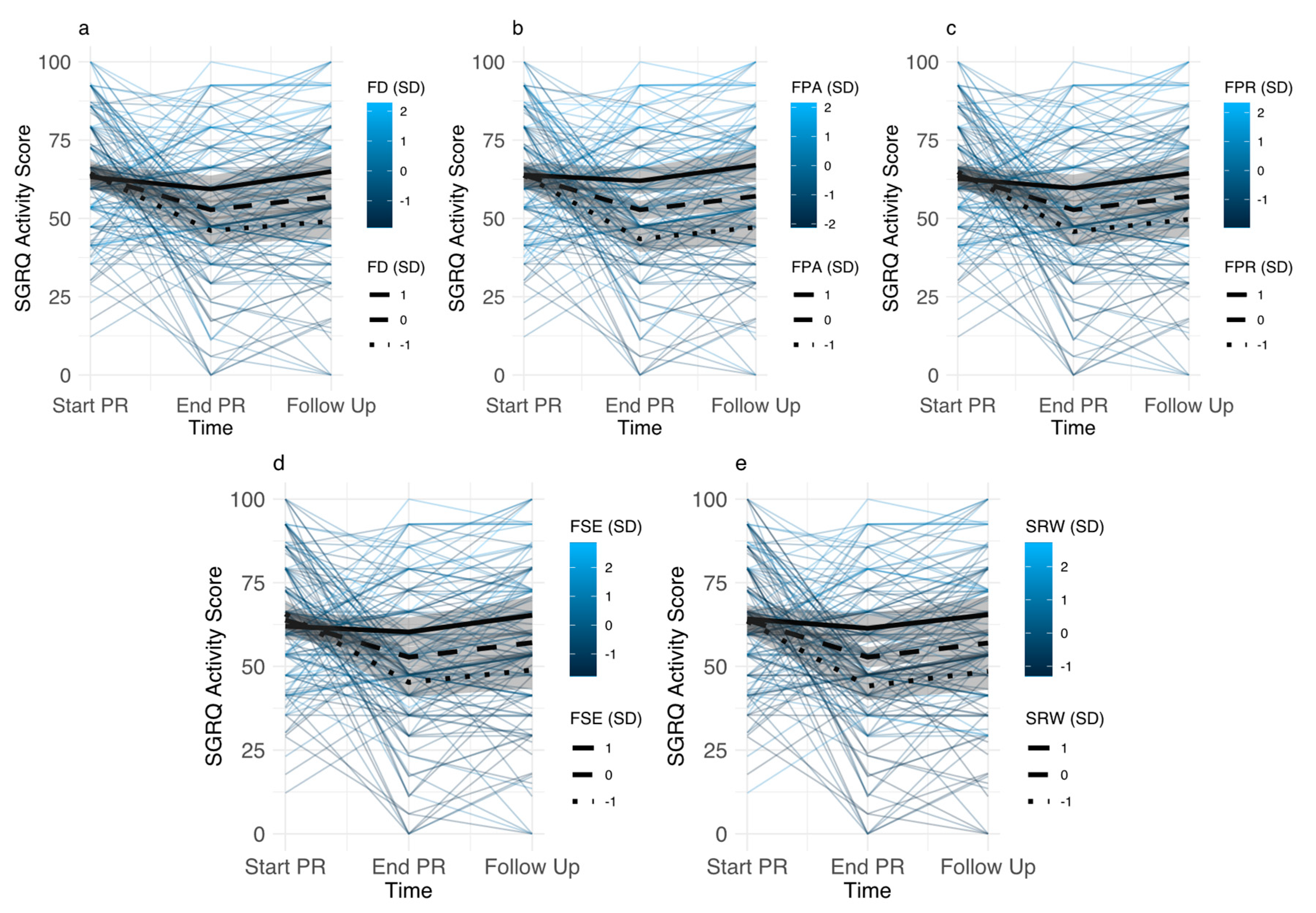
3.3.1. Baseline Levels of Disease-Specific Fears
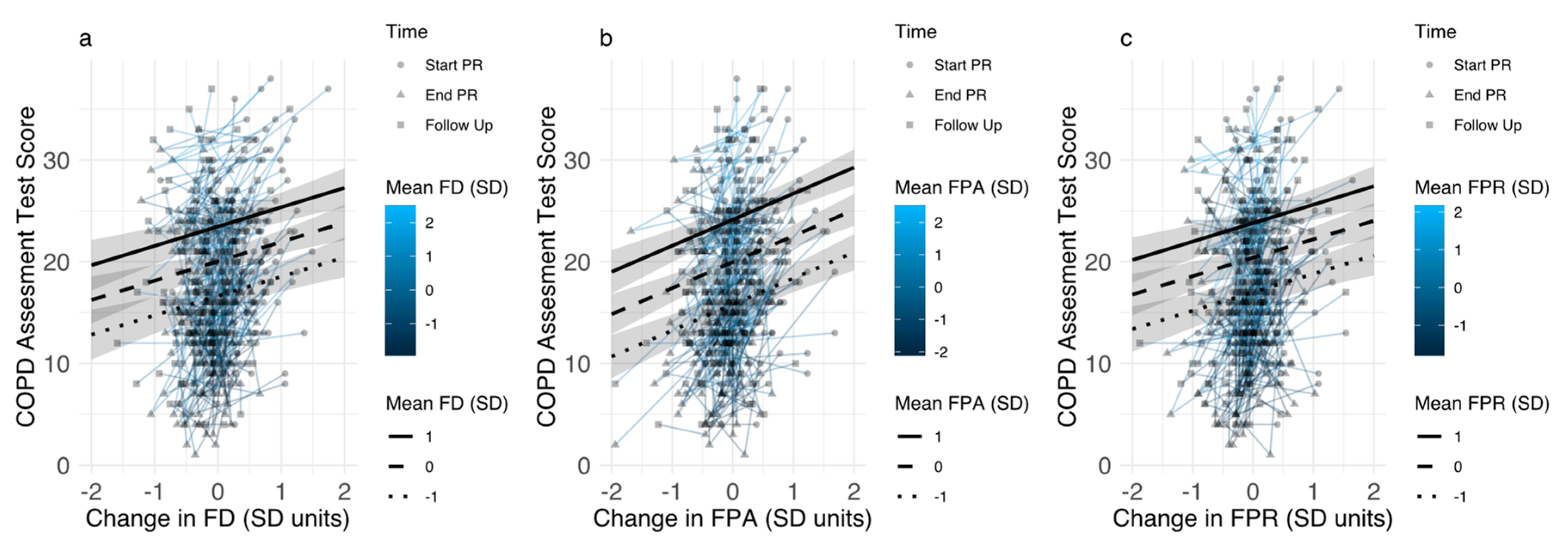
3.3.2. Associations between Changes in Disease-Specific Fears and Pulmonary Rehabilitation Outcomes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Global Initiative for Chronic Obstructive Lung Disease (GOLD). Global Strategy for the Diagnosis, Management and Prevention of COPD. Available online: https://goldcopd.org/gold-reports/ (accessed on 23 August 2019).
- Spruit, M.A.; Pitta, F.; McAuley, E.; ZuWallack, R.L.; Nici, L. Pulmonary Rehabilitation and Physical Activity in Patients with Chronic Obstructive Pulmonary Disease. Am. J. Respir. Crit. Care Med. 2015, 192, 924–933. [Google Scholar] [CrossRef] [PubMed]
- Coventry, P.A.; Hind, D. Comprehensive pulmonary rehabilitation for anxiety and depression in adults with chronic obstructive pulmonary disease: Systematic review and meta-analysis. J. Psychosom. Res. 2007, 63, 551–565. [Google Scholar] [CrossRef] [PubMed]
- McCarthy, B.; Casey, D.; DeVane, D.; Murphy, K.; Murphy, E.; Lacasse, Y. Pulmonary rehabilitation for chronic obstructive pulmonary disease. Cochrane Database Syst. Rev. 2015, CD003793. [Google Scholar] [CrossRef] [PubMed]
- Spruit, M.A.; Singh, S.J.; Garvey, C.; ZuWallack, R.; Nici, L.; Rochester, C.; Hill, K.; Holland, A.E.; Lareau, S.C.; Man, W.D.-C.; et al. An Official American Thoracic Society/European Respiratory Society Statement: Key Concepts and Advances in Pulmonary Rehabilitation. Am. J. Respir. Crit. Care Med. 2013, 188, e13–e64. [Google Scholar] [CrossRef] [PubMed]
- Matte, D.L.; Pizzichini, M.M.; Hoepers, A.T.; Diaz, A.P.; Karloh, M.; Dias, M.; Pizzichini, E. Prevalence of depression in COPD: A systematic review and meta-analysis of controlled studies. Respir. Med. 2016, 117, 154–161. [Google Scholar] [CrossRef] [PubMed]
- Panagioti, M.; Scott, C.; Blakemore, A.; A Coventry, P. Overview of the prevalence, impact, and management of depression and anxiety in chronic obstructive pulmonary disease. Int. J. Chronic Obstr. Pulm. Dis. 2014, 9, 1289–1306. [Google Scholar]
- Ratcliff, C.G.; Barrera, T.L.; Petersen, N.J.; Sansgiry, S.; Kauth, M.R.; Kunik, M.E.; Stanley, M.A.; Cully, J.A. Recognition of anxiety, depression, and PTSD in patients with COPD and CHF: Who gets missed? Gen. Hosp. Psychiatry 2017, 47, 61–67. [Google Scholar] [CrossRef]
- Yohannes, A.M.; Alexopoulos, G.S. Depression and anxiety in patients with COPD. Eur. Respir. Rev. 2014, 23, 345–349. [Google Scholar] [CrossRef]
- Willgoss, T.G.; Yohannes, A.M. Anxiety Disorders in Patients With COPD: A Systematic Review. Respir. Care 2013, 58, 858–866. [Google Scholar]
- Schuler, M.; Strohmayer, M.; Mühlig, S.; Schwaighofer, B.; Wittmann, M.; Faller, H.; Schultz, K. Assessment of depression before and after inpatient rehabilitation in COPD patients: Psychometric properties of the German version of the Patient Health Questionnaire (PHQ-9/PHQ-2). J. Affect. Disord. 2018, 232, 268–275. [Google Scholar] [CrossRef]
- Kim, H.F.S.; Kunik, M.E.; Molinari, V.A.; Hillman, S.L.; Lalani, S.; Orengo, C.A.; Petersen, N.J.; Nahas, Z.; Goodnight-White, S. Functional Impairment in COPD Patients: The Impact of Anxiety and Depression. J. Psychosom. Res. 2000, 41, 465–471. [Google Scholar] [CrossRef] [PubMed]
- Kunik, M.E.; Roundy, K.; Veazey, C.; Souchek, J.; Richardson, P.; Wray, N.P.; Stanley, M.A. Surprisingly High Prevalence of Anxiety and Depression in Chronic Breathing Disorders. Chest 2005, 127, 1205. [Google Scholar] [CrossRef]
- Blakemore, A.; Dickens, C.; Guthrie, E.; Bower, P.; Kontopantelis, E.; Afzal, C.; Coventry, P.A. Depression and anxiety predict health-related quality of life in chronic obstructive pulmonary disease: Systematic review and meta-analysis. Int. J. Chronic Obstr. Pulm. Dis. 2014, 9, 501. [Google Scholar] [CrossRef] [PubMed]
- Schwab, P.; Dhamane, A.D.; Hopson, S.D.; Moretz, C.; Annavarapu, S.; Burslem, K.; Renda, A.; Kaila, S. Impact of comorbid conditions in COPD patients on health care resource utilization and costs in a predominantly Medicare population. Int. J. Chronic Obstr. Pulm. Dis. 2017, 12, 735. [Google Scholar] [CrossRef] [PubMed]
- Wacker, M.E.; Kitzing, K.; Jörres, R.A.; Leidl, R.; Schulz, H.; Karrasch, S.; Karch, A.; Koch, A.; Vogelmeier, C.F.; Holle, R. The contribution of symptoms and comorbidities to the economic impact of COPD: An analysis of the German COSYCONET cohort. Int. J. Chronic Obstr. Pulm. Dis. 2017, 12, 3437. [Google Scholar] [CrossRef] [PubMed]
- Laforest, L.; Roche, N.; Devouassoux, G.; Belhassen, M.; Chouaid, C.; Ginoux, M.; Van Ganse, E. Frequency of comorbidities in chronic obstructive pulmonary disease, and impact on all-cause mortality: A population-based cohort study. Respir. Med. 2016, 117, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Divo, M.; Cote, C.; de Torres, J.P.; Casanova, C.; Marin, J.M.; Pinto-Plata, V.; Zulueta, J.; Cabrera, C.; Zagaceta, J.; Hunninghake, G.; et al. Comorbidities and Risk of Mortality in Patients with Chronic Obstructive Pulmonary Disease. Am. J. Respir. Crit. Care Med. 2012, 186, 155–161. [Google Scholar] [CrossRef] [PubMed]
- Lou, P.; Zhu, Y.; Chen, P.; Zhang, P.; Yu, J.; Zhang, N.; Chen, N.; Zhang, L.; Wu, H.; Zhao, J. Prevalence and correlations with depression, anxiety, and other features in outpatients with chronic obstructive pulmonary disease in China: A cross-sectional case control study. BMC Pulm. Med. 2012, 12, 53. [Google Scholar] [CrossRef]
- Von Leupoldt, A.; Taube, K.; Lehmann, K.; Fritzsche, A.; Magnussen, H. The Impact of Anxiety and Depression on Outcomes of Pulmonary Rehabilitation in Patients With COPD. Chest 2011, 140, 730–736. [Google Scholar] [CrossRef]
- Papaioannou, V.E.; Pneumatikos, I.; Maglaveras, N. Association of heart rate variability and inflammatory response in patients with cardiovascular diseases: Current strengths and limitations. Front. Physiol. 2013, 4, 4. [Google Scholar] [CrossRef]
- A Coventry, P.; Gemmell, I.; Todd, C.J. Psychosocial risk factors for hospital readmission in COPD patients on early discharge services: A cohort study. BMC Pulm. Med. 2011, 11, 49. [Google Scholar] [CrossRef] [PubMed]
- Laurin, C.; Moullec, G.; Bacon, S.L.; Lavoie, K.L. Impact of Anxiety and Depression on Chronic Obstructive Pulmonary Disease Exacerbation Risk. Am. J. Respir. Crit. Care Med. 2012, 185, 918–923. [Google Scholar] [CrossRef] [PubMed]
- Pikoula, M.; Quint, J.K.; Nissen, F.; Hemingway, H.; Smeeth, L.; Denaxas, S. Identifying clinically important COPD sub-types using data-driven approaches in primary care population based electronic health records. BMC Med. Inform. Decis. Mak. 2019, 19, 86. [Google Scholar] [CrossRef] [PubMed]
- Khdour, M.R.; Hawwa, A.F.; Kidney, J.C.; Smyth, B.M.; McElnay, J.C. Potential risk factors for medication non-adherence in patients with chronic obstructive pulmonary disease (COPD). Eur. J. Clin. Pharmacol. 2012, 68, 1365–1373. [Google Scholar] [CrossRef] [PubMed]
- Paine, N.J.; Bacon, S.L.; Bourbeau, J.; Tan, W.C.; Lavoie, K.L.; Aaron, S.D.; Chapman, K.R.; FitzGerald, J.M.; Hernandez, P.; Marciniuk, D.D.; et al. Psychological distress is related to poor health behaviours in COPD and non-COPD patients: Evidence from the CanCOLD study. Respir. Med. 2018, 146, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Von Leupoldt, A.; Janssens, T. Could targeting disease specific fear and anxiety improve COPD outcomes? Expert Rev. Respir. Med. 2016, 10, 835–837. [Google Scholar] [CrossRef] [PubMed]
- Wortz, K.; Cade, A.; Menard, J.R.; Lurie, S.; Lykens, K.; Bae, S.; Jackson, B.; Su, F.; Singh, K.; Coultas, D. A qualitative study of patients’ goals and expectations for self-management of COPD. Prim. Care Respir. J. 2012, 21, 384–391. [Google Scholar] [CrossRef] [PubMed]
- De Peuter, S.; Janssens, T.; Van Diest, I.; Stans, L.; Troosters, T.; Decramer, M.; Van den Bergh, O.; Vlaeyen, J.W.S. Dyspnea-related anxiety: The Dutch version of the Breathlessness Beliefs Questionnaire. Chronic Respir. Dis. 2011, 8, 11–19. [Google Scholar] [CrossRef]
- Keil, D.C.; Stenzel, N.M.; Kühl, K.; Vaske, I.; Mewes, R.; Rief, W.; Kenn, K. The impact of chronic obstructive pulmonary disease-related fears on disease-specific disability. Chronic Respir. Dis. 2014, 11, 31–40. [Google Scholar] [CrossRef]
- Vlaeyen, J.W.S.; Crombez, G.; Linton, S.J. The fear-avoidance model of pain. Pain 2016, 157, 1588–1589. [Google Scholar] [CrossRef]
- Lethem, J.; Slade, P.; Troup, J.; Bentley, G. Outline of a fear-avoidance model of exaggerated pain perception—I. Behav. Res. Ther. 1983, 21, 401–408. [Google Scholar] [CrossRef]
- Kühl, K.; Kuhn, C.; Kenn, K.; Rief, W. Der COPD-Angst-Fragebogen (CAF): Ein neues Instrument zur Erfassung krankheitsspezifischer Ängste bei COPD-Patienten. PPmP-Psychother. Psychosom. Med. Psychol. 2011, 61, e1–e9. [Google Scholar]
- Vercoulen, J.H.; Daudey, L.; Molema, J.; Vos, P.J.; Peters, J.B.; Top, M.; Folgering, H. An integral assessment framework of health status in chronic obstructive pulmonary disease (COPD). Int. J. Behav. Med. 2008, 15, 263–279. [Google Scholar] [CrossRef] [PubMed]
- Peters, J.B.; Daudey, L.; Heijdra, Y.F.; Molema, J.; Dekhuijzen, P.N.R.; Vercoulen, J.H. Development of a battery of instruments for detailed measurement of health status in patients with COPD in routine care: The Nijmegen Clinical Screening Instrument. Qual. Life Res. 2009, 18, 901–912. [Google Scholar] [CrossRef] [PubMed]
- Janssens, T.; De Peuter, S.; Stans, L.; Verleden, G.; Troosters, T.; Decramer, M.; Van den Bergh, O. Dyspnea Perception in COPD Association Between Anxiety, Dyspnea-Related Fear, and Dyspnea in a Pulmonary Rehabilitation Program. Chest 2011, 140, 618–625. [Google Scholar] [CrossRef]
- Reijnders, T.; Schuler, M.; Wittmann, M.; Jelusic, D.; Troosters, T.; Janssens, W.; Stenzel, N.M.; Schultz, K.; von Leupoldt, A. The Impact of Disease-Specific Fears on Outcome Measures of Pulmonary Rehabilitation in Patients with COPD. Respir. Med. 2018, 146, 87–95. [Google Scholar] [CrossRef]
- Esser, R.W.; Stoeckel, M.C.; Kirsten, A.; Watz, H.; Taube, K.; Lehmann, K.; Petersen, S.; Magnussen, H.; Von Leupoldt, A. Structural Brain Changes in Patients With COPD. Chest 2016, 149, 426–434. [Google Scholar] [CrossRef]
- Harris, D.; Hayter, M.; Allender, S. Improving the uptake of pulmonary rehabilitation in patients with COPD: Qualitative study of experiences and attitudes. Br. J. Gen. Pract. 2008, 58, 703–710. [Google Scholar] [CrossRef]
- Peters, J.B.; Boer, L.M.; Molema, J.; Heijdra, Y.F.; Prins, J.B.; Vercoulen, J.H. Integral Health Status-Based Cluster Analysis in Moderate–Severe COPD Patients Identifies Three Clinical Phenotypes: Relevant for Treatment as Usual and Pulmonary Rehabilitation. Int. J. Behav. Med. 2017, 24, 571–583. [Google Scholar] [CrossRef]
- Stenzel, N.M.; Vaske, I.; Kühl, K.; Kenn, K.; Rief, W. Prediction of end-of-life fears in COPD—Hoping for the best but preparing for the worst. Psychol. Health 2015, 30, 1–18. [Google Scholar] [CrossRef]
- Herigstad, M.; Faull, O.K.; Hayen, A.; Evans, E.; Hardinge, F.M.; Wiech, K.; Pattinson, K.T. Treating breathlessness via the brain: Changes in brain activity over a course of pulmonary rehabilitation. Eur. Respir. J. 2017, 50, 1701029. [Google Scholar] [CrossRef] [PubMed]
- Solomon, B.K.; Wilson, K.G.; Henderson, P.R.; Poulin, P.A.; Kowal, J.; McKim, D.A. A Breathlessness Catastrophizing Scale for chronic obstructive pulmonary disease. J. Psychosom. Res. 2015, 79, 62–68. [Google Scholar] [CrossRef] [PubMed]
- Zoeckler, N.; Kenn, K.; Kuehl, K.; Stenzel, N.; Rief, W. Illness perceptions predict exercise capacity and psychological well-being after pulmonary rehabilitation in COPD patients. J. Psychosom. Res. 2014, 76, 146–151. [Google Scholar] [CrossRef] [PubMed]
- Fischer, M.J.; Scharloo, M.; Abbink, J.; van ’t Hul, A.; van Ranst, D.; Rudolphus, A.; Weinman, J.; Rabe, K.F.; Kaptein, A.A. Concerns About Exercise Are Related to Walk Test Results in Pulmonary Rehabilitation for Patients with COPD. Int. J. Behav. Med. 2012, 19, 39–47. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Geidl, W.; Semrau, J.; Streber, R.; Lehbert, N.; Wingart, S.; Tallner, A.; Wittmann, M.; Wagner, R.; Schultz, K.; Pfeifer, K. Effects of a brief, pedometer-based behavioral intervention for individuals with COPD during inpatient pulmonary rehabilitation on 6-week and 6-month objectively measured physical activity: Study protocol for a randomized controlled trial. Trials 2017, 18, 396. [Google Scholar] [CrossRef] [PubMed]
- Miller, M.R. Standardisation of spirometry. Eur. Respir. J. 2005, 26, 319–338. [Google Scholar] [CrossRef] [PubMed]
- Quanjer, P.H.; Stanojevic, S.; Cole, T.J.; Baur, X.; Hall, G.L.; Culver, B.H.; Enright, P.L.; Hankinson, J.L.; Ip, M.S.; Zheng, J.; et al. multi-ethnic reference values for spirometry for the 3–95 year age range: The global lung function 2012 equations. Eur. Respir. J. 2012, 40, 1324–1343. [Google Scholar] [CrossRef] [PubMed]
- ATS Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories. ATS Statement Guidelines for the Six-Minute Walk Test. Am. J. Respir. Crit. Care Med. 2002, 166, 111–117. [Google Scholar] [CrossRef]
- Wingart, S.; Lehbert, N.; Krämer, B.; Huber, V.; Fuchs, S.; Wittmann, M.; Jelusic, D.; Schuler, M.; Schultz, K. Is a double 6-min walk test required as part of the routine assessment of pulmonary rehabilitation in COPD patients? Eur. Respir. J. 2014, 44, P1293. [Google Scholar]
- Jenkins, S.C. 6-Minute walk test in patients with COPD: Clinical applications in pulmonary rehabilitation. Physiotherapy 2007, 93, 175–182. [Google Scholar]
- Jones, P.W.; Harding, G.; Berry, P.; Wiklund, I.; Chen, W.-H.; Leidy, N.K. Development and first validation of the COPD Assessment Test. Eur. Respir. J. 2009, 34, 648–654. [Google Scholar] [CrossRef]
- Ringbaek, T.; Martinez, G.; Lange, P. A Comparison of the Assessment of Quality of Life with CAT, CCQ, and SGRQ in COPD Patients Participating in Pulmonary Rehabilitation. COPD J. Chronic Obstr. Pulm. Dis. 2012, 9, 12–15. [Google Scholar] [CrossRef] [PubMed]
- Gupta, N.; Pinto, L.M.; Morogan, A.; Bourbeau, J. The COPD assessment test: A systematic review. Eur. Respir. J. 2014, 44, 873–884. [Google Scholar] [CrossRef] [PubMed]
- Alma, H.; de Jong, C.; Jelusic, D.; Wittmann, M.; Schuler, M.; Blok, B.F.; Kocks, J.; Schultz, K.; van der Molen, T. Health status instruments for patients with COPD in pulmonary rehabilitation: Defining a minimal clinically important difference. NPJ Prim. Care Resp. Med. 2016, 26, 16041. [Google Scholar] [CrossRef] [PubMed]
- Von Leupoldt, A.; Reijnders, T.; Schuler, M.; Wittmann, M.; Jelusic, D.; Schultz, K. Validity of a Self-administered Questionnaire Version of the Transition Dyspnea Index in Patients with COPD. COPD J. Chronic Obstr. Pulm. Dis. 2017, 14, 66–71. [Google Scholar] [CrossRef] [PubMed]
- Witek, T.; Mahler, D. Minimal important difference of the transition dyspnoea index in a multinational clinical trial. Eur. Respir. J. 2003, 21, 267–272. [Google Scholar] [CrossRef] [PubMed]
- Mahler, D.A.; Weinberg, D.H.; Wells, C.K.; Feinstein, A.R. The Measurement of Dyspnea: Contents, Interobserver Agreement, and Physiologic Correlates of Two New Clinical Indexes. Chest 1984, 85, 751–758. [Google Scholar] [CrossRef] [PubMed]
- Jones, P.W.; Quirk, F.H.; Baveystock, C.M. The St George’s Respiratory Questionnaire. Respir. Med. 1991, 85, 25–31. [Google Scholar] [CrossRef]
- Gräfe, K.; Zipfel, S.; Herzog, W.; Löwe, B. Screening psychischer Störungen mit dem “Gesundheitsfragebogen für Patienten (PHQ-D)”. Diagnostica 2004, 50, 171–181. [Google Scholar] [CrossRef]
- Bates, D.; Mächler, M.; Bolker, B.; Walker, S. Fitting Linear Mixed-Effects Models Using lme4. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- Lenth, R.; Singmann, H.; Love, J.; Buerkner, P.; Herve, M. Emmeans: Estimated Marginal Means, aka Least-Squares Means. Available online: https://CRAN.R-project.org/package=emmeans (accessed on 23 August 2019).
- Kuznetsova, A.; Brockhoff, P.B.; Christensen, R.H.B. lmerTest: Tests in Linear Mixed Effects Models. Available online: https://CRAN.R-project.org/package=lmerTest (accessed on 21 August 2019).
- Carrieri-Kohlman, V.; Gormley, J.M.; Douglas, M.K.; Paul, S.M.; Stulbarg, M.S. Exercise Training Decreases Dyspnea and the Distress and Anxiety Associated with It. Chest 1996, 110, 1526–1535. [Google Scholar] [CrossRef]
- Wadell, K.; Webb, K.A.; Preston, M.E.; Amornputtisathaporn, N.; Samis, L.; Patelli, J.; Guenette, J.A.; O’Donnell, D.E. Impact of Pulmonary Rehabilitation on the Major Dimensions of Dyspnea in COPD. COPD J. Chronic Obstr. Pulm. Dis. 2013, 10, 425–435. [Google Scholar] [CrossRef] [PubMed]
- McNicholas, W.T.; Verbraecken, J.; Marín, J.M. Sleep disorders in COPD: The forgotten dimension. Eur. Respir. Rev. 2013, 22, 365–375. [Google Scholar] [CrossRef] [PubMed]
- Scharf, S.M.; Maimon, N.; Simon-Tuval, T.; Bernhard-Scharf, B.J.; Reuveni, H.; Tarasiuk, A. Sleep quality predicts quality of life in chronic obstructive pulmonary disease. Int. J. Chronic Obstruct. Pulm. Dis. 2011, 6, 1. [Google Scholar] [CrossRef] [PubMed]
- Zeidler, M.R.; Martin, J.L.; Kleerup, E.C.; Schneider, H.; Mitchell, M.N.; Hansel, N.N.; Sundar, K.; Schotland, H.; Basner, R.C.; Wells, J.M.; et al. Sleep disruption as a predictor of quality of life among patients in the subpopulations and intermediate outcome measures in COPD study (SPIROMICS). Sleep 2018, 41, zsy044. [Google Scholar] [CrossRef] [PubMed]
- Reijnders, T.; Schuler, M.; Jelusic, D.; Troosters, T.; Janssens, W.; Schultz, K.; Leupoldt, A. von The Impact of Loneliness on Outcomes of Pulmonary Rehabilitation in Patients with COPD. COPD J. Chronic Obstr. Pulm. Dis. 2018, 15, 446–453. [Google Scholar] [CrossRef] [PubMed]
- Halding, A.-G.; Wahl, A.; Heggdal, K. ‘Belonging’. ‘Patients’ experiences of social relationships during pulmonary rehabilitation. Disabil. Rehabil. 2010, 32, 1272–1280. [Google Scholar] [CrossRef] [PubMed]
- Spruit, M.A.; Pitta, F.; Garvey, C.; ZuWallack, R.L.; Roberts, C.M.; Collins, E.G.; Goldstein, R.; McNamara, R.; Surpas, P.; Atsuyoshi, K.; et al. Differences in content and organisational aspects of pulmonary rehabilitation programmes. Eur. Respir. J. 2014, 43, 1326–1337. [Google Scholar] [CrossRef] [PubMed]
- Desveaux, L.; Janaudis-Ferreira, T.; Goldstein, R.; Brooks, D. An International Comparison of Pulmonary Rehabilitation: A Systematic Review. COPD J. Chronic Obstr. Pulm. Dis. 2015, 12, 144–153. [Google Scholar] [CrossRef]
- Livermore, N.; Sharpe, L.; McKenzie, D. Prevention of panic attacks and panic disorder in COPD. Eur. Respir. J. 2010, 35, 557–563. [Google Scholar] [CrossRef]
- Heslop-Marshall, K.; Baker, C.; Carrick-Sen, D.; Newton, J.; Echevarria, C.; Stenton, C.; Jambon, M.; Gray, J.; Pearce, K.; Burns, G.; et al. Randomised controlled trial of cognitive behavioural therapy in COPD. ERJ Open Res. 2018, 4, 00094–2018. [Google Scholar] [CrossRef]
- Barrera, T.L.; Grubbs, K.M.; Kunik, M.E.; Teng, E.J. A Review of Cognitive Behavioral Therapy for Panic Disorder in Patients with Chronic Obstructive Pulmonary Disease: The Rationale for Interoceptive Exposure. J. Clin. Psychol. Med. Settings 2014, 21, 144–154. [Google Scholar] [CrossRef] [PubMed]
- Wouters, E.F.; Wouters, B.B.; Augustin, I.M.; Houben-Wilke, S.; Vanfleteren, L.E.; Franssen, F.M. Personalised pulmonary rehabilitation in COPD. Eur. Respir. Rev. 2018, 27, 170125. [Google Scholar] [CrossRef] [PubMed]
- Agusti, A.; Bel, E.; Thomas, M.; Vogelmeier, C.; Brusselle, G.; Holgate, S.; Humbert, M.; Jones, P.; Gibson, P.G.; Vestbo, J.; et al. Treatable traits: Toward precision medicine of chronic airway diseases. Eur. Respir. J. 2016, 47, 410–419. [Google Scholar] [CrossRef] [PubMed]

| Variable | N | Data | Association with Baseline Disease-Specific Fears p < 0.05 |
|---|---|---|---|
| Age, mean (SD) | 146 | 57.5 (4.4) | ns |
| Gender, n (%) | 146 | ns | |
| Males | 98 (67) | ||
| Females | 48 (33) | ||
| Smoking status, n (%) | 146 | SRW | |
| Active smokers | 66 (45) | ||
| Non-smokers | 80 (55) | ||
| Pack years, mean (SD) | 142 | 44.4 (22.8) | ns |
| Exacerbation history (past year), n (%) | 140 | FD, FP, FSE | |
| 0 or 1 exacerbation | 68 (49) | ||
| 2 or more exacerbations | 72 (51) | ||
| Inhaled Medication, n (%) | 146 | ns | |
| ICS + LABA + LAMA | 64 (44) | ||
| ICS + LABA/LAMA | 15 (10) | ||
| LABA + LAMA | 50 (34) | ||
| LABA/LAMA monotherapy | 10 (7) | ||
| SABA or no inhaled medication | 7 (5) | ||
| FEV1% predicted, mean (SD) | 142 | 55.4 (16.6) | FD, FPA, FSE |
| 6MWT, mean (SD) | 144 | 460.8 (102.5) | FD, FPA, FSE |
| Baseline PHQ-9, mean (SD) | 138 | 7.9 (5.4) | FD, FPA, FP, FSE, SRW |
| Start PR | End PR | Follow-Up | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Variable | Mean | SE | Mean | SE | Mean | SE | F | df | p | |
| Disease-Specific Fears (CAF) | ||||||||||
| Fear of Social Exclusion | 10.74 a | 0.542 | 9.03 b | 0.555 | 10.09 a | 0.582 | 8.376 | (2, 146.16) | 0.0004 | |
| Fear of Dyspnea | 9.86 a | 0.44 | 6.67 b | 0.372 | 6.72 b | 0.337 | 53.203 | (2, 146) | <0.0001 | |
| Fear of Physical Activity | 10.4 a | 0.425 | 7.75 b | 0.413 | 8.22 b | 0.414 | 31.614 | (2, 145.98) | <0.0001 | |
| Fear of Progression | 10.90 a | 0.489 | 8.91 b | 0.488 | 9.25 b | 0.507 | 14.391 | (2, 146) | <0.0001 | |
| Sleep-Related Worries | 2.51 a | 0.17 | 1.89 b | 0.158 | 2.18 ab | 0.16 | 10.759 | (2, 146) | <0.0001 | |
| Symptom Burden (CAT) | 20.8 a | 0.579 | 15.2 b | 0.603 | 18.3 c | 0.646 | 70.322 | (2, 145.98) | <0.0001 | |
| Quality of Life (SGRQ) | ||||||||||
| Symptoms | 61.1 a | 1.81 | 36.6 b | 1.91 | 52.9 c | 2.03 | 35.853 | (2,142.28) | <0.0001 | |
| Activity | 63.8 a | 1.47 | 52.7 b | 1.62 | 57.0 b | 2.15 | 16.289 | (2, 144.91) | <0.0001 | |
| Impact | 41.8 a | 1.37 | 27.7 b | 1.44 | 31.8 c | 1.81 | 39.083 | (2, 145.69) | <0.0001 | |
| Transition Dyspnea Index (TDI) | 1.92 a | 0.107 | 1.04 b | 0.135 | 45.484 | (1, 169.9) | <0.0001 | |||
| Depressive Symptoms (PHQ-9) | 7.9 a | 0.422 | 5.02 b | 0.406 | 7.12 a | 0.407 | 31.592 | (2, 143.24) | <0.0001 | |
| 6 Min walking test Distance (m) | 461 a | 6.68 | 542 b | 9.24 | 81.711 | (1, 226.17) | <0.0001 | |||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Janssens, T.; Van de Moortel, Z.; Geidl, W.; Carl, J.; Pfeifer, K.; Lehbert, N.; Wittmann, M.; Schultz, K.; von Leupoldt, A. Impact of Disease-Specific Fears on Pulmonary Rehabilitation Trajectories in Patients with COPD. J. Clin. Med. 2019, 8, 1460. https://doi.org/10.3390/jcm8091460
Janssens T, Van de Moortel Z, Geidl W, Carl J, Pfeifer K, Lehbert N, Wittmann M, Schultz K, von Leupoldt A. Impact of Disease-Specific Fears on Pulmonary Rehabilitation Trajectories in Patients with COPD. Journal of Clinical Medicine. 2019; 8(9):1460. https://doi.org/10.3390/jcm8091460
Chicago/Turabian StyleJanssens, Thomas, Zora Van de Moortel, Wolfgang Geidl, Johannes Carl, Klaus Pfeifer, Nicola Lehbert, Michael Wittmann, Konrad Schultz, and Andreas von Leupoldt. 2019. "Impact of Disease-Specific Fears on Pulmonary Rehabilitation Trajectories in Patients with COPD" Journal of Clinical Medicine 8, no. 9: 1460. https://doi.org/10.3390/jcm8091460
APA StyleJanssens, T., Van de Moortel, Z., Geidl, W., Carl, J., Pfeifer, K., Lehbert, N., Wittmann, M., Schultz, K., & von Leupoldt, A. (2019). Impact of Disease-Specific Fears on Pulmonary Rehabilitation Trajectories in Patients with COPD. Journal of Clinical Medicine, 8(9), 1460. https://doi.org/10.3390/jcm8091460





