Management and Treatment of Varicocele in Children and Adolescents: An Endocrinologic Perspective
Abstract
1. Introduction
2. Pathogenesis of Testicular Damage
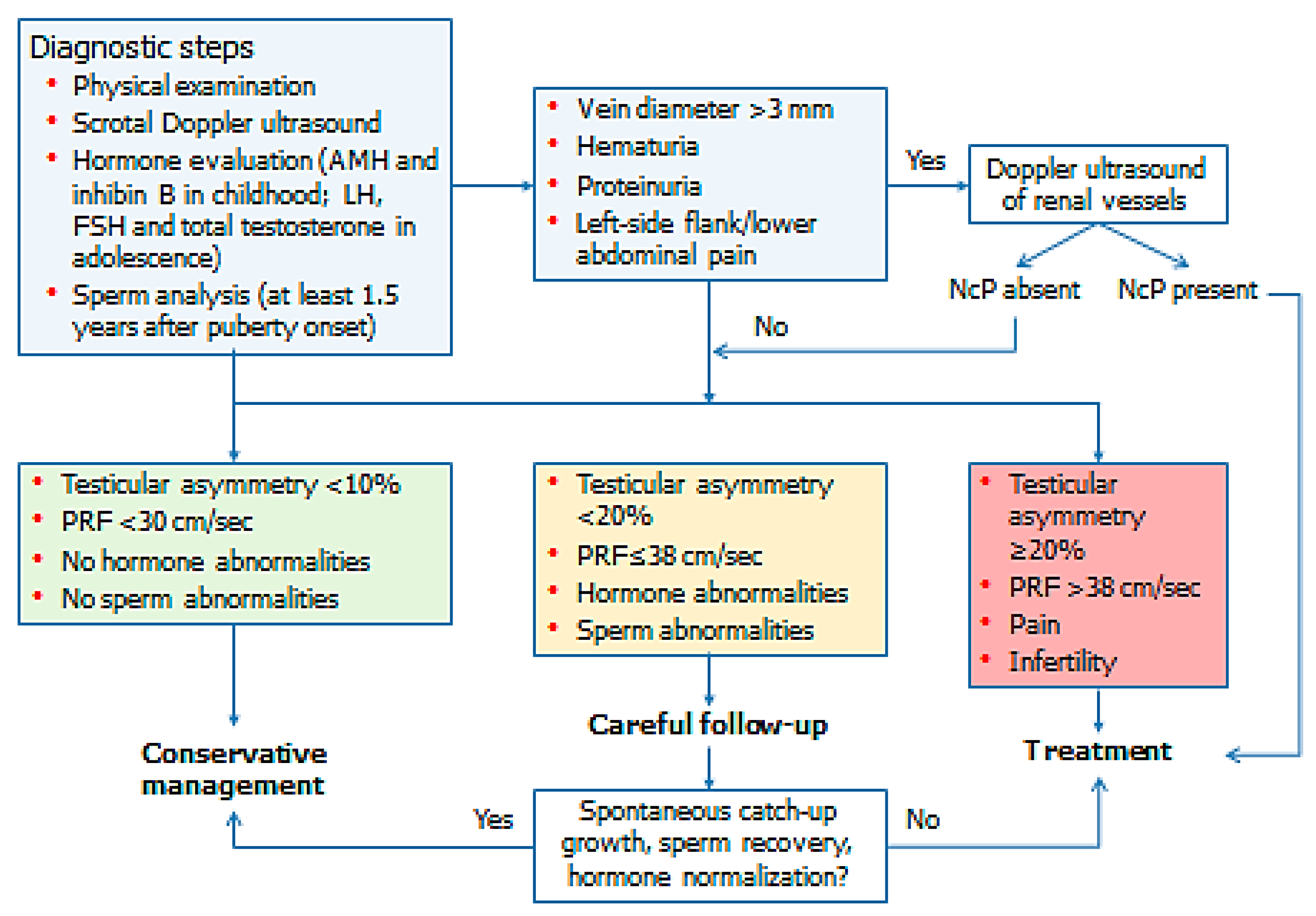
3. Evaluation
4. Management
5. Treatment Options
6. Established Guidelines and Societies’ Positions
7. Conclusions and Authors’ Recommendations
Author Contributions
Conflicts of Interest
References
- Alsaikhan, B.; Alrabeeah, K.; Delouya, G.; Zini, A. Epidemiology of varicocele. Asian J. Androl. 2016, 18, 179–181. [Google Scholar] [PubMed]
- Damsgaard, J.; Joensen, U.N.; Carlsen, E.; Erenpreiss, J.; Blomberg Jensen, M.; Matulevicius, V.; Zilaitiene, B.; Olesen, I.A.; Perheentupa, A.; Punab, M.; et al. Varicocele is associated with impaired semen quality and reproductive hormone levels: A study of 7035 healthy young men from six European countries. Eur. Urol. 2016, 70, 1019–1029. [Google Scholar] [CrossRef] [PubMed]
- Akbay, E.; Cayan, S.; Doruk, E.; Duce, M.N.; Bozlu, M. The prevalence of varicocele and varicocele-related testicular atrophy in Turkish children and adolescents. BJU Int. 2000, 86, 490–493. [Google Scholar] [CrossRef] [PubMed]
- Mongioì, L.M.; Mammino, L.; Compagnone, M.; Condorelli, R.A.; Basile, A.; Alamo, A.; La Vignera, S.; Morgia, G.; Russo, G.I.; Calogero, A.E. Effects of varicocele treatment on sperm conventional parameters: surgical varicocelectomy versus sclerotherapy. Cardiovasc. Intervent. Radiol. 2019, 42, 396–404. [Google Scholar] [CrossRef] [PubMed]
- Kirby, E.W.; Wiener, L.E.; Rajanahally, S.; Crowell, K.; Coward, R.M. Undergoing varicocele repair before assisted reproduction improves pregnancy rate and live birth rate in azoospermic and oligospermic men with a varicocele: A systematic review and meta-analysis. Fertil. Steril. 2016, 106, 1338–1343. [Google Scholar] [CrossRef]
- La Vignera, S.; Condorelli, R.; Vicari, E.; D’Agata, R.; Calogero, A.E. Effects of varicocelectomy on sperm DNA fragmentation, mitochondrial function, chromatin condensation, and apoptosis. J. Androl. 2012, 33, 389–396. [Google Scholar] [CrossRef]
- Li, F.; Yue, H.; Yamaguchi, K.; Okada, K.; Matsushita, K.; Ando, M.; Chiba, K.; Fujisawa, M. Effect of surgical repair on testosterone production in infertile men with varicocele: A meta-analysis. Int. J. Urol. 2012, 19, 149–154. [Google Scholar] [CrossRef]
- Cho, C.L.; Esteves, S.C.; Agarwal, A. Indications and outcomes of varicocele repair. Panminerva Medica 2019, 61, 152–163. [Google Scholar] [CrossRef]
- Glassberg, K.I.; Korets, R. Update on the management of adolescent varicocele. F1000 Med. Rep. 2010, 12, 2. [Google Scholar] [CrossRef]
- Chung, J.M.; Lee, S.D. Current issues in adolescent varicocele: Pediatric urological perspectives. World J. Men’s Health. 2018, 36, 123–131. [Google Scholar] [CrossRef]
- Nork, J.J.; Berger, J.H.; Crain, D.S.; Christman, M.S. Youth varicocele and varicocele treatment: A meta-analysis of semen outcomes. Fertil. Steril. 2014, 102, 381–387. [Google Scholar] [CrossRef] [PubMed]
- Diamond, D.A.; Zurakowski, D.; Bauer, S.B.; Borer, J.G.; Peters, C.A.; Cilento, B.G., Jr.; Paltiel, H.J.; Rosoklija, I.; Retik, A.B. Relationship of varicocele grade and testicular hypotrophy to semen parameters in adolescents. J. Urol. 2007, 178, 1584–1588. [Google Scholar] [CrossRef] [PubMed]
- Niu, X.B.; Tang, J.; Wang, H.B.; Yan, L.; Zhang, C.Y.; Wang, G.C.; Liang, J.; Dou, X.Y.; Fu, G.B. Inhibin B level helps evaluate the testicular function of prepubertal patients with varicocele. Zhonghua Nan KeXue 2018, 24, 618–621. [Google Scholar]
- Gökçe, A.; Davarci, M.; Yalçinkaya, F.R.; Güven, E.O.; Kaya, Y.S.; Helvaci, M.R.; Balbay, M.D. Hereditary behavior of varicocele. J. Androl. 2010, 31, 288–290. [Google Scholar] [CrossRef] [PubMed]
- Hassanin, A.M.; Ahmed, H.H.; Kaddah, A.N. A global view of the pathophysiology of varicocele. Andrology 2018, 6, 654–661. [Google Scholar] [CrossRef] [PubMed]
- Condorelli, R.A.; Cannarella, R.; Calogero, A.E.; La Vignera, S. Evaluation of testicular function in prepubertal children. Endocrine 2018, 62, 274–280. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.T.; Shao, C.H.; Wang, P.T.; Liu, Y.; Hao, W.Y.; Feng, Y.L.; Liu, S.H.; Wang, X.S. High temperature reduces the proliferation of and occludin expression in rat Sertoli cells in vitro. Zhonghua Nan KeXue 2012, 18, 920–924. [Google Scholar]
- Abdel-Meguid, T.A.; Al-Sayyad, A.; Tayib, A.; Farsi, H.M. Does varicocele repair improve male infertility? An evidence-based perspective from a randomized, controlled trial. Eur. Urol. 2011, 59, 455–461. [Google Scholar] [CrossRef]
- Macey, M.R.; Owen, R.C.; Ross, S.S.; Coward, R.M. Best practice in the diagnosis and treatment of varicocele in children and adolescents. Ther. Adv. Urol. 2018, 10, 273–282. [Google Scholar] [CrossRef]
- Dubin, L.; Amelar, R.D. Varicocele size and results of varicocelectomy in selected subfertile men with varicocele. Fertil. Steril. 1970, 21, 606–609. [Google Scholar] [CrossRef]
- Condorelli, R.A.; Calogero, A.E.; Vicari, E.; Mongioi’, L.; Burgio, G.; Cannarella, R.; Giacone, F.; Iacoviello, L.; Morgia, G.; Favilla, V.; et al. Reduced seminal concentration of CD45pos cells after follicle-stimulating hormone treatment in selected patients with idiopathic oligoasthenoteratozoospermia. Int. J. Endocrinol. 2014, 2014, 372060. [Google Scholar] [CrossRef] [PubMed]
- Pauroso, S.; Di Leo, N.; Fulle, I.; Di Segni, M.; Alessi, S.; Maggini, E. Varicocele: Ultrasonographic assessment in dailyclinical practice. J. Ultrasound. 2011, 14, 199–204. [Google Scholar] [CrossRef] [PubMed]
- Englund, K.M.; Rayment, M. Nutcracker syndrome: A proposed ultrasound protocol. Australasian J. Ultrasound Med. Banner. 2018, 21, 75–78. [Google Scholar] [CrossRef]
- Hannick, J.H.; Blais, A.S.; Kim, J.K.; Traubici, J.; Shiff, M.; Book, R.; Lorenzo, A.J. Prevalence, doppler ultrasound findings, and clinical implications of the nutcracker phenomenon in pediatric varicoceles. Urology 2019, 128, 78–83. [Google Scholar] [CrossRef] [PubMed]
- Jedrzejewski, G.; Osemlak, P.; Wieczorek, A.P.; Nachulewicz, P. Prognostic values of shear wave elastography in adolescent boys with varicocele. J. Pediatr. Urol. 2019, 15, e1–e5. [Google Scholar] [CrossRef]
- Dabaja, A.A.; Wosnitzer, M.S.; Bolyakov, A.; Schlegel, P.N.; Paduch, D.A. When to ask male adolescents to provide semen sample for fertility preservation? Transl. Androl. Urol. 2014, 3, 2–8. [Google Scholar] [PubMed]
- Fine, R.G.; Gitlin, J.; Reda, E.F.; Palmer, L.S. Barriers to use of semen analysis in the adolescent with a varicocele: Survey of patient, parental, and practitioner attitudes. J. Pediatr. Urol. 2016, 12, 41.e1–41.e6. [Google Scholar] [CrossRef]
- Haans, L.C.; Laven, J.S.; Mali, W.P.; te Velde, E.R.; Wensing, C.J. Testis volumes, semen quality, and hormonal patterns in adolescents with and without a varicocele. Fertil. Steril. 1991, 56, 731–736. [Google Scholar] [CrossRef]
- Paduch, D.A.; Niedzielski, J. Semen analysis in young men with varicocele: Preliminary study. J. Urol. 1996, 156, 788–790. [Google Scholar] [CrossRef]
- Romeo, C.; Arrigo, T.; Impellizzeri, P.; Manganaro, A.; Antonuccio, P.; Di Pasquale, G.; Messina, M.F.; Marseglia, L.; Formica, I.; Zuccarello, B. Alteredseruminhibin b levels in adolescents with varicocele. J. Pediatr. Surg. 2007, 42, 390–394. [Google Scholar] [CrossRef]
- Trigo, R.V.; Bergadá, I.; Rey, R.; Ballerini, M.G.; Bedecarrás, P.; Bergadá, C.; Gottlieb, S.; Campo, S. Altered serum profile of inhibin B, Pro-αC and anti-Müllerian hormone in prepubertal and pubertal boys with varicocele. Clin. Endocrinol. 2004, 60, 758–764. [Google Scholar] [CrossRef] [PubMed]
- Chu, D.I.; Zderic, S.A.; Shukla, A.R.; Srinivasan, A.K.; Tasian, G.E.; Weiss, D.A.; Long, C.J.; Canning, D.A.; Kolon, T.F. The natural history of semen parameters in untreated asymptomatic adolescent varicocele patients: A retrospective cohort study. J. Pediatr. Urol. 2017, 13, 77.e1–77.e5. [Google Scholar] [CrossRef] [PubMed]
- Kolon, T.F.; Clement, M.R.; Cartwright, L.; Bellah, R.; Carr, M.C.; Canning, D.A.; Snyder, H.M. Transient asynchronous testicular growth in adolescent males with a varicocele. J. Urol. 2008, 180, 1111–1114. [Google Scholar] [CrossRef] [PubMed]
- Kozakowski, K.A.; Gjertson, C.K.; Decastro, G.J.; Poon, S.; Gasalberti, A.; Glassberg, K.I. Peak retrograde flow: A novel predictor of persistent, progressive and new onset asymmetry in adolescent varicocele. J. Urol. 2009, 181, 2717–2722. [Google Scholar] [CrossRef] [PubMed]
- Van Batavia, J.P.; Badalato, G.; Fast, A.; Glassberg, K.I. Adolescent varicocele-is the 20/38 harbinger a durable predictor of testicular asymmetry? J. Urol. 2013, 189, 1897–1901. [Google Scholar] [CrossRef] [PubMed]
- Cimador, M.; Castagnetti, M.; Gattuccio, I.; Pensabene, M.; Sergio, M.; De Grazia, E. The hemodynamic approach to evaluating adolescent varicocele. Nat. Rev. Urol. 2012, 9, 247–257. [Google Scholar] [CrossRef] [PubMed]
- Glassberg, K.I. My indications for treatment of the adolescent varicocele (and why?). Trans. Androl. Urol. 2014, 3, 402–412. [Google Scholar]
- Zhou, T.; Zhang, W.; Chen, Q.; Li, L.; Cao, H.; Xu, C.L.; Sun, Y.H. Effect of varicocelectomy on testis volume and semen parameters in adolescents: A meta-analysis. Asian J. Androl. 2015, 17, 1012–1016. [Google Scholar] [PubMed]
- Locke, J.A.; Maryam, N.; Kourosh, A. Treatment of varicocele in children and adolescents: A systematic review and meta-analysis of randomized controlled trials. J. Pediatr. Urol. 2017, 13, 437–445. [Google Scholar] [CrossRef] [PubMed]
- Silay, M.S.; Hoen, L.; Quadackaers, J.; Undre, S.; Bogaert, G.; Dogan, H.S.; Kocvara, R.; Nijman, R.J.M.; Radmayr, C.; Tekgul, S.; et al. Treatment of varicocele in children and adolescents: A systematic review and meta-analysis from the European association of urology/European Society for Paediatric Urology guidelines panel. Eur. Urol. 2019, 75, 448–461. [Google Scholar] [CrossRef] [PubMed]
- Schwentner, C.; Radmayr, C.; Lunacek, A.; Gozzi, C.; Pinggera, G.M.; Neururer, R.; Oswald, J. Laparoscopic varicocele ligation in children and adolescents using isosulphan blue: A prospective randomized trial. BJU Int. 2006, 98, 861–865. [Google Scholar] [CrossRef] [PubMed]
- Shiraishi, K.; Oka, S.; Matsuyama, H. Surgical comparison of subinguinal and high inguinal microsurgical varicocelectomy for adolescent varicocele. Int. J. Urol. 2016, 23, 338–342. [Google Scholar] [CrossRef] [PubMed]
- Laven, J.S.; Haans, L.C.; Mali, W.P.; te Velde, E.R.; Wensing, C.J.; Eimers, J.M. Effects of varicocele treatment in adolescents: A randomized study. Fertil. Steril. 1992, 58, 756–762. [Google Scholar] [CrossRef]
- Moursy, E.E.; El Dahshoury, M.Z.; Hussein, M.M.; Mourad, M.Z.; Badawy, A.A. Dilemma of adolescent varicocele: Long-term outcome in patients managed surgically and in patients managed expectantly. J. Pediatr. Urol. 2013, 9, 1018–1022. [Google Scholar] [CrossRef] [PubMed]
- Paduch, D.A.; Niedzielski, J. Repair versus observation in adolescent varicocele: A prospective study. J. Urol. 1997, 12, 410–413. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, M.; Hibi, H.; Katsuno, S.; Miyake, K. Effects of varicocelectomy on testis volume and semen parameters in adolescents: A randomized prospective study. Nagoya J. Med. Sci. 1995, 58, 127–132. [Google Scholar] [PubMed]
- Çayan, S.; Şahin, S.; Akbay, E. Paternity rates and time to conception in adolescents with varicocele undergoing microsurgical varicocele repair vs observation only: A single institution experience with 408 patients. J. Urol. 2017, 198, 195–201. [Google Scholar] [CrossRef] [PubMed]
- Bogaert, G.; Orye, C.; De Win, G. Pubertal screening and treatment for varicocele do not improve chance of paternity as adult. J. Urol. 2013, 189, 2298–2303. [Google Scholar] [CrossRef]
- Dong, W.; Yao, Y.; Huang, H.; Han, J.; Zhao, X.; Huang, J. Surgical management of nutcracker phenomenon presenting as left varicocele in adolescents: A novel approach. J. Pediatr. Urol. 2014, 10, 424–429. [Google Scholar] [CrossRef]
- Practice committee of the American society for reproductive medicine; Society for male reproduction and urology. Report on varicocele and infertility: A committee opinion. Fertil. Steril. 2014, 102, 1556–1560. [Google Scholar] [CrossRef]
- Jungwirth, A.; Giwercman, A.; Tournaye, H.; Diemer, T.; Kopa, Z.; Dohle, G.; Krausz, C. European Association of Urology working group on male infertility. European Association of Urology guidelines on male infertility: The 2012 update. Eur. Urol. 2012, 62, 324–332. [Google Scholar] [CrossRef] [PubMed]
| Scale | Degree | Description |
|---|---|---|
| Sarteschi | I | Reflux detected only during the Valsalva maneuver, in the absence of evident scrotal varicosity during US study. |
| II | Small posterior varicosity that extends to the superior pole of the testes. Their diameter increases and the reflux becomes detectable in the supratesticular region only during the Valsalva maneuver. | |
| III | Vessels appear enlarged in the superior pole only in the standing position. No enlargement can be detected in the supine position. Reflux is observed only during the Valsalva maneuver. | |
| IV | Vessels appear enlarged in the supine position. Dilatation is more marked during the Valsalva maneuver. | |
| V | Venus ectasia is detected in the prone and supine position. Reflux occurs at rest and it does not increase during the Valsalva maneuver. | |
| Dubin | 0 | Moderate and transient venous reflux during the Valsalva maneuver. |
| I | Persistent venous reflux that ends before the Valsalva maneuver is completed. | |
| II | Persistent venous reflux through the entire Valsalva maneuver. | |
| III | Venous reflux is basally detected and does not change during the Valsalva manuever |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cannarella, R.; Calogero, A.E.; Condorelli, R.A.; Giacone, F.; Aversa, A.; La Vignera, S. Management and Treatment of Varicocele in Children and Adolescents: An Endocrinologic Perspective. J. Clin. Med. 2019, 8, 1410. https://doi.org/10.3390/jcm8091410
Cannarella R, Calogero AE, Condorelli RA, Giacone F, Aversa A, La Vignera S. Management and Treatment of Varicocele in Children and Adolescents: An Endocrinologic Perspective. Journal of Clinical Medicine. 2019; 8(9):1410. https://doi.org/10.3390/jcm8091410
Chicago/Turabian StyleCannarella, Rossella, Aldo E. Calogero, Rosita A. Condorelli, Filippo Giacone, Antonio Aversa, and Sandro La Vignera. 2019. "Management and Treatment of Varicocele in Children and Adolescents: An Endocrinologic Perspective" Journal of Clinical Medicine 8, no. 9: 1410. https://doi.org/10.3390/jcm8091410
APA StyleCannarella, R., Calogero, A. E., Condorelli, R. A., Giacone, F., Aversa, A., & La Vignera, S. (2019). Management and Treatment of Varicocele in Children and Adolescents: An Endocrinologic Perspective. Journal of Clinical Medicine, 8(9), 1410. https://doi.org/10.3390/jcm8091410









