The QT Interval Dynamic in a Human Experimental Model of Controlled Heart Rate and QRS Widening
Abstract
:1. Introduction
2. Experimental Section
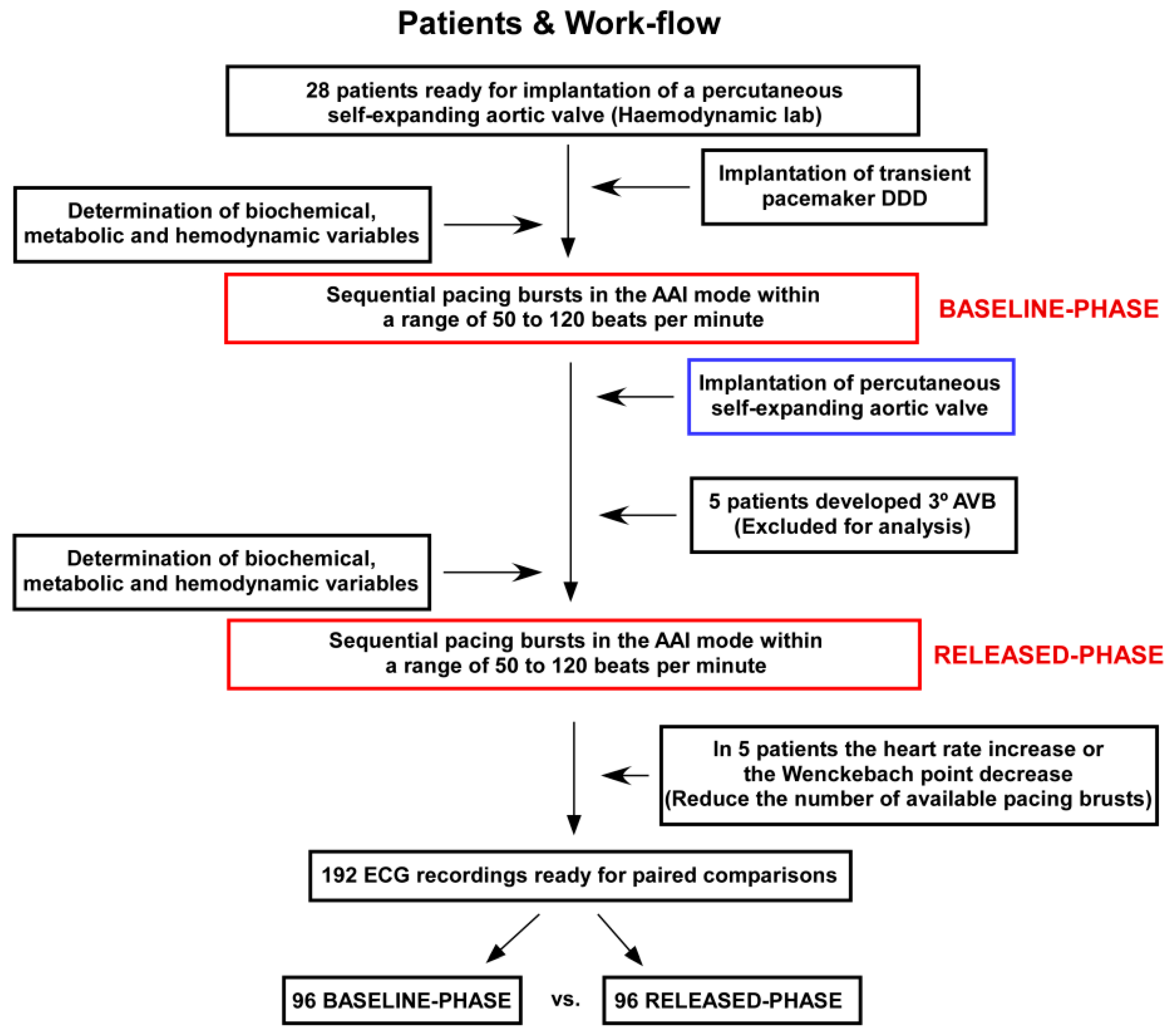
2.1. Patients and Experimental Protocol
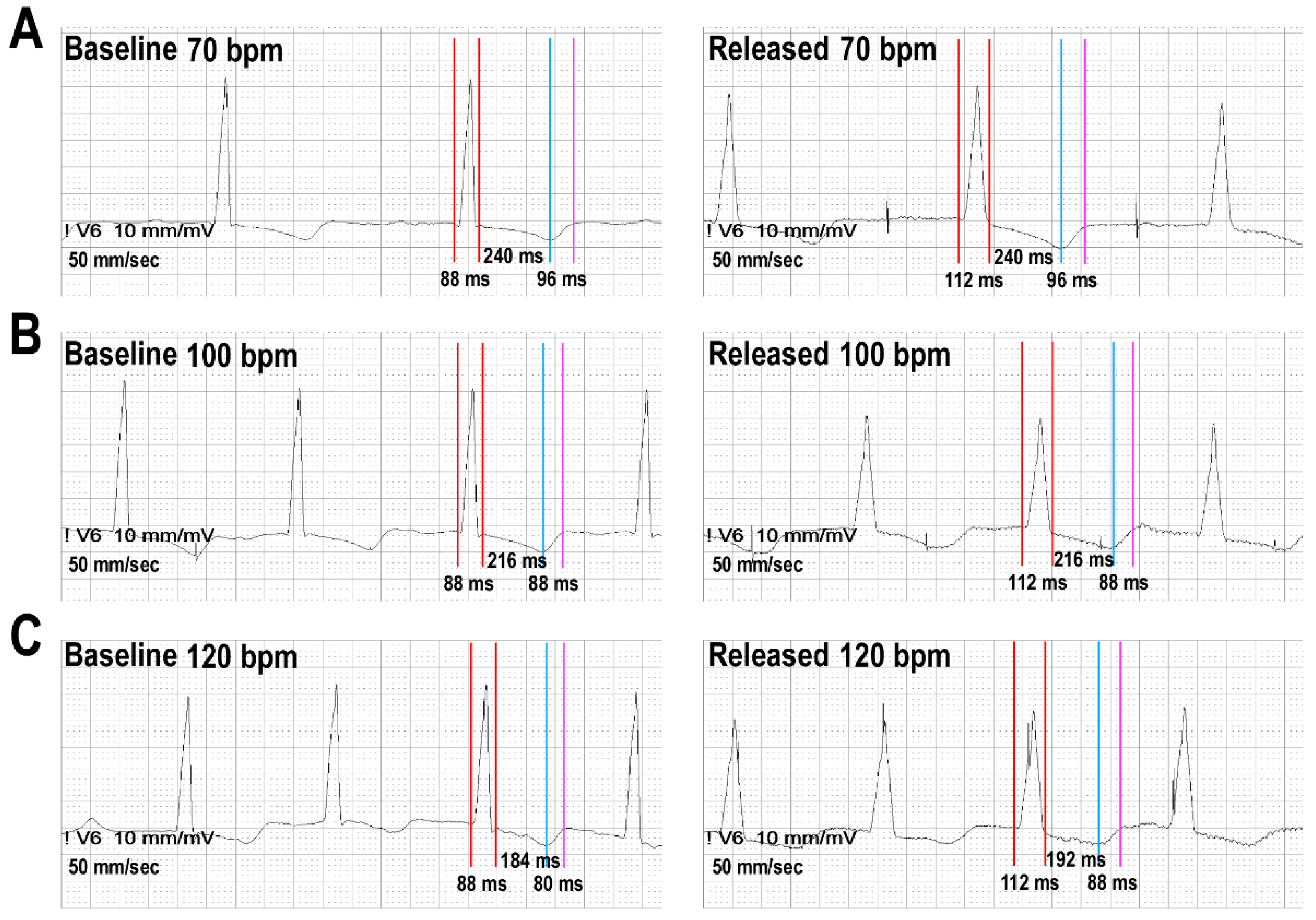
2.2. ECG Measurements, Frequency Correction and Definitions
2.3. Statistics and Data Analysis
3. Results
3.1. Patients and the Dynamic of ECG Intervals
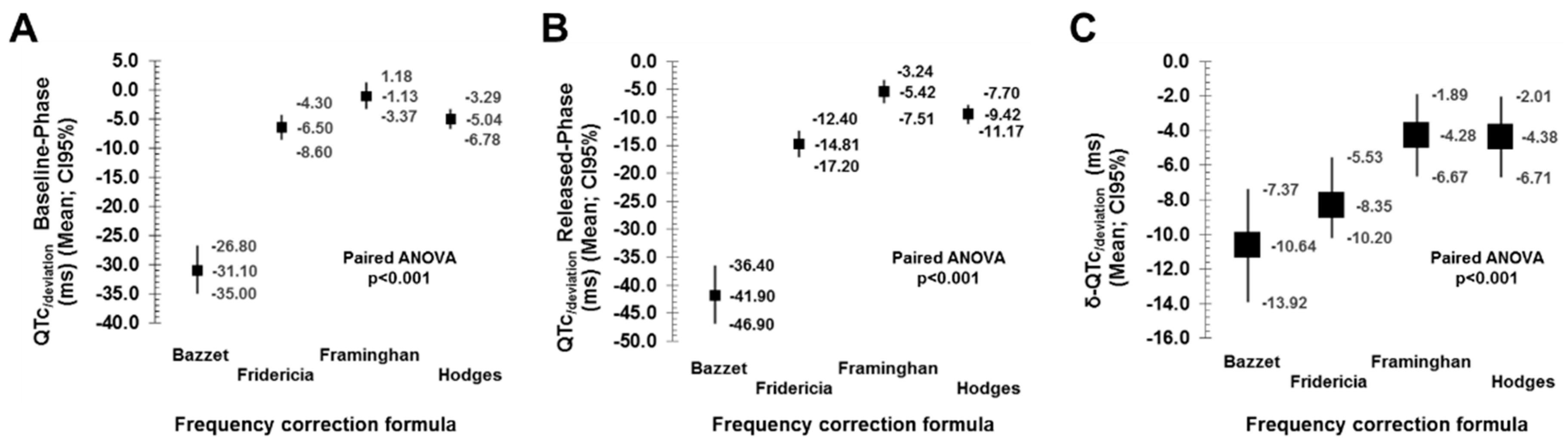
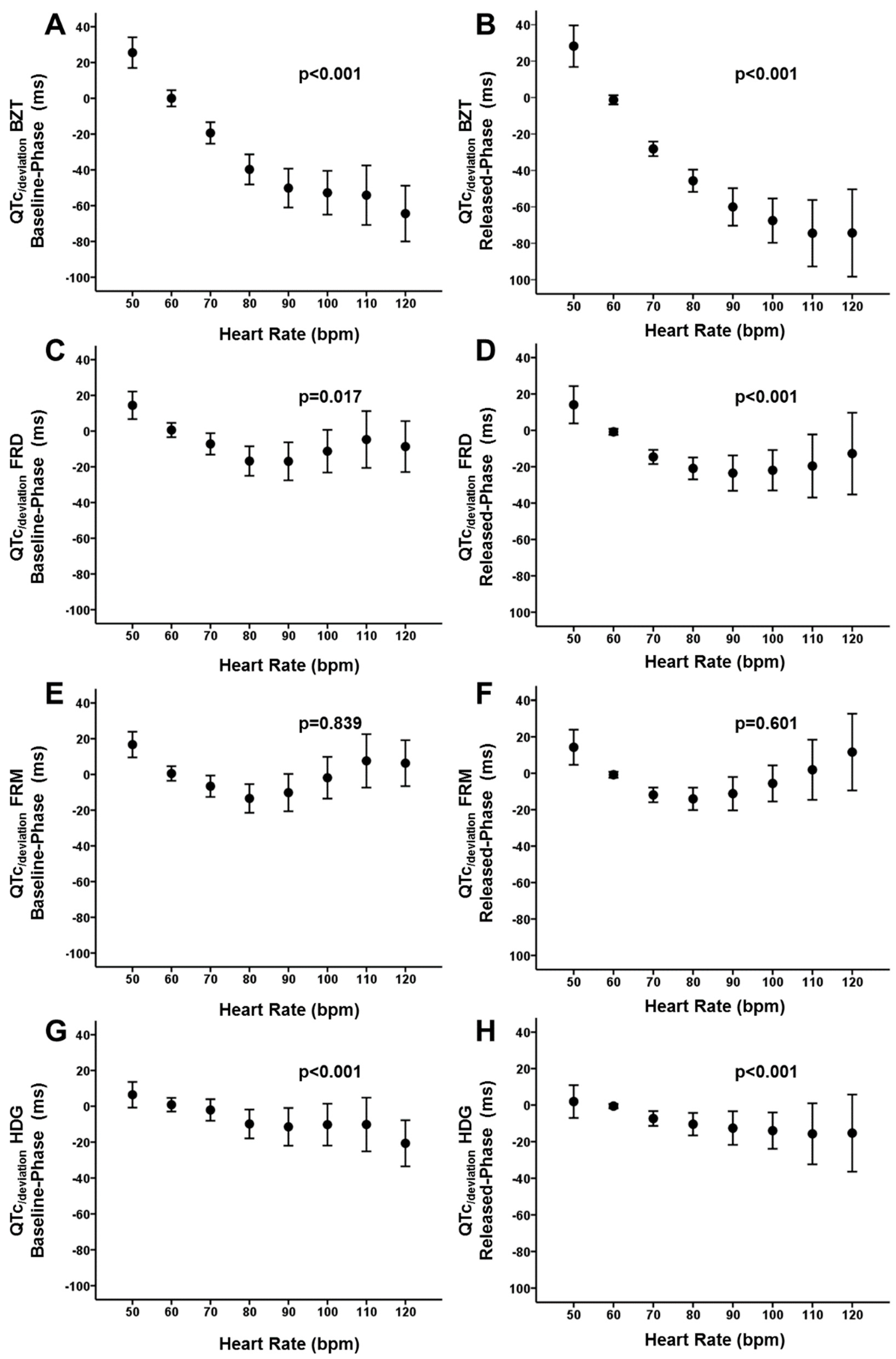
3.2. Performance of Frequency-Correction Formulas
4. Discussion
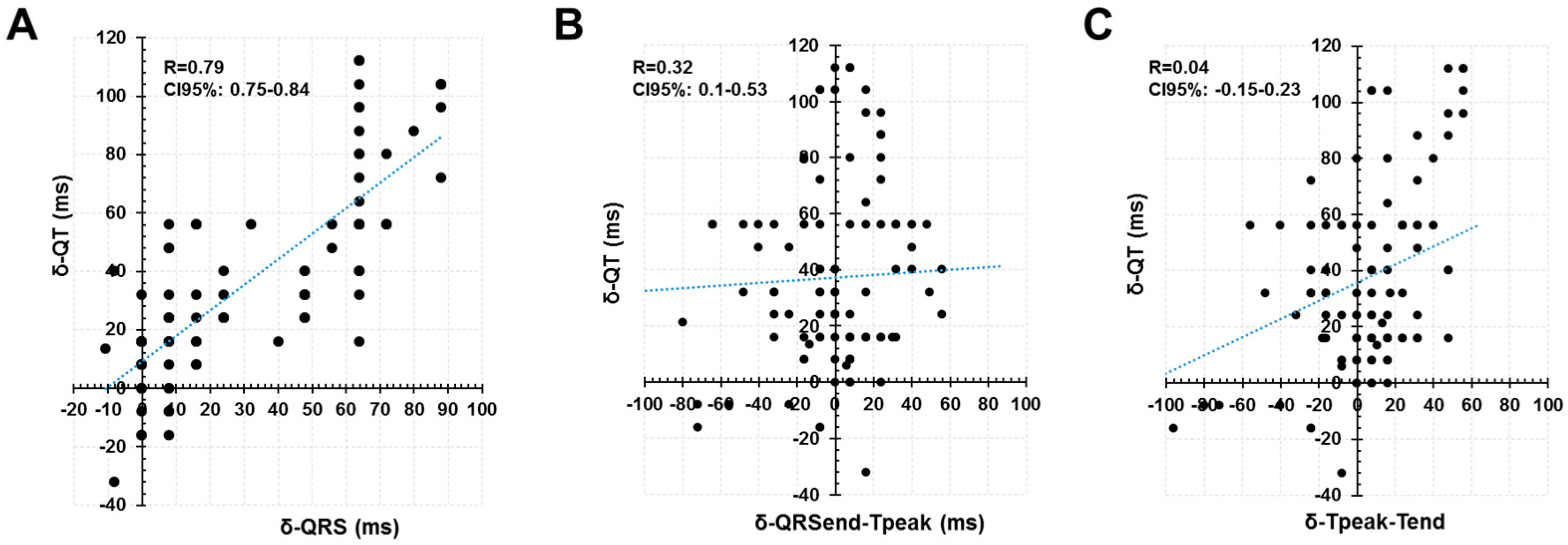
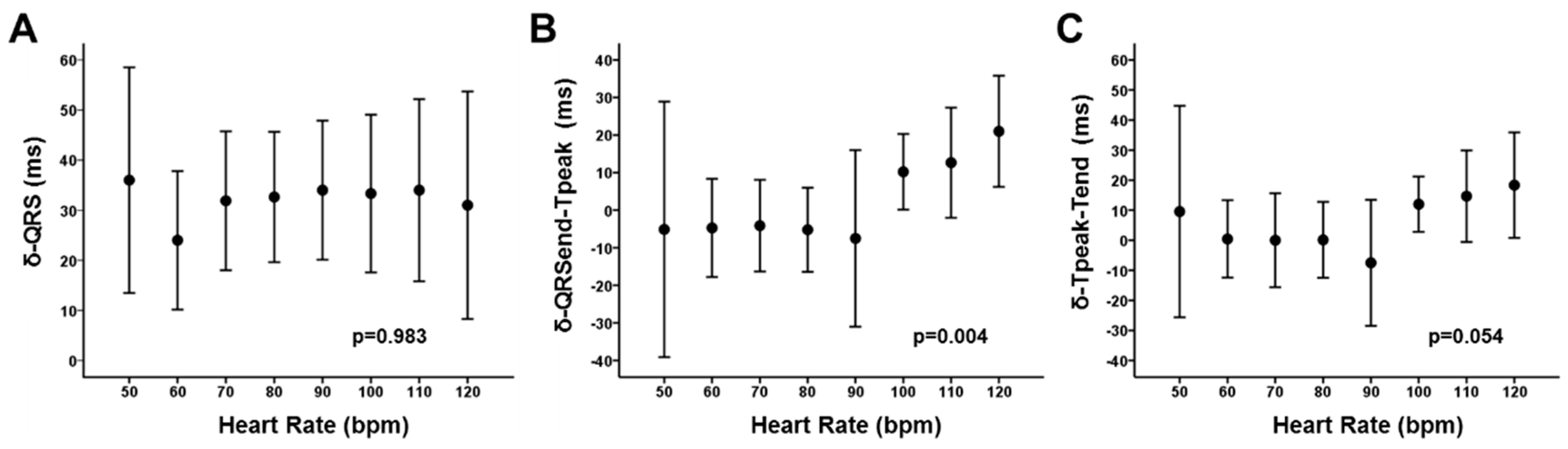
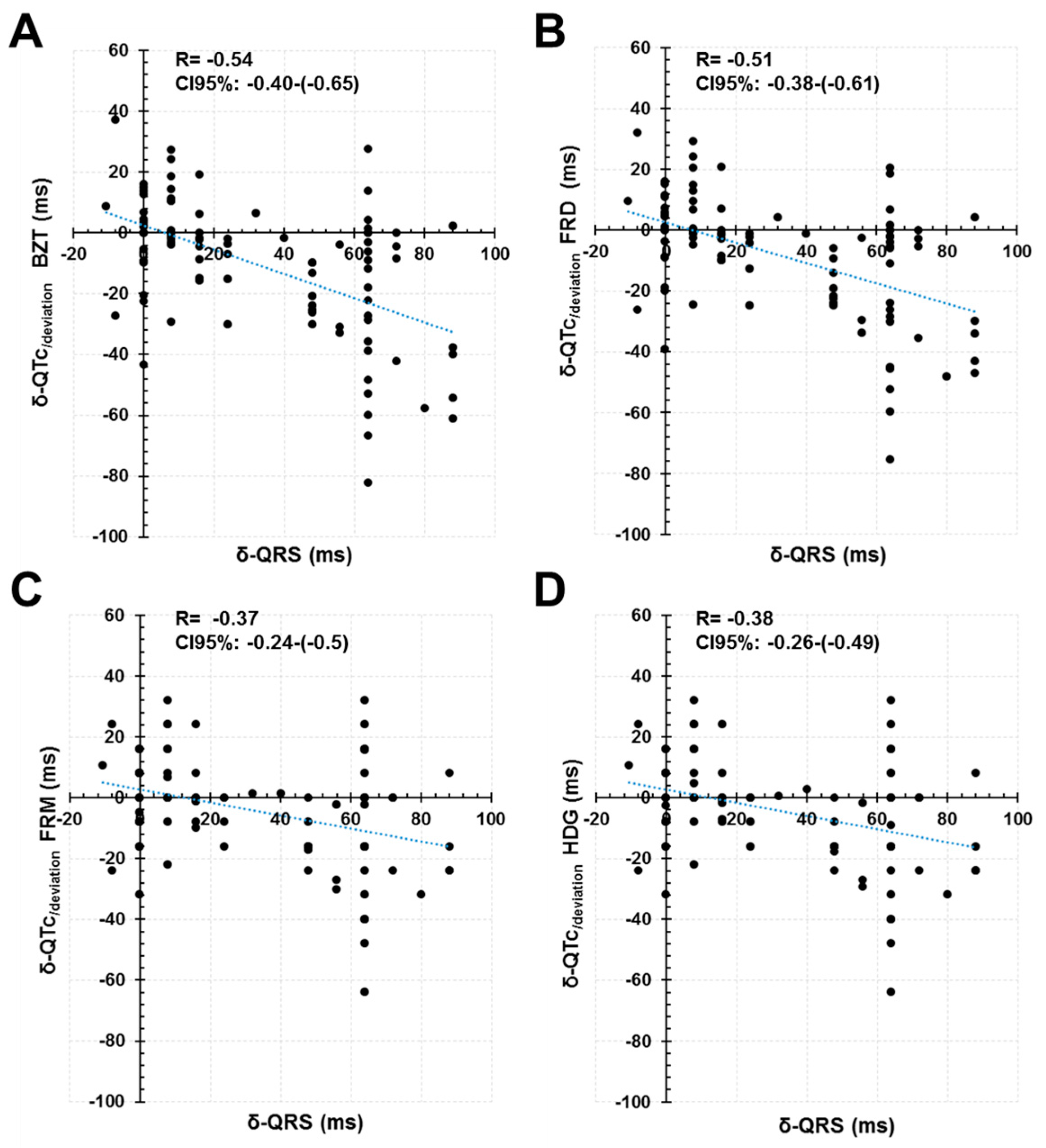
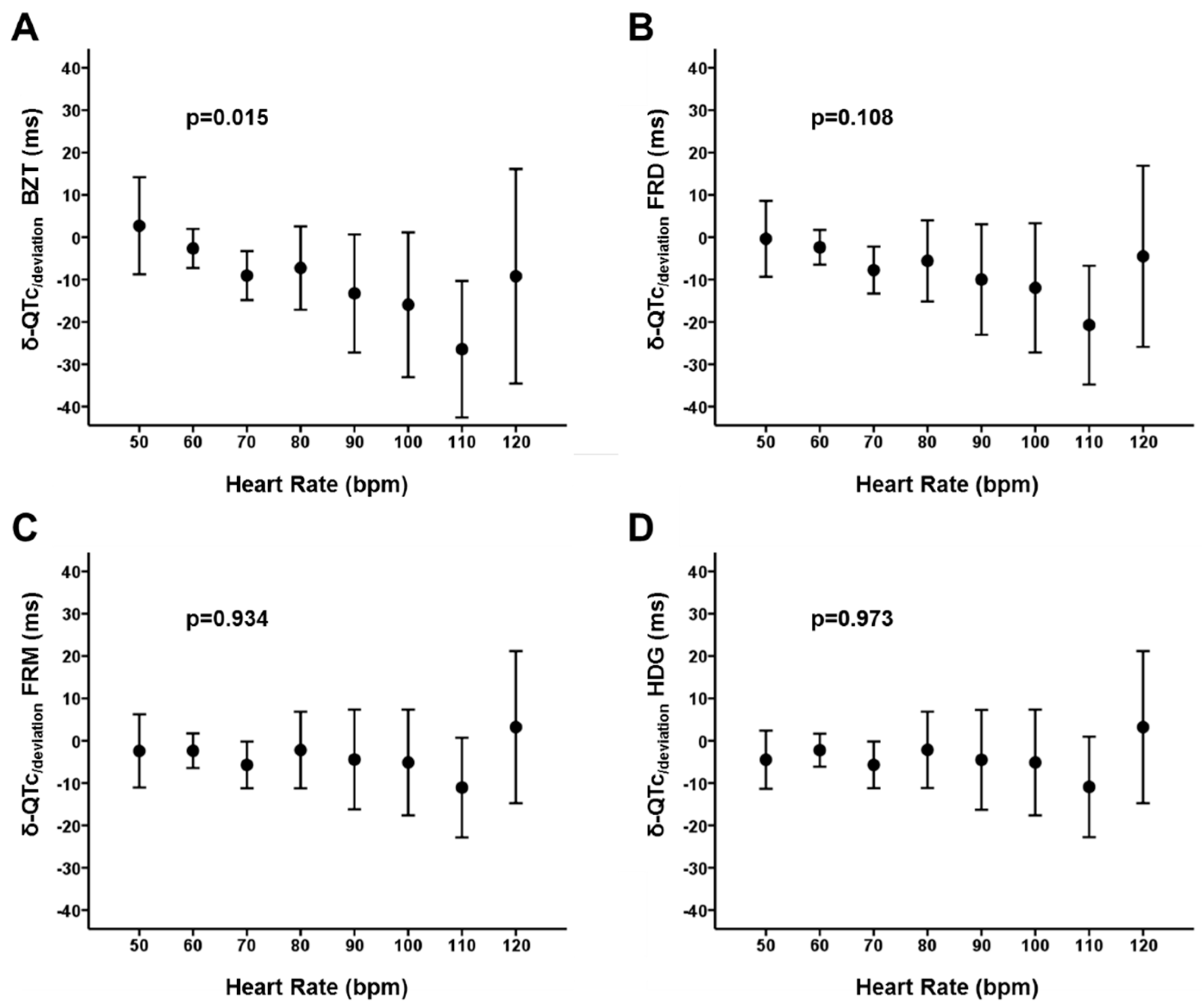
4.1. Increased QRS Duration and Its Relationship with the QT Interval Dynamics
4.2. Increased QRS Duration and Its Relationship with Frequency-Correction Formulas
4.3. Limitations
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Moss, A.J. Measurement of the QT interval and the risk associated with QTc interval prolongation: A review. Am. J. Cardiol. 1993, 72, 23B–25B. [Google Scholar] [CrossRef]
- Rautaharju, P.M.; Surawicz, B.; Gettes, L.S.; Bailey, J.J.; Childers, R.; Deal, B.J.; Gorgels, A.; Hancock, E.W.; Josephson, M.; Kligfield, P.; et al. AHA/ACCF/HRS recommendations for the standardization and interpretation of the electrocardiogram: Part IV: The ST segment, T and U waves, and the QT interval: A scientific statement from the American Heart Association Electrocardiography and Arrhythmias Committee, Council on Clinical Cardiology; the American College of Cardiology Foundation; and the Heart Rhythm Society: Endorsed by the International Society for Computerized Electrocardiology. Circulation 2009, 119, e241–e250. [Google Scholar] [PubMed]
- Bogossian, H.; Frommeyer, G.; Ninios, I.; Hasan, F.; Nguyen, Q.S.; Karosiene, Z.; Mijic, D.; Kloppe, A.; Suleiman, H.; Bandorski, D.; et al. New formula for evaluation of the QT interval in patients with left bundle branch block. Heart Rhythm 2014, 11, 2273–2277. [Google Scholar] [CrossRef] [PubMed]
- Sriwattanakomen, R.; Mukamal, K.J.; Shvilkin, A. A novel algorithm to predict the QT interval during intrinsic atrioventricular conduction from an electrocardiogram obtained during ventricular pacing. Heart Rhythm 2016, 13, 2076–2082. [Google Scholar] [CrossRef] [PubMed]
- Yankelson, L.; Hochstadt, A.; Sadeh, B.; Pick, B.; Finkelstein, A.; Rosso, R.; Viskin, S. New formula for defining ‘normal’ and ‘prolonged’ QT in patients with bundle branch block. J. Electrocardiol. 2018, 51, 481–486. [Google Scholar] [CrossRef]
- Rubín, J.M.; Avanzas, P.; del Valle, R.; Renilla, A.; Ríos, E.; Calvo, D.; Lozano, I.; Anguera, I.; Díaz-Molina, B.; Cequier, A.; et al. Atrioventricular conduction disturbance characterization in transcatheter aortic valve implantation with the CoreValve prosthesis. Circ. Cardiovasc. Interv. 2011, 4, 280–286. [Google Scholar] [CrossRef]
- Rubín, J.M.; Avanzas, P.; Calvo, D.; Morís, C. Intra-Hisian block during transcatheter aortic valve implantation with the CoreValve prosthesis. Revista Española de Cardiología 2011, 64, 168–169. [Google Scholar]
- Postema, P.G.; De Jong, J.S.S.G.; Van der Bilt, I.A.C.; Wilde, A.A.M. Accurate electrocardiographic assessment of the QT interval: Teach the tangent. Heart Rhythm 2008, 5, 1015–1018. [Google Scholar] [CrossRef]
- Bazett, H. An analysis of the time-relations of electrocardiograms. Heart 1920, 7, 353–367. [Google Scholar] [CrossRef]
- Fridericia, L.S. The duration of systole in an electrocardiogram in normal humans and in patients with heart disease. 1920. Ann. Noninvasive Electrocardiol. 2003, 8, 343–351. [Google Scholar] [CrossRef]
- Sagie, A.; Larson, M.G.; Goldberg, R.J.; Bengtson, J.R.; Levy, D. An improved method for adjusting the QT interval for heart rate (the Framingham Heart Study). Am. J. Cardiol. 1992, 70, 797–801. [Google Scholar] [CrossRef]
- Hodges, M.; Salerno, D.; Erlien, D. Bazett’s QT correction reviewed. Evidence that a linear QT correction for heart is better. J. Am. Coll. Cardiol. 1983, 1, 694. [Google Scholar]
- Efron B TR. An. Introduction to the Bootstrap; Chapman & Hall: New York, NY, USA, 1993. [Google Scholar]
- Martínez-Camblor, P.; Corral, N. A general bootstrap algorithm for hypothesis testing. J. Stat. Plan. Inference 2012, 142, 589–600. [Google Scholar] [CrossRef]
- Chiladakis, J.; Kalogeropoulos, A.; Arvanitis, P.; Koutsogiannis, N.; Zagli, F.; Alexopoulos, D. Heart rate-dependence of QTc intervals assessed by different correction methods in patients with normal or prolonged repolarization. Pacing Clin. Electrophysiol. 2010, 33, 553–560. [Google Scholar] [CrossRef] [PubMed]
- Nerbonne, J.M.; Kass, R.S. Molecular physiology of cardiac repolarization. Physiol. Rev. 2005, 85, 1205–1253. [Google Scholar] [CrossRef] [PubMed]
- Yan, G.X.; Antzelevitch, C. Cellular basis for the Brugada syndrome and other mechanisms of arrhythmogenesis associated with ST-segment elevation. Circulation 1999, 100, 1660–1666. [Google Scholar] [CrossRef] [PubMed]
- Yan, G.X.; Antzelevitch, C. Cellular basis for the normal T wave and the electrocardiographic manifestations of the long-QT syndrome. Circulation 1998, 98, 1928–1936. [Google Scholar] [CrossRef]
- Das, G. QT interval and repolarization time in patients with intraventricular conduction delay. J. Electrocardiol. 1990, 23, 49–52. [Google Scholar] [CrossRef]
- Rautaharju, P.M.; Zhang, Z.-M.; Prineas, R.; Heiss, G. Assessment of prolonged QT and JT intervals in ventricular conduction defects. Am. J. Cardiol. 2004, 93, 1017–1021. [Google Scholar] [CrossRef]
- Crow, R.S.; Hannan, P.J.; Folsom, A.R. Prognostic significance of corrected QT and corrected JT interval for incident coronary heart disease in a general population sample stratified by presence or absence of wide QRS complex: The ARIC Study with 13 years of follow-up. Circulation 2003, 108, 1985–1989. [Google Scholar] [CrossRef]
- Simonson, E.; Cady, L.D.; Woodbury, M. The normal Q-T interval. Am. Heart J. 1962, 63, 747–753. [Google Scholar] [CrossRef]
- Malik, M.; Färbom, P.; Batchvarov, V.; Hnatkova, K.; Camm, A.J. Relation between QT and RR intervals is highly individual among healthy subjects: Implications for heart rate correction of the QT interval. Heart 2002, 87, 220–228. [Google Scholar] [CrossRef] [PubMed]
- Luo, S.; Michler, K.; Johnston, P.; Macfarlane, P.W. A comparison of commonly used QT correction formulae: The effect of heart rate on the QTc of normal ECGs. J. Electrocardiol. 2004, 37, 81–90. [Google Scholar] [CrossRef] [PubMed]
- Rabkin, S.W.; Cheng, X.B. Nomenclature, categorization and usage of formulae to adjust QT interval for heart rate. World J. Cardiol. 2015, 7, 315–325. [Google Scholar] [CrossRef] [PubMed]
- Rabkin, S.W.; Szefer, E.; Thompson, D.J.S. A New QT Interval Correction Formulae to Adjust for Increases in Heart Rate. JACC Clin. Electrophysiol. 2017, 3, 756–766. [Google Scholar] [CrossRef] [PubMed]
- Patel, P.J.; Borovskiy, Y.; Killian, A.; Verdino, R.J.; Epstein, A.E.; Callans, D.J.; Marchlinski, F.E.; Deo, R. Optimal QT interval correction formula in sinus tachycardia for identifying cardiovascular and mortality risk: Findings from the Penn Atrial Fibrillation Free study. Heart Rhythm 2016, 13, 527–535. [Google Scholar] [CrossRef]
- Vandenberk, B.; Vandael, E.; Robyns, T.; Vandenberghe, J.; Garweg, C.; Foulon, V.; Ector, J.; Willems, R. QT correction across the heart rate spectrum, in atrial fibrillation and ventricular conduction defects. Pacing Clin. Electrophys. 2018, 41, 1101–1108. [Google Scholar] [CrossRef]
- Nordin, C.; Siri, F.; Aronson, R.S. Electrophysiologic characteristics of single myocytes isolated from hypertrophied guinea-pig hearts. J. Mol. Cell. Cardiol. 1989, 21, 729–739. [Google Scholar] [CrossRef]
- Mason, J.W.; Badilini, F.; Vaglio, M.; Lux, R.L.; Aysin, B.; Moon, T.E.; Heinz, B.; Strachan, I. A fundamental relationship between intraventricular conduction and heart rate. J. Electrocardiol. 2016, 49, 362–370. [Google Scholar] [CrossRef]
- Mason, J.W.; Strauss, D.G.; Vaglio, M.; Badilini, F. Correction of the QRS duration for heart rate. J. Electrocardiol. 2019, 54, 1–4. [Google Scholar] [CrossRef]
- Datino, T.; Almendral, J.; Gonzalez-Torrecilla, E.; Atienza, F.; García-Fernández, F.J.; Arenal, A.; Atea, L.; Fernandez-Aviles, F. Rate-related changes in QRS morphology in patients with fixed bundle branch block: Implications for differential diagnosis of wide QRS complex tachycardia. Eur. Heart J. 2008, 29, 2351–2358. [Google Scholar] [CrossRef] [PubMed]
| Characteristics | N = 23 | ||
| Age (years) | 84 (±4) | ||
| Weight (Kg) | 70.29 (±12.98) | ||
| Height (cm) | 158 (±8) | ||
| Body mass index | 28.06 (±4, 9) | ||
| Male Gender (N; %) | 11 (48%) | ||
| Cardiovascular Risk Factors | |||
| Hypertension (N; %) | 20 (87%) | ||
| Diabetes (N; %) | 7 (30%) | ||
| Dyslipidemia (N; %) | 17 (74%) | ||
| Smoking (N; %) | 0 (0%) | ||
| Former | 5 (22%) | ||
| Coronary artery disease (N; %) | 8 (35%) | ||
| Peripheral vascular disease (N; %) | 6 (26%) | ||
| Chronic Kidney disease (N; %) | 10 (44%) | ||
| Dialysis treatment | 0 (0%) | ||
| Echocardiographics findings | |||
| IVS (cm) | 1.6 (±0.3) | ||
| LVED Diameter (cm) | 4,8 (±0.8) | ||
| LV mass (g/m2) | 167.76 (±55.18) | ||
| RWT | 0.59 (±0.19) | ||
| LV ejection fraction (%) | 56 (±14) | ||
| Aortic mean gradient (mmHg) | 50 (±14) | ||
| Antiarrhythmics | |||
| Class I (N; %) | 0 (0%) | ||
| Class II (N; %) | 12 (52%) | ||
| Class III (N; %) | 0 (0%) | ||
| Class IV (N; %) | 1 (4%) | ||
| Ivabradine (N; %) | 1 (4%) | ||
| Baseline-Phase | Released-Phase | δ Released-Baseline (IC95%) | p-Value | ||
|---|---|---|---|---|---|
| QRS (ms) | 98.50 (±21.78) | 131.0 (±25.77) | 32.5 (31.5–33.6) | p < 0.001 | |
| Axis (degrees) | 8.55° (±36.2) | 3.18º (±43.64) | p = 0.376 | ||
| LBBB % (N) | 0 | 39% (9) | p = 0.001 | ||
| RBBB % (N) | 9% (2) | 17% (4) | p = 0.162 | ||
| LAFH % (N) | 13% (3) | 13% (3) | |||
| LPFH % (N) | 0 | 0 | |||
| QT (ms) | 409.2 (±37.63) | 444.6 (±45.41) | 35.4 (33.4–37.4) | p < 0.001 | |
| 50bpm | 469.33 (±27.09) | 520.0 (±17.53) | |||
| 60bpm | 448.53 (±26.44) | 477.16 (±37.80) | |||
| 70bpm | 432.44 (±22.96) | 468.0 (±31.79) | |||
| 80bpm | 417.37 (±24.59) | 454.48 (±32.06) | |||
| 90bpm | 401.60 (±19.53) | 435.60 (±34.58) | |||
| 100bpm | 382.22 (±2.08) | 417.33 (±33.97) | |||
| 110bpm | 368.67 (±20.60) | 404.67 (±30.60) | |||
| 120bpm | 359.67 (±17.31) | 388.0 (±31.42) | |||
| QTpeak (ms) | 322.2 (±32.12) | 353.6 (±44.46) | 31.3 (27.8–35.2) | p = 0.001 | |
| Tpeak-Tend (ms) | 87.0 (±19.31) | 91.07 (±28.35) | 4.07 (−0.16–7.90) | p = 0.044 | |
| QRSend-Tpeak (ms) | 223.7 (±34.64) | 222.5 (±43.92) | −1.18 (−4.84–2.61) | p = 0.563 | |
| QTc Bazett’s (ms) | 480.9 (±31.47) | 522.5 (±4.21) | 41.6 (38.6–44.5) | p < 0.001 | |
| QTc Fridericia (ms) | 454.5 (±23.92) | 493.8 (±35.16) | 39.3 (36.8–41.8) | p < 0.001 | |
| QTc Framingham (ms) | 449.2 (±22.35) | 484.4 (±31.95) | 35.3 (33.2–37.4) | p < 0.001 | |
| QTc Hodges (ms) | 453.6 (±21.65) | 488.9 (±31.19) | 35.4 (33.4–37.4) | p < 0.001 | |
| Baseline-Phase (SD) | Released-Phase (SD) | p-Value | |
|---|---|---|---|
| Telesystolic LV presure (mmHg) | 197.44 (±30.77) | 159.61 (±33.65) | <0.001 |
| Telediastolic LV presure mmHg | 3.17 (±7.98) | 17.06 (±17.85) | 0.006 |
| Temperature (°C) | 36.77 (±0.4) | 36.9 (±0.27) | 0.047 |
| Na+ (mmol/l) | 141.61 (±3.09) | 138.72 (±2.78) | 0.053 |
| K+ (mmol/l) | 3.70 (±0.29) | 3.70 (±0.37) | 0.929 |
| Ca2+ (mmol/l) | 1.17 (±0.55) | 1.12 (±0.52) | 0.061 |
| Cl−(mmol/l) | 106.94 (±3.70) | 106.17 (±3.94) | 0.255 |
| Glucose (mg/dl) | 108.39 (±35.92) | 107.56 (±34.23) | 0.697 |
| pH | 7.37 (±0.33) | 7.34 (±0.44) | 0.049 |
| pCO2 (mmHg) | 46.83 (±6.03) | 46.85 (±6.52) | 0.981 |
| HCO3− (mmol/l) | 27.32 (±3.25) | 25.30 (±3.42) | 0.051 |
| Hemoglobin (g/dl) | 11.76 (±1.60) | 11.05 (±1.69) | 0.069 |
| Lactate (mmol/l) | 0.55 (±0.22) | 0.57 (±0.29) | 0.959 |
| QTc/deviation * (ms; CI95%) | Correlation Coefficient with QT60 (CI95%) | |||
|---|---|---|---|---|
| Basaline-Phase | Released-Phase | Basaline-Phase | Released-Phase | |
| Bazett | −31.1 (−35,0 – [−26.8]) | −41.8 (−46.9 – [−36.4]) | 0.462 (0.344–0.574) | 0.613 (0.505–0.706) |
| Fridericia | −6.46 (−8.61 – [−4.26]) | −14.8 (−17.2 – [−12.4]) | 0.694 (0.628–0.755) | 0.825 (0.783–0.862) |
| Framinghan | −1.14 (−3.38 – 1.19) | −5.42 (−7.51 – [−3.24]) | 0.698 (0.633–0.761) | 0.843 (0.796–0.888) |
| Hodges | −5.05 (−6.78 – [−3.29]) | −9.43 (−11.2 – [−7.70]) | 0.722 (0.651–0.789) | 0.867 (0.836–0.897) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Colunga, S.; Padrón, R.; García-Iglesias, D.; Rubín, J.M.; Pérez, D.; del Valle, R.; Avanzas, P.; Morís, C.; Calvo, D. The QT Interval Dynamic in a Human Experimental Model of Controlled Heart Rate and QRS Widening. J. Clin. Med. 2019, 8, 1417. https://doi.org/10.3390/jcm8091417
Colunga S, Padrón R, García-Iglesias D, Rubín JM, Pérez D, del Valle R, Avanzas P, Morís C, Calvo D. The QT Interval Dynamic in a Human Experimental Model of Controlled Heart Rate and QRS Widening. Journal of Clinical Medicine. 2019; 8(9):1417. https://doi.org/10.3390/jcm8091417
Chicago/Turabian StyleColunga, Santiago, Remigio Padrón, Daniel García-Iglesias, José Manuel Rubín, Diego Pérez, Raquel del Valle, Pablo Avanzas, César Morís, and David Calvo. 2019. "The QT Interval Dynamic in a Human Experimental Model of Controlled Heart Rate and QRS Widening" Journal of Clinical Medicine 8, no. 9: 1417. https://doi.org/10.3390/jcm8091417
APA StyleColunga, S., Padrón, R., García-Iglesias, D., Rubín, J. M., Pérez, D., del Valle, R., Avanzas, P., Morís, C., & Calvo, D. (2019). The QT Interval Dynamic in a Human Experimental Model of Controlled Heart Rate and QRS Widening. Journal of Clinical Medicine, 8(9), 1417. https://doi.org/10.3390/jcm8091417






