Consensus-Derived Quality Performance Indicators for Neuroendocrine Tumour Care
Abstract
1. Introduction
2. Methods
2.1. Participants
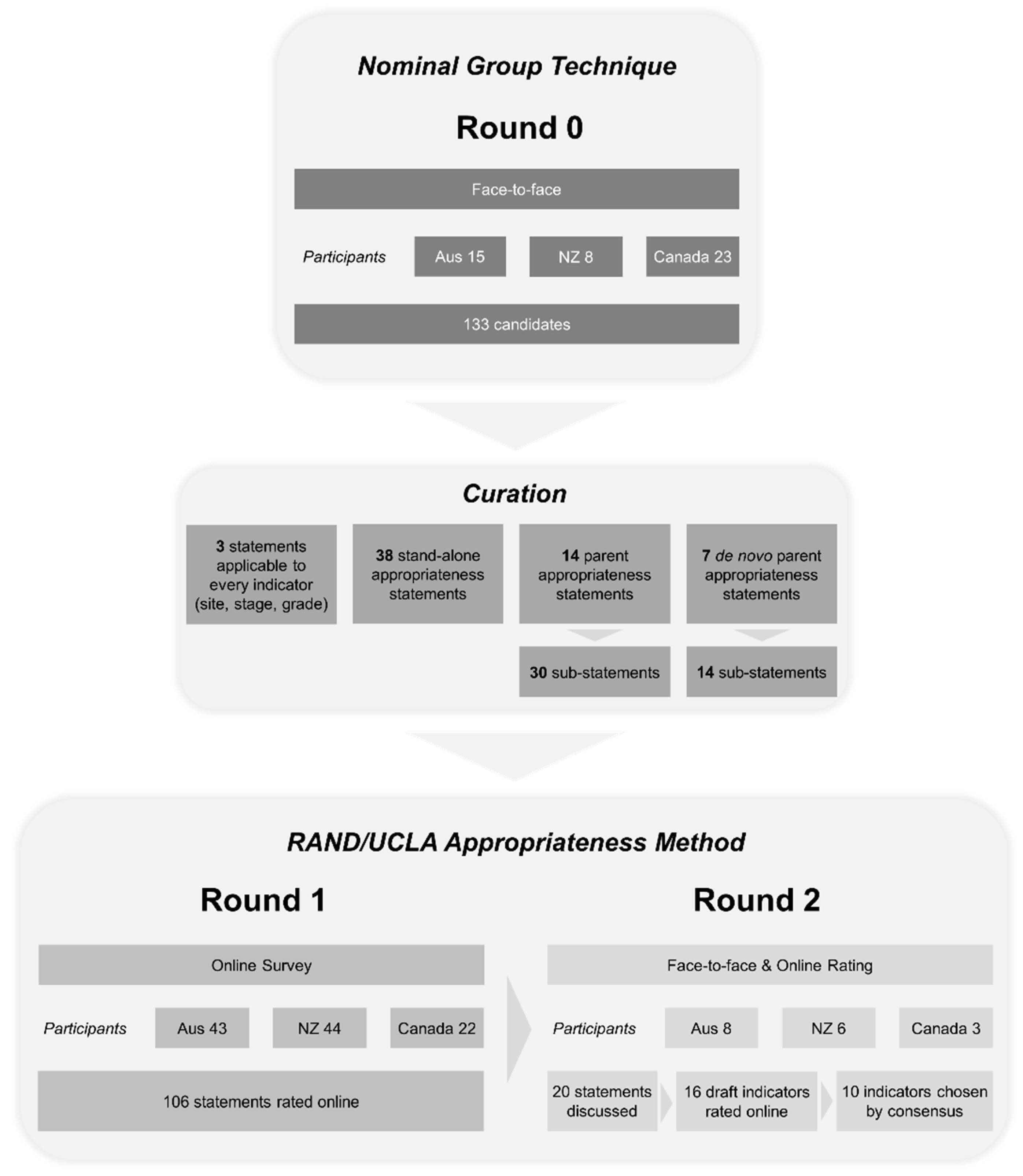
2.2. Round 0—The Generation of Candidate Statements
2.3. The Conversion of Candidate Statements into Appropriateness Statements
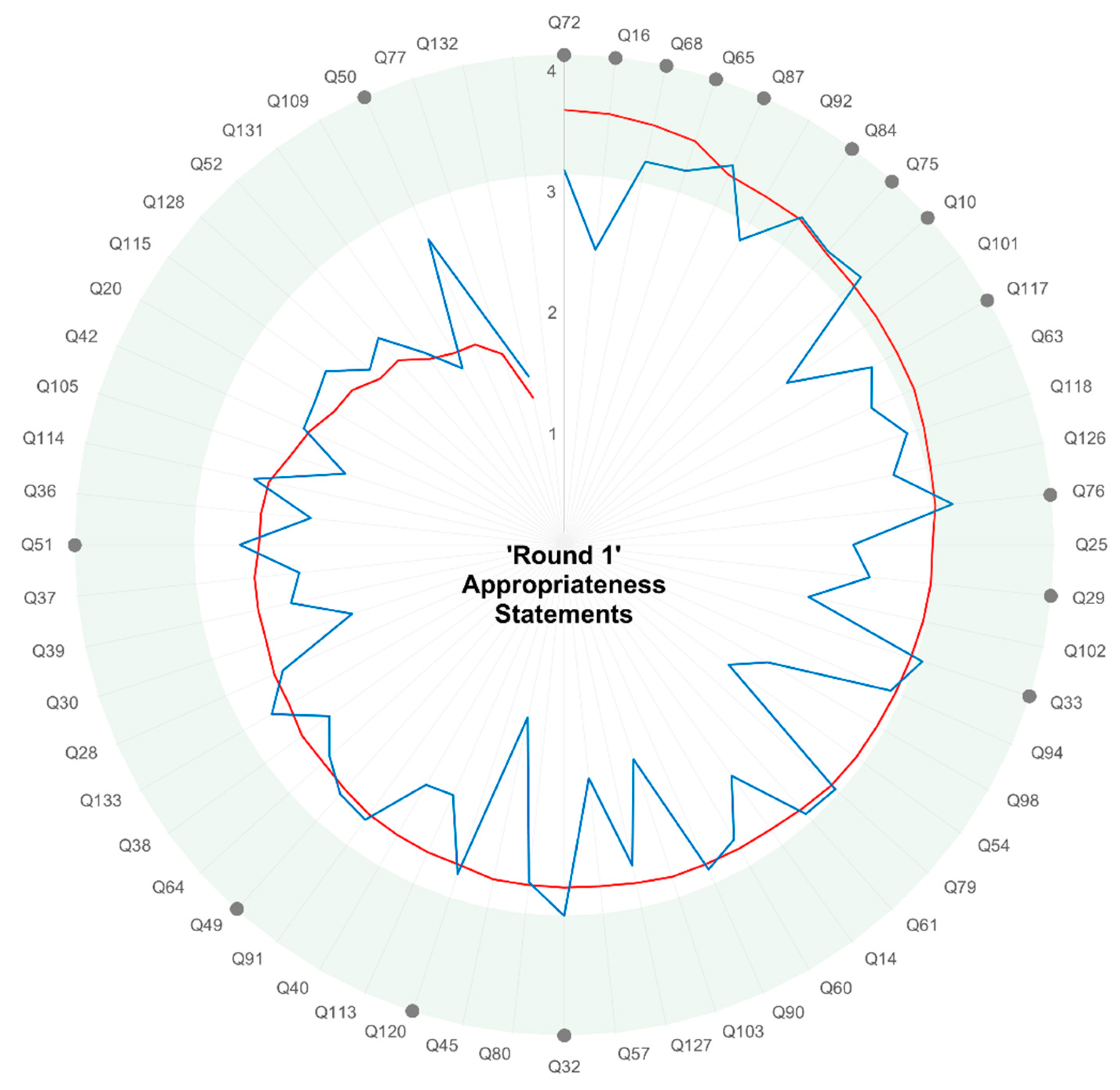
2.4. Round 1—Online Survey
2.5. Round 2—Modified RAND/UCLA Delphi Consensus Expert Group Ranking
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- New Zealand Ministry of Health. Bowel Cancer Quality Performance Indicators: Descriptions; Ministry of Health: Wellington, New Zealand, 2019. [Google Scholar]
- Cancer Australia. National Cancer Control Indicators (NCCI). Available online: https://ncci.canceraustralia.gov.au (accessed on 20 June 2019).
- New Zealand Ministry of Health Cancer Services. Review of the National Tumour Standards. Available online: https://www.health.govt.nz/our-work/diseases-and-conditions/national-cancer-programme/cancer-initiatives/review-national-tumour-standards (accessed on 20 June 2019).
- Cancer Quality Council of Ontario. Cancer System Quality Index (CSQI). Available online: https://www.csqi.on.ca/indicators (accessed on 20 June 2019).
- The Scottish Government. Healthcare and Healthcare Improvement—National Cancer Quality Programme. Available online: https://www.sehd.scot.nhs.uk/mels/CEL2012_06.pdf (accessed on 20 June 2019).
- National Quality Forum. NQF Endorses Cancer Measures. Available online: https://www.qualityforum.org/News_And_Resources/Press_Releases/2012/NQF_Endorses_Cancer_Measures.aspx (accessed on 20 June 2019).
- Stordeur, S.; Vrijens, F.; Beirens, K.; Vlayen, J.; Devriese, S.; Van Eycken, E. Quality Indicators in Oncology: Breast Cancer. Good Clinical Practice (GCP); Belgian Health Care Knowledge Centre (KCE): Brussels, Belgium, 2010. [Google Scholar]
- Walpole, E.T.; Theile, D.E.; Philpot, S.; Youl, P.H. Development and Implementation of a Cancer Quality Index in Queensland, Australia: A Tool for Monitoring Cancer Care. J. Oncol. Pract. 2019, 15, e636–e643. [Google Scholar] [CrossRef] [PubMed]
- Scottish Cancer Taskforce and National Cancer Quality Steering Group. Lung Cancer Clinical Quality Performance Indicators. Available online: http://www.healthcareimprovementscotland.org/our_work/cancer_care_improvement/cancer_qpis/quality_performance_indicators.aspx (accessed on 20 Jun 2019).
- Biganzoli, L.; Marotti, L.; Hart, C.D.; Cataliotti, L.; Cutuli, B.; Kuhn, T.; Mansel, R.E.; Ponti, A.; Poortmans, P.; Regitnig, P.; et al. Quality indicators in breast cancer care: An update from the EUSOMA working group. Eur. J. Cancer 2017, 86, 59–81. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, T.; Mikami, M.; Katabuchi, H.; Kato, S.; Kaneuchi, M.; Takahashi, M.; Nakai, H.; Nagase, S.; Niikura, H.; Mandai, M.; et al. Quality indicators for cervical cancer care in Japan. J. Gynecol. Oncol. 2018, 29, e83. [Google Scholar] [CrossRef] [PubMed]
- Maharaj, A.D.; Ioannou, L.; Croagh, D.; Zalcberg, J.; Neale, R.E.; Goldstein, D.; Merrett, N.; Kench, J.G.; White, K.; Pilgrim, C.H.C.; et al. Monitoring quality of care for patients with pancreatic cancer: A modified Delphi consensus. HPB (Oxford) 2019, 21, 444–455. [Google Scholar] [CrossRef] [PubMed]
- New Zealand Ministry of Health. Bowel Cancer Quality Performance Indicator Specifications. Available online: https://www.health.govt.nz/publication/bowel-cancer-quality-performance-indicator-specifications (accessed on 29 July 2019).
- Gagliardi, A.R.; Simunovic, M.; Langer, B.; Stern, H.; Brown, A.D. Development of quality indicators for colorectal cancer surgery, using a 3-step modified Delphi approach. Can. J. Surg. 2005, 48, 441–452. [Google Scholar] [PubMed]
- Di Valentin, T.; Biagi, J.; Bourque, S.; Butt, R.; Champion, P.; Chaput, V.; Colwell, B.; Cripps, C.; Dorreen, M.; Edwards, S.; et al. Eastern Canadian Colorectal Cancer Consensus Conference: Standards of care for the treatment of patients with rectal, pancreatic, and gastrointestinal stromal tumours and pancreatic neuroendocrine tumours. Curr. Oncol. 2013, 20, e455–e464. [Google Scholar] [CrossRef] [PubMed]
- Khare, S.R.; Batist, G.; Bartlett, G. Identification of performance indicators across a network of clinical cancer programs. Curr. Oncol. 2016, 23, 81–90. [Google Scholar] [CrossRef]
- Turner, N.H.; Wong, H.L.; Field, K.; Wong, R.; Shapiro, J.; Yip, D.; Nott, L.; Tie, J.; Kosmider, S.; Tran, B.; et al. Novel quality indicators for metastatic colorectal cancer management identify significant variations in these measures across treatment centers in Australia. Asia Pac. J. Clin. Oncol. 2015, 11, 262–271. [Google Scholar] [CrossRef]
- Man, D.; Wu, J.; Shen, Z.; Zhu, X. Prognosis of patients with neuroendocrine tumor: A SEER database analysis. Cancer Manag. Res. 2018, 10, 5629–5638. [Google Scholar] [CrossRef]
- Dasari, A.; Shen, C.; Halperin, D.; Zhao, B.; Zhou, S.; Xu, Y.; Shih, T.; Yao, J.C. Trends in the Incidence, Prevalence, and Survival Outcomes in Patients With Neuroendocrine Tumors in the United States. JAMA Oncol. 2017, 3, 1335–1342. [Google Scholar] [CrossRef]
- Hallet, J.; Law, C.H.; Cukier, M.; Saskin, R.; Liu, N.; Singh, S. Exploring the rising incidence of neuroendocrine tumors: A population-based analysis of epidemiology, metastatic presentation, and outcomes. Cancer 2015, 121, 589–597. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, B.; Gustafsson, B.I.; Chan, A.; Svejda, B.; Kidd, M.; Modlin, I.M. The epidemiology of gastroenteropancreatic neuroendocrine tumors. Endocrinol. Metab. Clin. N. Am. 2011, 40, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Perez, E.A.; Koniaris, L.G.; Snell, S.E.; Gutierrez, J.C.; Sumner, W.E., III; Lee, D.J.; Hodgson, N.C.; Livingstone, A.S.; Franceschi, D. 7201 carcinoids: Increasing incidence overall and disproportionate mortality in the elderly. World J. Surg. 2007, 31, 1022–1030. [Google Scholar] [CrossRef] [PubMed]
- Yao, J.C.; Hassan, M.; Phan, A.; Dagohoy, C.; Leary, C.; Mares, J.E.; Abdalla, E.K.; Fleming, J.B.; Vauthey, J.N.; Rashid, A.; et al. One hundred years after “carcinoid”: Epidemiology of and prognostic factors for neuroendocrine tumors in 35,825 cases in the United States. J. Clin. Oncol. 2008, 26, 3063–3072. [Google Scholar] [CrossRef] [PubMed]
- Hallet, J.; Coburn, N.G.; Singh, S.; Beyfuss, K.; Koujanian, S.; Liu, N.; Law, C.H.L. Access to care and outcomes for neuroendocrine tumours: Does socioeconomic status matter? Curr. Oncol. 2018, 25, e356–e364. [Google Scholar] [CrossRef] [PubMed]
- Fitch, K.; Bernstein, S.J.; Aguilar, M.D.; Burnand, B.; LaCalle, J.R. The RAND/UCLA Appropriateness Method User’s Manual; RAND Corporation: Santa Monica, CA, USA, 2001. [Google Scholar]
- American Society for Quality. About Nominal Group Technique. Available online: https://asq.org/quality-resources/nominal-group-technique (accessed on 23 May 2019).
- Segelov, E.; Chan, D.; Lawrence, B.; Pavlakis, N.; Kennecke, H.F.; Jackson, C.; Law, C.; Singh, S. Identifying and Prioritizing Gaps in Neuroendocrine Tumor Research: A Modified Delphi Process With Patients and Health Care Providers to Set the Research Action Plan for the Newly Formed Commonwealth Neuroendocrine Tumor Collaboration. J. Glob. Oncol. 2017, 3, 380–388. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Moody, L.; Chan, D.L.; Metz, D.C.; Strosberg, J.; Asmis, T.; Bailey, D.L.; Bergsland, E.; Brendtro, K.; Carroll, R.; et al. Follow-up Recommendations for Completely Resected Gastroenteropancreatic Neuroendocrine Tumors. JAMA Oncol. 2018, 4, 1597–1604. [Google Scholar] [CrossRef] [PubMed]
- Delle Fave, G.; O’Toole, D.; Sundin, A.; Taal, B.; Ferolla, P.; Ramage, J.K.; Ferone, D.; Ito, T.; Weber, W.; Zheng-Pei, Z.; et al. ENETS Consensus Guidelines Update for Gastroduodenal Neuroendocrine Neoplasms. Neuroendocrinology 2016, 103, 119–124. [Google Scholar] [CrossRef] [PubMed]
- Pavel, M.; O’Toole, D.; Costa, F.; Capdevila, J.; Gross, D.; Kianmanesh, R.; Krenning, E.; Knigge, U.; Salazar, R.; Pape, U.F.; et al. ENETS Consensus Guidelines Update for the Management of Distant Metastatic Disease of Intestinal, Pancreatic, Bronchial Neuroendocrine Neoplasms (NEN) and NEN of Unknown Primary Site. Neuroendocrinology 2016, 103, 172–185. [Google Scholar] [CrossRef]
- Strosberg, J.R.; Halfdanarson, T.R.; Bellizzi, A.M.; Chan, J.A.; Dillon, J.S.; Heaney, A.P.; Kunz, P.L.; O’Dorisio, T.M.; Salem, R.; Segelov, E.; et al. The North American Neuroendocrine Tumor Society Consensus Guidelines for Surveillance and Medical Management of Midgut Neuroendocrine Tumors. Pancreas 2017, 46, 707–714. [Google Scholar] [CrossRef]
- Howe, J.R.; Cardona, K.; Fraker, D.L.; Kebebew, E.; Untch, B.R.; Wang, Y.Z.; Law, C.H.; Liu, E.H.; Kim, M.K.; Menda, Y.; et al. The Surgical Management of Small Bowel Neuroendocrine Tumors: Consensus Guidelines of the North American Neuroendocrine Tumor Society. Pancreas 2017, 46, 715–731. [Google Scholar] [CrossRef] [PubMed]
- Perren, A.; Couvelard, A.; Scoazec, J.Y.; Costa, F.; Borbath, I.; Delle Fave, G.; Gorbounova, V.; Gross, D.; Grossma, A.; Jense, R.T.; et al. ENETS Consensus Guidelines for the Standards of Care in Neuroendocrine Tumors: Pathology: Diagnosis and Prognostic Stratification. Neuroendocrinology 2017, 105, 196–200. [Google Scholar] [CrossRef] [PubMed]
- Kunz, P.L.; Reidy-Lagunes, D.; Anthony, L.B.; Bertino, E.M.; Brendtro, K.; Chan, J.A.; Chen, H.; Jensen, R.T.; Kim, M.K.; Klimstra, D.S.; et al. Consensus guidelines for the management and treatment of neuroendocrine tumors. Pancreas 2013, 42, 557–577. [Google Scholar] [CrossRef] [PubMed]

| Core statements |
| Grade |
| Stage |
| Primary site |
| Important and measurable statements |
| Quality of pathology reports |
| Pathology involvement in MDM review * |
| MDM review * |
| Proportion patients with structural imaging |
| Proportion of patients with functional imaging in staging |
| Proportion of histopathology reports presented in a synoptic report |
| Survival after diagnosis |
| Complete synoptic reporting to College of American Pathologists standards |
| ‘Last chance’ statements |
| Proportion of patients receiving systemic treatment |
| Proportion of patients with surgical consultation for consideration of resection |
| Proportion of patients who receive surgery with curative intent |
| Proportion of patients getting resection is an important and measurable indicator of NET care quality |
| Patient reported quality of life |
| All cases reported to national registry |
| Proportion of patients with functional symptom control |
| Proportion of carcinoid patients who have cardiac imaging |
| Proportion of NET patients diagnosed with carcinoid heart disease (using echocardiogram) |
| Primary site reported |
| Proliferative index reported * |
| Distant metastases reported (M0/M1) |
| 5-year overall survival |
| 10-year overall survival |
| Differentiation reported * |
| Structured pathology report |
| Tumour board review |
| 5-year disease-free survival |
| 10-year disease-free survival |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Woodhouse, B.; Pattison, S.; Segelov, E.; Singh, S.; Parker, K.; Kong, G.; Macdonald, W.; Wyld, D.; Meyer-Rochow, G.; Pavlakis, N.; et al. Consensus-Derived Quality Performance Indicators for Neuroendocrine Tumour Care. J. Clin. Med. 2019, 8, 1455. https://doi.org/10.3390/jcm8091455
Woodhouse B, Pattison S, Segelov E, Singh S, Parker K, Kong G, Macdonald W, Wyld D, Meyer-Rochow G, Pavlakis N, et al. Consensus-Derived Quality Performance Indicators for Neuroendocrine Tumour Care. Journal of Clinical Medicine. 2019; 8(9):1455. https://doi.org/10.3390/jcm8091455
Chicago/Turabian StyleWoodhouse, Braden, Sharon Pattison, Eva Segelov, Simron Singh, Kate Parker, Grace Kong, William Macdonald, David Wyld, Goswin Meyer-Rochow, Nick Pavlakis, and et al. 2019. "Consensus-Derived Quality Performance Indicators for Neuroendocrine Tumour Care" Journal of Clinical Medicine 8, no. 9: 1455. https://doi.org/10.3390/jcm8091455
APA StyleWoodhouse, B., Pattison, S., Segelov, E., Singh, S., Parker, K., Kong, G., Macdonald, W., Wyld, D., Meyer-Rochow, G., Pavlakis, N., Conroy, S., Gordon, V., Koea, J., Kramer, N., Michael, M., Wakelin, K., Asif, T., Lo, D., Price, T., ... on behalf of the Commonwealth Neuroendocrine Tumour Collaboration (CommNETs). (2019). Consensus-Derived Quality Performance Indicators for Neuroendocrine Tumour Care. Journal of Clinical Medicine, 8(9), 1455. https://doi.org/10.3390/jcm8091455






