The Outcomes of Pancreatic Transplantation from Pediatric Donors–A Single Institution Experience
Abstract
1. Introduction
2. Patients and Methods
2.1. Patients
2.2. The Study Design
2.3. Transplantation Methods and Immunosuppression Protocols
2.4. Statistical Analyses
2.5. Ethical Aspects
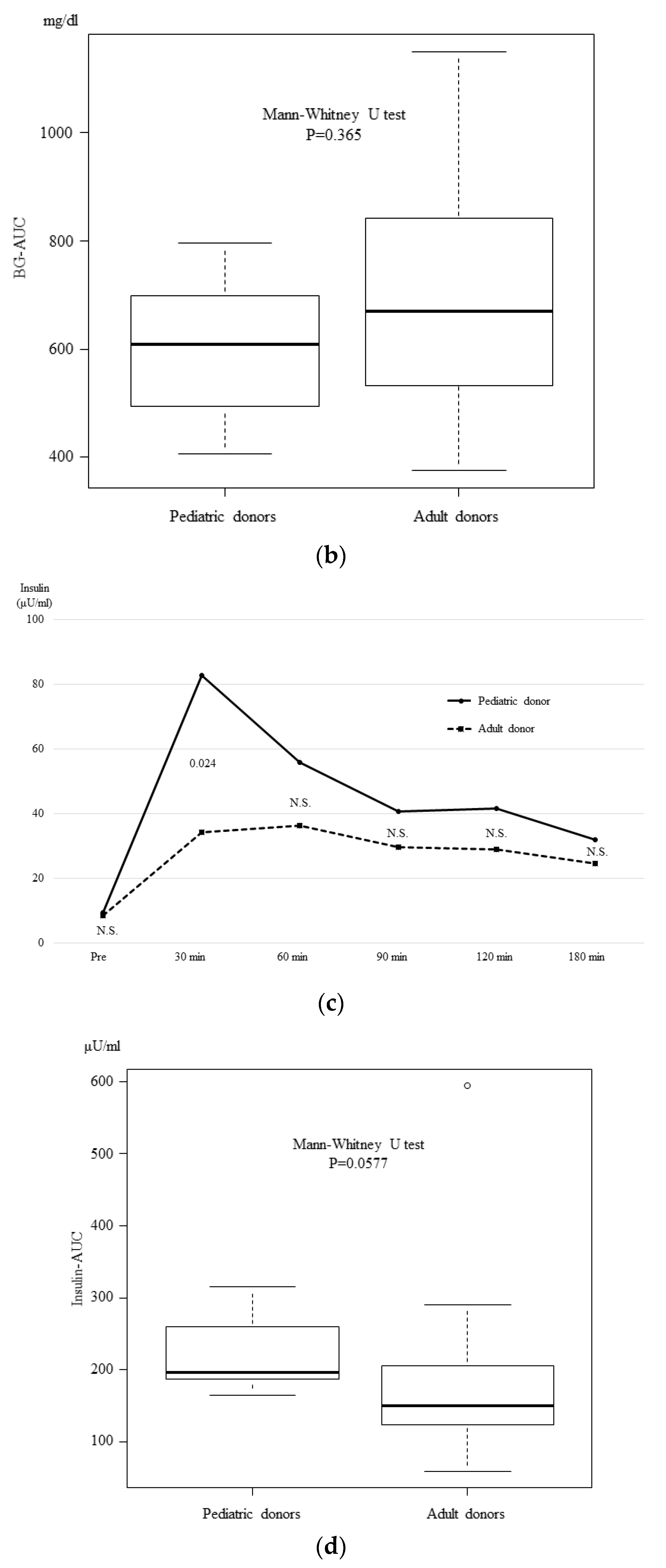
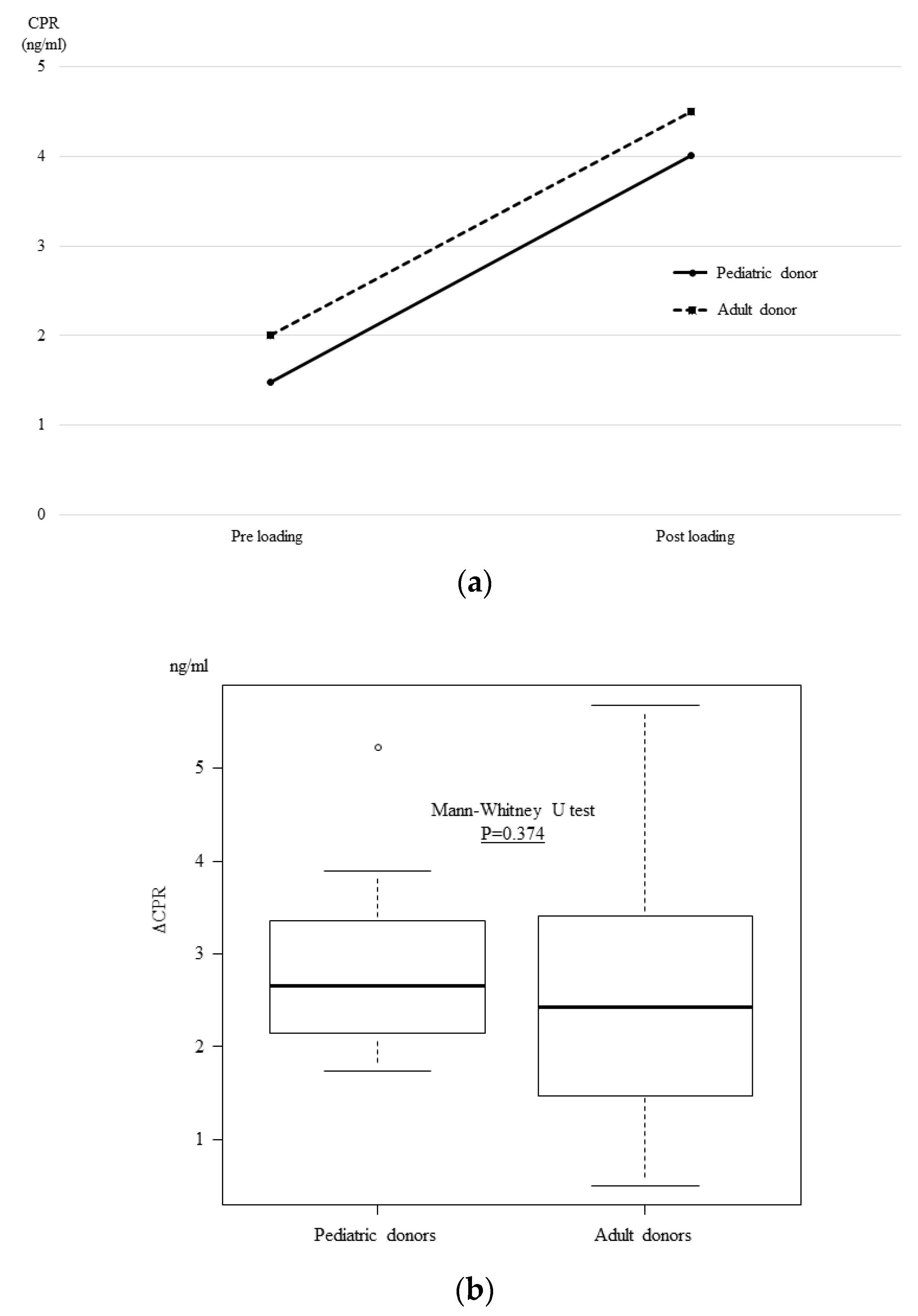
3. Results
3.1. Background Factors and the Outcomes of Transplantation from Pediatric Donors
3.2. Background Factors of the Pediatric Donor and Adult Donor Groups
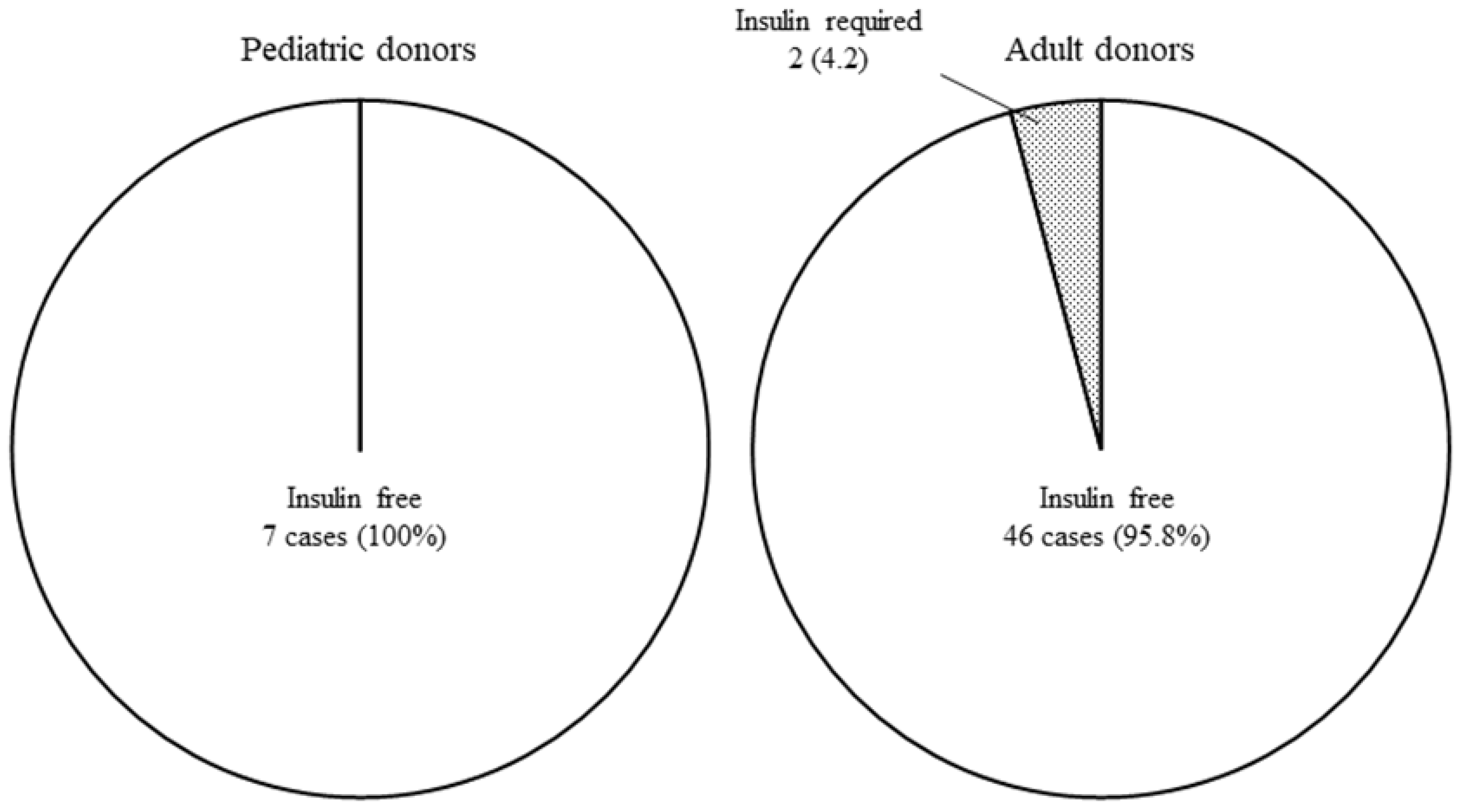
3.3. Pancreatic Graft Survival
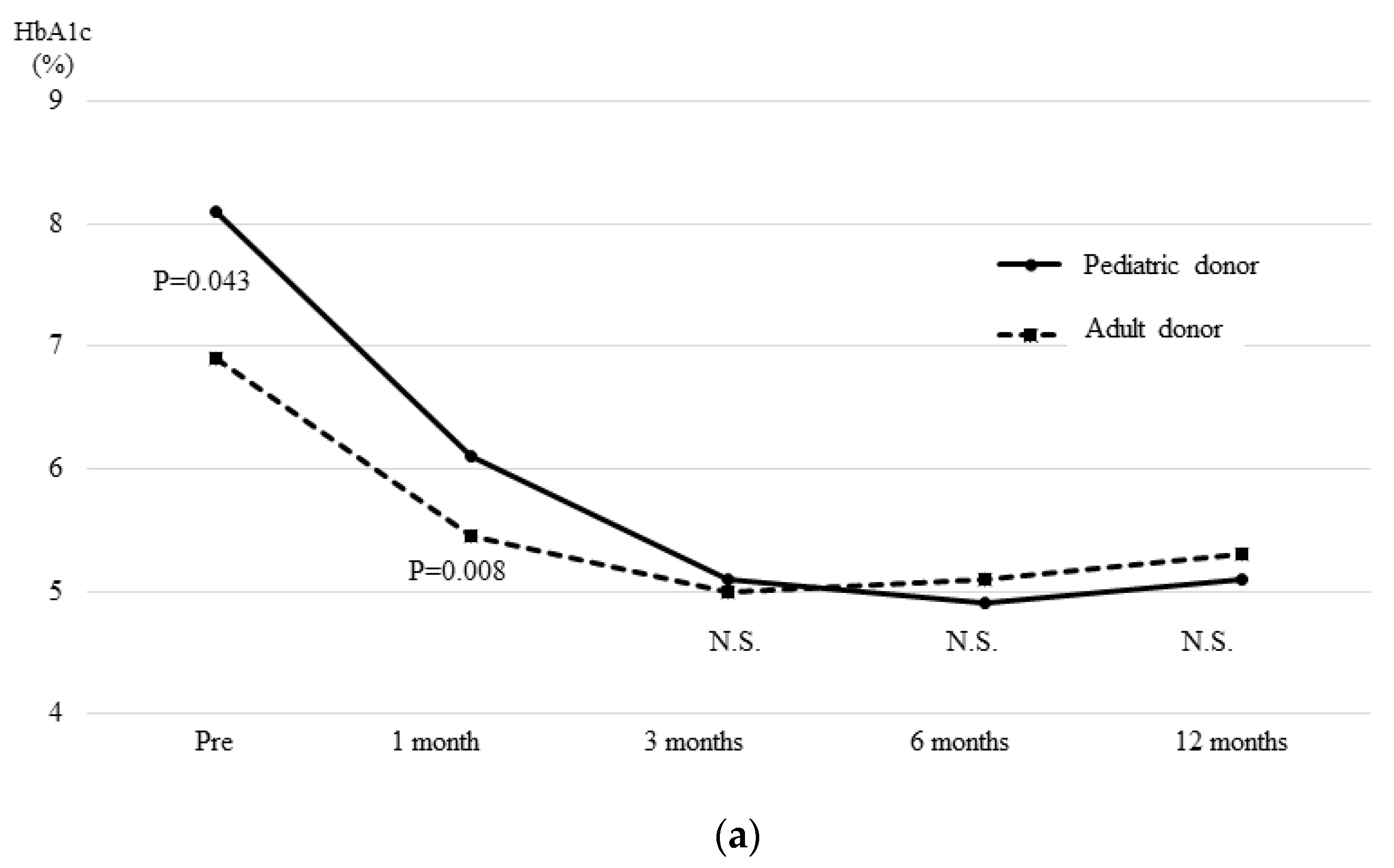
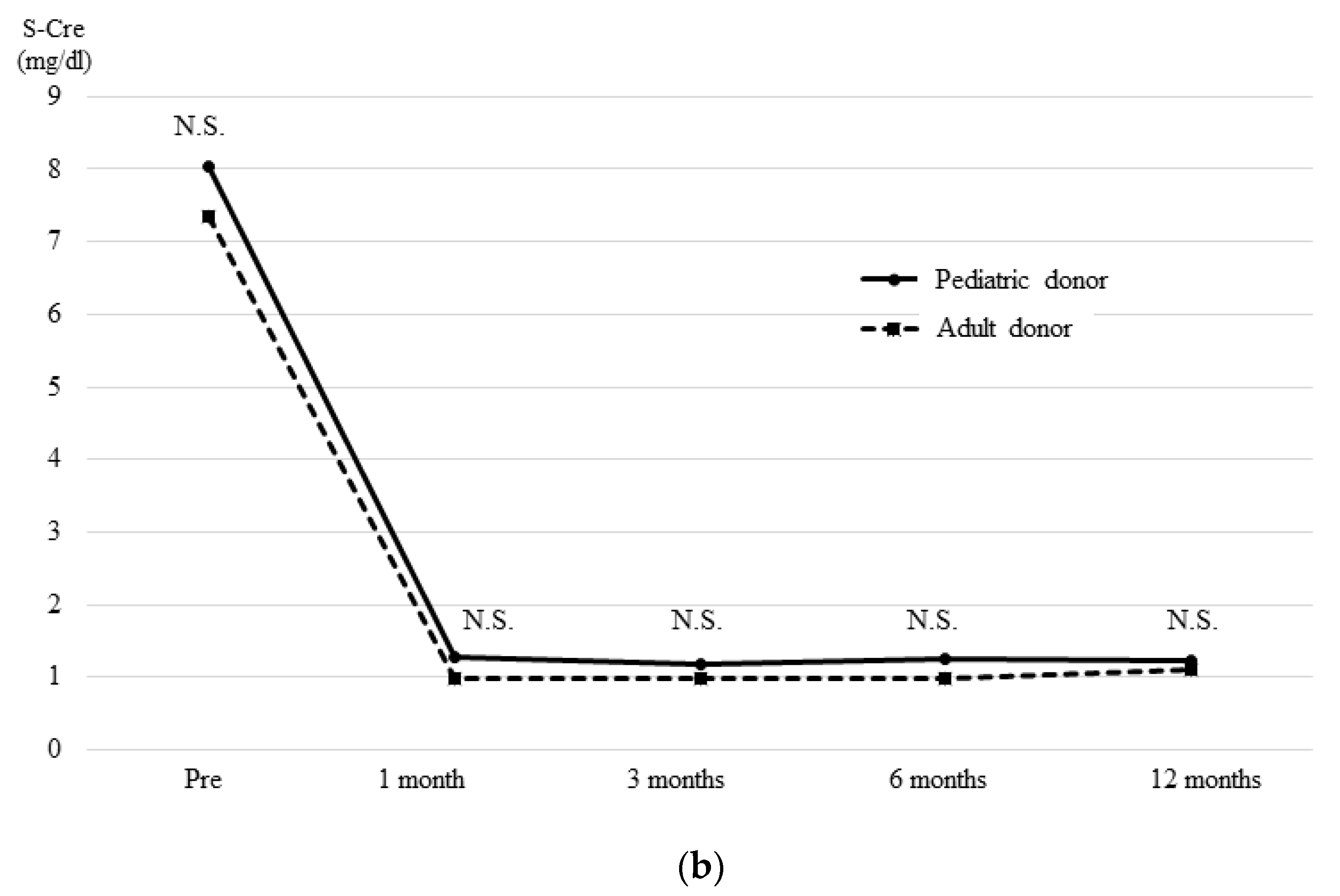
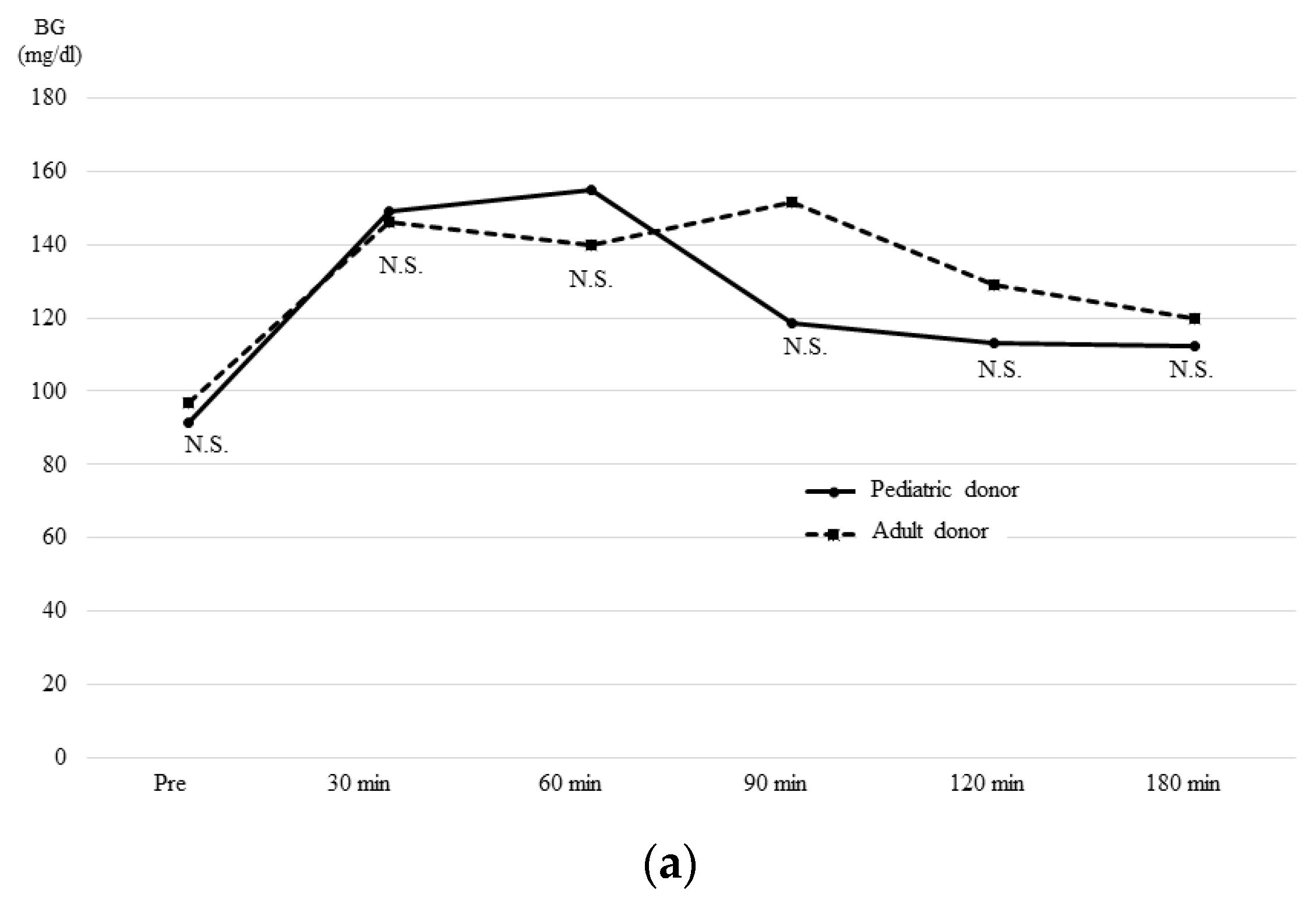
3.4. The OGTT and Glucagon Stimulation Test Results at One Month after Transplantation
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
References
- Asaoka, T.; Ito, T.; Kenmochi, T. The Japan Society for Pancreas and Islet Transplantation, The registry of Japanese pancreas and islet transplantation 2018. Ishoku 2018, 53, 139–147. [Google Scholar]
- Buggenhout, A.; Hoang, A.D.; Hut, F.; Lekeufack, J.B.; Bali, M.A.; De Pauw, L. Pediatric en bloc dual kidney-pancreas transplantation into an adult recipient: A simplified technique. Benefits of the en bloc kidney-pancreas transplantation technique in pediatric donors. Am. J. Transpl. 2004, 4, 663–665. [Google Scholar] [CrossRef] [PubMed]
- Sageshima, J.; Ciancio, G.; Chen, L.; Selvaggi, G.; Nishida, S.; Akpinar, E.; Nesher, E.; Romano, A.; Misawa, R.; Burke, G.W., 3rd. Combined pancreas and en bloc kidney transplantation using a bladder patch technique from very small pediatric donors. Am. J. Transpl. 2010, 10, 2168–2172. [Google Scholar] [CrossRef] [PubMed]
- Waldner, M.; Bachler, T.; Schadde, E.; Schiesser, M.; Immer, F.; Clavien, P.A.; Brockmann, J.G. New surgical technique for pediatric en-bloc kidney and pancreas transplantation: The pancreas piggy-back. Transpl. Int. 2013, 26, 30–33. [Google Scholar] [CrossRef] [PubMed]
- Kanda, Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transpl. 2013, 48, 452–458. [Google Scholar] [CrossRef] [PubMed]
- Nghiem, D.D.; Corry, R.J.; Cottington, E.M. Function of simultaneous kidney and pancreas transplants from pediatric donors. Transplantation 1989, 47, 1075–1079. [Google Scholar] [PubMed]
- Abouna, G.M.; Kumar, M.S.; Miller, J.L.; Rose, L.I.; Brezin, J.; Chvala, R.; Lyons, P.; Katz, S.M.; McSorley, M. Combined kidney and pancreas transplantation from pediatric donors into adult diabetic recipients. Transpl. Proc. 1994, 26, 441–442. [Google Scholar]
- Van der Werf, W.J.; Odorico, J.; D’Alessandro, A.M.; Knechtle, S.; Becker, Y.; Collins, B.; Pirsch, J.; Hoffman, R.; Sollinger, H.W. Utilization of pediatric donors for pancreas transplantation. Transpl. Proc. 1999, 31, 610–611. [Google Scholar] [CrossRef]
- Rhein, T.; Metzner, R.; Uhlmann, D.; Serr, F.; Caca, K.; Weinert, D.; Hauss, J.; Witzigmann, H. Pediatric donor organs for pancreas transplantation: An underutilized resource? Transplant. Proc. 2003, 35, 2145–2146. [Google Scholar] [CrossRef]
- Fernandez, L.A.; Turgeon, N.A.; Odorico, J.S.; Leverson, G.; Pirsch, J.D.; Becker, B.N.; Chin, L.T.; Becker, Y.T.; Knechtle, S.J.; Foley, D.P.; et al. Superior long-term results of simultaneous pancreas-kidney transplantation from pediatric donors. Am. J. Transpl. 2004, 4, 2093–2101. [Google Scholar] [CrossRef]
- Pelletier, S.J.; Guidinger, M.K.; Merion, R.M.; Englesbe, M.J.; Wolfe, R.A.; Magee, J.C.; Sollinger, H.W. Recovery and utilization of deceased donor kidneys from small pediatric donors. Am. J. Transpl. 2006, 6, 1646–1652. [Google Scholar] [CrossRef] [PubMed]
- Mazor, R.; Baden, H.P. Trends in pediatric organ donation after cardiac death. Pediatrics 2007, 120, e960–e966. [Google Scholar] [CrossRef] [PubMed]
- Illanes, H.G.; Quarin, C.M.; Maurette, R.; Sanchez, N.G.; Reniero, L.; Casadei, D.H. Use of small donors (<28 kg) for pancreas transplantation. Transpl. Proc. 2009, 41, 2199–2201. [Google Scholar] [CrossRef] [PubMed]
- Schenker, P.; Flecken, M.; Vonend, O.; Wunsch, A.; Traska, T.; Viebahn, R. En bloc retroperitoneal pancreas-kidney transplantation with duodenoduodenostomy using pediatric organs. Transpl. Proc. 2009, 41, 2643–2645. [Google Scholar] [CrossRef] [PubMed]
- Socci, C.; Orsenigo, E.; Santagostino, I.; Caumo, A.; Caldara, R.; Parolini, D.; Aldrighetti, L.; Castoldi, R.; Frasson, M.; Carvello, M.; et al. Pancreata from pediatric donors restore insulin independence in adult insulin-dependent diabetes mellitus recipients. Transpl. Proc. 2010, 42, 2068–2070. [Google Scholar] [CrossRef]
- Biglarnia, A.R.; Bennet, W.; Nilsson, T.; Larsson, E.; Magnusson, A.; Yamamoto, S.; Lorant, T.; Sedigh, A.; von Zur-Muhlen, B.; Backman, L.; et al. Utilization of small pediatric donors including infants for pancreas and kidney transplantation: Exemplification of the surgical technique and the surveillance. Ann. Surg. 2014, 260, e5–e7. [Google Scholar] [CrossRef]
- Fisher, R.A. Commentary on “Utilization of small pediatric donors including infants for pancreas and kidney transplantation”. Ann. Surg. 2014, 260, e8. [Google Scholar] [CrossRef]
- Chiari, D.; Bissolati, M.; Gazzetta, P.G.; Guarneri, G.; Tomanin, D.; Maffi, P.; Secchi, A.; Rosati, R.; Socci, C. Pancreas Transplantation from Very Small Pediatric Donor Using the “Cephalic Placement” Technique: A Case Report. Transpl. Proc. 2016, 48, 435–437. [Google Scholar] [CrossRef]
- Spaggiari, M.; Bissing, M.; Campara, M.; Yeh, C.C.; Tzvetanov, I.; Jeon, H.; Benedetti, E. Pancreas Transplantation from Pediatric Donors: A United Network for Organ Sharing Registry Analysis. Transplantation 2017, 101, 2484–2491. [Google Scholar] [CrossRef]
- Christensen, K.; Kennedy, A.; Kim, R.; Martinez, E.; Campsen, J. Pancreatic Grafts from Pediatric Donors Do Not Appear to Grow After Transplantation into Adults. Cureus 2018, 10, e3363. [Google Scholar] [CrossRef]
- Spaggiari, M.; Di Bella, C.; Di Cocco, P.; Campara, M.; Galen, K.; Gheza, F.; Oberholzer, J.; Benedetti, E.; Tzvetanov, I. Pancreas Transplantation from Pediatric Donors: A Single-Center Experience. Transplantation 2018, 102, 1732–1739. [Google Scholar] [CrossRef] [PubMed]
- Dobbs, S.; Shapey, I.M.; Summers, A.; Moinuddin, Z.; van Dellen, D.; Augustine, T. Simultaneous en-bloc pancreas and kidney transplantation from a small pediatric donor after circulatory death. Am. J. Transpl. 2019, 19, 929–932. [Google Scholar] [CrossRef] [PubMed]
- Aida, N.; Kenmochi, T.; Ito, T.; Nishikawa, T.; Hiratsuka, I.; Shibata, M.; Suzuki, A.; Hasegawa, M.; Kawai, A.; Kusaka, M.; et al. Prediction of Insulin Secretion Ability with Microcirculation Evaluated by Contrast-enhanced Ultrasonography in Pancreas Transplantation. Pancreas 2018, 47, 617–624. [Google Scholar] [CrossRef] [PubMed]
- Kayler, L.K.; Magliocca, J.; Fujita, S.; Kim, R.D.; Zendejas, I.; Hemming, A.W.; Howard, R.; Schold, J.D. Recovery factors affecting utilization of small pediatric donor kidneys. Am. J. Transpl. 2009, 9, 210–216. [Google Scholar] [CrossRef] [PubMed]
- Kayler, L.K.; Magliocca, J.; Kim, R.D.; Howard, R.; Schold, J.D. Single kidney transplantation from young pediatric donors in the United States. Am. J. Transpl. 2009, 9, 2745–2751. [Google Scholar] [CrossRef] [PubMed]

| Case | Age | Sex | Cause of Death | BW (kg) | HbA1c (%) | s-Cre (mg/dl) | Number of HLA Mismatches | TIT (Pancreas, min) | TIT (Kidney, min) | Graft Weight (Pancreas, g) |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 6 | Male | Hypoxia | 20.0 | 4.7 | 0.33 | 3 | 891 | 676 | 95 |
| 2 | 5 | Female | Trauma | 20.8 | 5.5 | 0.19 | 3 | 630 | N/A | 71 |
| 3 | 9 | Male | Trauma | 27.0 | 5.4 | 0.41 | 5 | 714 | 597 | 64 |
| 4 | 11 | Female | Hypoxia | 43.0 | 5.3 | 0.82 | 2 | 969 | 704 | 114 |
| 5 | 5 | Male | Hypoxia | 21.0 | 4.9 | 0.22 | 2 | 729 | 522 | 63 |
| 6 | 4 | Female | Hypoxia | 18.5 | 5.2 | 0.17 | 3 | 974 | 680 | 61 |
| 7 | 10 | Female | CVA | 29.1 | 5.4 | 0.47 | 2 | 801 | 606 | 75 |
| Case | Recipient Age | Recipient Sex | Recipient BW (kg) | Operation Type | Arterial Reconstruction | Portal Vein Elongation | Episode of Rejection | Graft Survival | HbA1c * | s-Cre * (mg/dl) | Basal CRP ** (ng/mL) | CPR after Glucagon Load ** (ng/mL) | ΔCPR ** (ng/mL) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (%) | |||||||||||||
| 1 | 61 | Male | 61.3 | SPK | Y-graft | + | - | Survive (43 M) | 5.2 | 1.42 | 2.44 | 5.1 | 2.66 |
| 2 | 34 | Male | 55.1 | PTA | Y-graft | + | + | Graft failure due to rejection (6 M) | 5.3 | N/A | 1.19 | 4.01 | 2.82 |
| 3 | 36 | Female | 49.3 | SPK | Carrel patch | SPV bypass | - | Survive (27 M) | 5.1 | 0.76 | 2.44 | 7.66 | 5.22 |
| 4 | 50 | Male | 59.8 | SPK | Carrel patch | - | - | Survive (14 M) | 4.7 | 1.47 | 1.48 | 3.22 | 1.74 |
| 5 | 34 | Female | 56 | SPK | Carrel patch | - | + | Survive (12 M) | 5.1 | 0.94 | 1.3 | 3.46 | 2.16 |
| 6 | 56 | Male | 65.7 | SPK | Carrel patch | - | - | Survive (5 M) | 5 | 1.4 | 1.79 | 5.68 | 3.89 |
| 7 | 44 | Female | 51.9 | SPK | Carrel patch | - | - | Survive (4 M) | 4.2 | 0.91 | 1.43 | 3.57 | 2.14 |
| Group | Pediatric Donors | Adult Donors | p Value | ||
|---|---|---|---|---|---|
| n | 7 | 53 | |||
| Donor factors | Age | 6 (4–11) | 48 (17–67) | <0.001 | |
| Sex | Male (%) | 3 (42.9) | 26 (49.1) | 1 | |
| Female (%) | 4 (57.1) | 27 (50.9) | |||
| BW (kg) | 21.0 (18.5–43.0) | 59.7 (40.0–94.1) | <0.001 | ||
| BMI (kg/m2) | 16.0 (12.8–20.8) | 22.2 (16.6–30.0) | <0.001 | ||
| Cause of death | CVA (%) | 1 (14.3) | 27 (52.9) | 0.104 | |
| Others (%) | 6 (85.7) | 24 (47.1) | |||
| ICU stay (days) | 24 (7–35) | 7 (2–34) | 0.005 | ||
| Preoperative HbA1c (%) | 5.3 (4.7–5.5) | 5.5 (4.9–6.3) | 0.017 | ||
| Preoperative BG (mg/dl) | 97 (80–117) | 128 (81–237) | 0.003 | ||
| Preoperative s-Cre (mg/dl) | 0.33 (0.17–0.82) | 0.68 (0.23–6.93) | 0.004 | ||
| Preoperative LDH (U/l) | 1249 (871–2211) | 688 (248–2323) | 0.002 | ||
| Preoperative Na (mmol/l) | 139 (130–143) | 141 (114–166) | 0.213 | ||
| Preoperative CRP (mg/dl) | 7.43 (0.15–22.53) | 17.51 (0.38–39.57) | 0.025 | ||
| Pancreatic graft weight (g) | 71 (61–114) | 191 (95–352) | <0.001 | ||
| TIT (pancreas, min) | 801 (630–974) | 886 (494–1383) | 0.189 | ||
| TIT (kidney, min) | 641 (522–704) | 706 (474–1124) | 0.244 | ||
| Recipient factors | Age | 44 (34–61) | 44 (31–62) | 0.926 | |
| Sex | Male (%) | 4 (57.1) | 17 (32.1) | 0.226 | |
| Female (%) | 3 (42.9) | 36 (67.9) | |||
| Preoperative HbA1c (%) | 8.1 (6.3–12.3) | 6.9 (4.9–9.8) | 0.043 | ||
| Period of diabetic history (year) | 29 (21–38) | 29 (11–43) | 0.926 | ||
| Period of hemodialysis history (year) | 6.0 (1.5–11.0) | 6.0 (0–20.0) | 0.893 | ||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ito, T.; Kenmochi, T.; Aida, N.; Kurihara, K.; Kawai, A.; Suzuki, A.; Shibata, M.; Hiratsuka, I.; Hasegawa, M. The Outcomes of Pancreatic Transplantation from Pediatric Donors–A Single Institution Experience. J. Clin. Med. 2019, 8, 1386. https://doi.org/10.3390/jcm8091386
Ito T, Kenmochi T, Aida N, Kurihara K, Kawai A, Suzuki A, Shibata M, Hiratsuka I, Hasegawa M. The Outcomes of Pancreatic Transplantation from Pediatric Donors–A Single Institution Experience. Journal of Clinical Medicine. 2019; 8(9):1386. https://doi.org/10.3390/jcm8091386
Chicago/Turabian StyleIto, Taihei, Takashi Kenmochi, Naohiro Aida, Kei Kurihara, Akihiro Kawai, Atsushi Suzuki, Megumi Shibata, Izumi Hiratsuka, and Midori Hasegawa. 2019. "The Outcomes of Pancreatic Transplantation from Pediatric Donors–A Single Institution Experience" Journal of Clinical Medicine 8, no. 9: 1386. https://doi.org/10.3390/jcm8091386
APA StyleIto, T., Kenmochi, T., Aida, N., Kurihara, K., Kawai, A., Suzuki, A., Shibata, M., Hiratsuka, I., & Hasegawa, M. (2019). The Outcomes of Pancreatic Transplantation from Pediatric Donors–A Single Institution Experience. Journal of Clinical Medicine, 8(9), 1386. https://doi.org/10.3390/jcm8091386





