Potential Protective Effect of Oleanolic Acid on the Components of Metabolic Syndrome: A Systematic Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Databases and Search Strategy
2.2. Selection of Papers. Eligibility Criteria
2.3. Data Extraction
2.4. Risk of Bias and Methodological Quality Assessment
3. Results
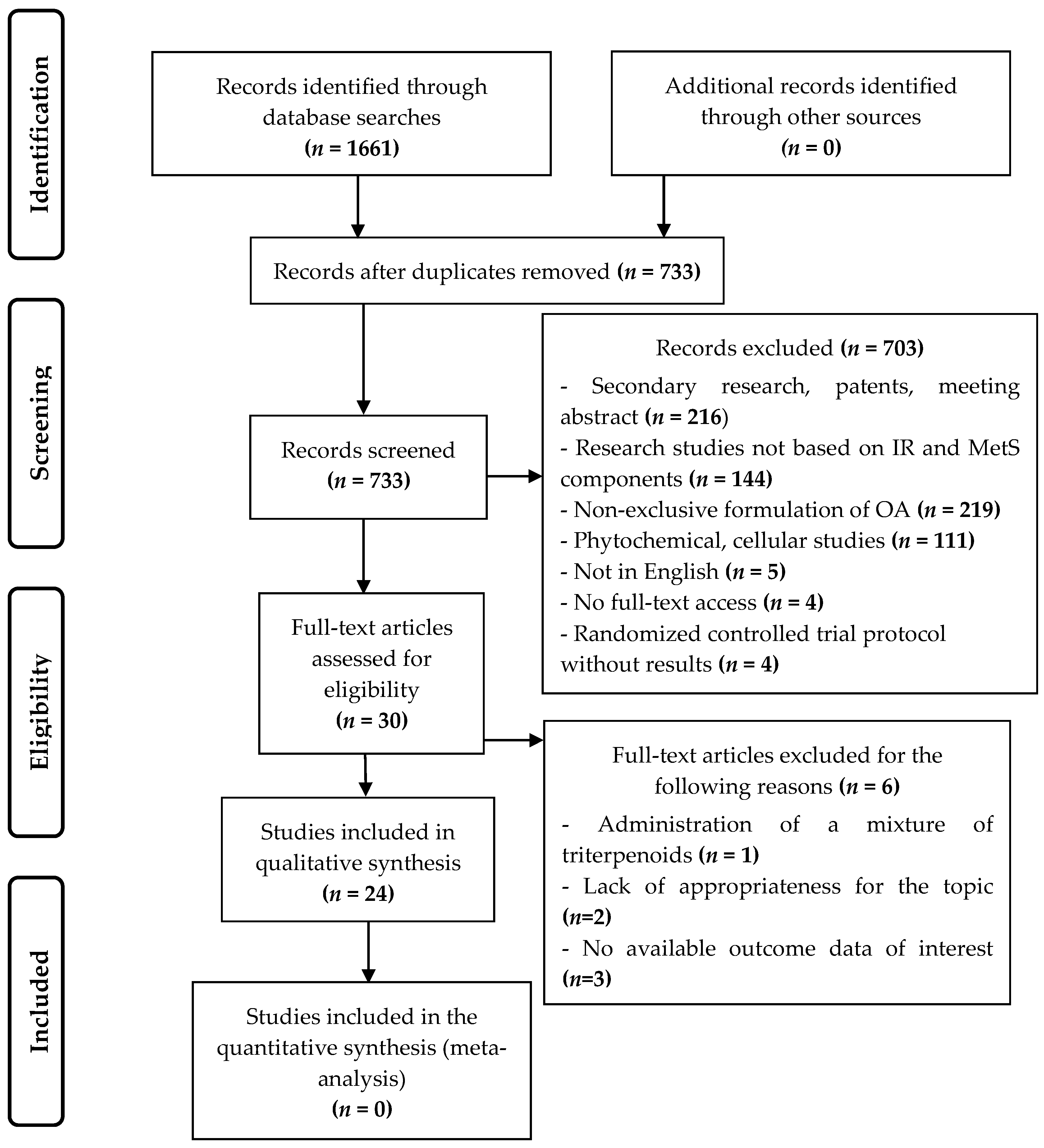
3.1. Study Selection
3.2. Characteristics of the Animal Studies Selected
3.3. Characteristics of the Clinical Trial Selected
3.4. Risk of Bias and Methodological Quality Assessment
3.5. OA Effects on Insulin Resistance and MetS Components in Animal Studies
3.5.1. Hypertension
3.5.2. Lipid Profile and Obesity
3.5.3. Hyperglycemia and Insulin Resistance
3.5.4. Inflammatory and oxidative stress biomarkers. Antioxidant enzymes
3.6. Hypolipidemic Effects of OA in Human Patients
4. Discussion
5. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- World Health Organization Obesity and Overweight. Available online: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight (accessed on 20 June 2019).
- Pan American Health Organization; World Health Organization PAHO/WHO|Obesity as a Precursor to Diabetes. Available online: https://www.paho.org/hq/index.php?option=com_content&view=article&id=6718:2012-obesity-as-precursor-diabetes&Itemid=39448&lang=en (accessed on 20 June 2019).
- International Diabetes Federation IDF Consensus Worldwide Definition of the Metabolic Syndromeand Tools. Available online: https://www.idf.org/our-activities/advocacy-awareness/resources-and-tools/60:idfconsensus-worldwide-definitionof-the-metabolic-syndrome.html (accessed on 20 June 2019).
- Lira Neto, J.C.G.; de Almeida Xavier, M.; Borges, J.W.P.; de Araújo, M.F.M.; Damasceno, M.M.C.; de Freitas, R.W.J.F. Prevalence of Metabolic Syndrome in individuals with Type 2 Diabetes Mellitus. Rev. Bras. Enferm. 2017, 70, 265–270. [Google Scholar] [CrossRef] [PubMed]
- McCullough, A.J. Epidemiology of the metabolic syndrome in the USA. J. Dig. Dis. 2011, 12, 333–340. [Google Scholar] [CrossRef] [PubMed]
- Weiss, R.; Bremer, A.A.; Lustig, R.H. What is metabolic syndrome, and why are children getting it? Ann. N. Y. Acad. Sci. 2013, 1281, 123–140. [Google Scholar] [CrossRef] [PubMed]
- Czech, M.P. Insulin action and resistance in obesity and type 2 diabetes. Nat. Med. 2017, 23, 804–814. [Google Scholar] [CrossRef] [PubMed]
- Pérez, M.R.; Medina-Gomez, G. Obesidad, adipogénesis y resistencia a la insulina. Endocrinol. y Nutr. 2011, 58, 360–369. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.C.; Cui, J.Y.; Klaassen, C.D. Beneficial Role of Nrf2 in Regulating NADPH Generation and Consumption. Toxicol. Sci. 2011, 123, 590–600. [Google Scholar] [CrossRef] [PubMed]
- Castellano, J.M.; Guinda, A.; Delgado, T.; Rada, M.; Cayuela, J.A. Biochemical Basis of the Antidiabetic Activity of Oleanolic Acid and Related Pentacyclic Triterpenes. Diabetes 2013, 62, 1791–1799. [Google Scholar] [CrossRef] [PubMed]
- Larsen, J.R.; Dima, L.; Correll, C.U.; Manu, P. The pharmacological management of metabolic syndrome. Expert Rev. Clin. Pharmacol. 2018, 11, 397–410. [Google Scholar] [CrossRef]
- Molepo, M.; Ayeleso, A.; Nyakudya, T.; Erlwanger, K.; Mukwevho, E. A Study on Neonatal Intake of Oleanolic Acid and Metformin in Rats (Rattus norvegicus) with Metabolic Dysfunction: Implications on Lipid Metabolism and Glucose Transport. Molecules 2018, 23, 2528. [Google Scholar] [CrossRef]
- Guinda, A.; Rada, M.; Delgado, T.; Gutiérrez-Adánez, P.; Castellano, J.M. Pentacyclic Triterpenoids from Olive Fruit and Leaf. J. Agric. Food Chem. 2010, 58, 9685–9691. [Google Scholar] [CrossRef]
- Ayeleso, T.B.; Matumba, M.G.; Mukwevho, E. Oleanolic Acid and Its Derivatives: Biological Activities and Therapeutic Potential in Chronic Diseases. Molecules 2017, 22, 1915. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Rodriguez, R. Oleanolic acid and related triterpenoids from olives on vascular function: Molecular mechanisms and therapeutic perspectives. Curr. Med. Chem. 2015, 22, 1414–1425. [Google Scholar] [CrossRef] [PubMed]
- Pols, T.W.H.; Noriega, L.G.; Nomura, M.; Auwerx, J.; Schoonjans, K. The bile acid membrane receptor TGR5 as an emerging target in metabolism and inflammation. J. Hepatol. 2011, 54, 1263–1272. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Han, Z.; Bei, W.; Rong, X.; Guo, J.; Hu, X. Oleanolic Acid Attenuates Insulin Resistance via NF-κB to Regulate the IRS1-GLUT4 Pathway in HepG2 Cells. Evid. Based Complement. Altern. Med. 2015, 2015, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef] [PubMed]
- Hooijmans, C.R.; Rovers, M.M.; De Vries, R.B.; Leenaars, M.; Ritskes-Hoitinga, M.; Langendam, M.W. SYRCLE’s risk of bias tool for animal studies. BMC Med. Res. Methodol. 2014, 14, 43. [Google Scholar] [CrossRef] [PubMed]
- Jadad, A.R.; Moore, R.; Carroll, D.; Jenkinson, C.; Reynolds, D.M.; Gavaghan, D.J.; McQuay, H.J. Assessing the quality of reports of randomized clinical trials: Is blinding necessary? Control. Clin. Trials 1996, 17, 1–12. [Google Scholar] [CrossRef]
- He, W.-F.; Liang, L.-F.; Cai, Y.-S.; Gao, L.-X.; Li, Y.-F.; Li, J.; Liu, H.-L.; Guo, Y.-W. Brominated polyunsaturated lipids with protein tyrosine phosphatase-1B inhibitory activity from Chinese marine sponge Xestospongia testudinaria. J. Asian Nat. Prod. Res. 2015, 17, 1–6. [Google Scholar] [CrossRef]
- He, W.-F.; Xue, D.-Q.; Yao, L.-G.; Li, J.; Liu, H.-L.; Guo, Y.-W. A new bioactive steroidal ketone from the South China Sea sponge Xestospongia testudinaria. J. Asian Nat. Prod. Res. 2016, 18, 195–199. [Google Scholar] [CrossRef]
- Liu, Y.L.A.Y.; Liu, Y. Editorial: Vascular Protection of Herbal Medicine: Roles and Mechanisms. Curr. Vasc. Pharmacol. 2017, 15, 502. [Google Scholar] [CrossRef]
- Jing, X.; Lin-hui, Z.; De-bin, W.; Xin, H.; Guang-Zhong, Y. Effect of oleanolic acid derivatives on improving insulin resistance and its molecular mechanism. Chin. Pharmacol. Bull. 2014, 30, 1585–1589. [Google Scholar]
- Sanchez-Rodriguez, E.; Biel-Glesson, S.; Fernandez-Navarro, J.R.; Calleja, M.A.; Espejo-Calvo, J.A.; Gil-Extremera, B.; De La Torre, R.; Fito, M.; Covas, M.-I.; Vilchez, P.; et al. Effects of Virgin Olive Oils Differing in Their Bioactive Compound Contents on Biomarkers of Oxidative Stress and Inflammation in Healthy Adults: A Randomized Double-Blind Controlled Trial. Nutrients 2019, 11, 561. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Rodriguez, E.; Lima-Cabello, E.; Biel-Glesson, S.; Fernandez-Navarro, J.R.; Calleja, M.A.; Roca, M.; Espejo-Calvo, J.A.; Gil-Extremera, B.; Soria-Florido, M.; De La Torre, R.; et al. Effects of Virgin Olive Oils Differing in Their Bioactive Compound Contents on Metabolic Syndrome and Endothelial Functional Risk Biomarkers in Healthy Adults: A Randomized Double-Blind Controlled Trial. Nutrients 2018, 10, 626. [Google Scholar] [CrossRef] [PubMed]
- Ahn, Y.M.; Choi, Y.H.; Yoon, J.J.; Lee, Y.J.; Cho, K.W.; Kang, D.G.; Lee, H.S. Oleanolic acid modulates the renin-angiotensin system and cardiac natriuretic hormone concomitantly with volume and pressure balance in rats. Eur. J. Pharmacol. 2017, 809, 231–241. [Google Scholar] [CrossRef] [PubMed]
- Bachhav, S.S.; Bhutada, M.S.; Patil, S.P.; Sharma, K.S.; Patil, S.D. Oleanolic Acid Prevents Increase in Blood Pressure and Nephrotoxicity in Nitric Oxide Dependent Type of Hypertension in Rats. Pharmacogn. Res. 2015, 7, 385–392. [Google Scholar]
- Madlala, H.P.; Van Heerden, F.R.; Mubagwa, K.; Musabayane, C.T. Changes in Renal Function and Oxidative Status Associated with the Hypotensive Effects of Oleanolic Acid and Related Synthetic Derivatives in Experimental Animals. PLoS ONE 2015, 10, e0128192. [Google Scholar] [CrossRef] [PubMed]
- Gamede, M.; Mabuza, L.; Ngubane, P.; Khathi, A. Plant-Derived Oleanolic Acid (OA) Ameliorates Risk Factors of Cardiovascular Diseases in a Diet-Induced Pre-Diabetic Rat Model: Effects on Selected Cardiovascular Risk Factors. Molecules 2019, 24, 340. [Google Scholar] [CrossRef]
- Chen, S.; Wen, X.; Zhang, W.; Wang, C.; Liu, J.; Liu, C. Hypolipidemic effect of oleanolic acid is mediated by the miR-98-5p/PGC-1β axis in high-fat diet–induced hyperlipidemic mice. FASEB J. 2017, 31, 1085–1096. [Google Scholar] [CrossRef]
- Jiang, Q.; Wang, D.; Han, Y.; Han, Z.; Zhong, W.; Wang, C. Modulation of oxidized-LDL receptor-1 (LOX1) contributes to the antiatherosclerosis effect of oleanolic acid. Int. J. Biochem. Cell Boil. 2015, 69, 142–152. [Google Scholar] [CrossRef]
- Luo, H.; Liu, J.; Ouyang, Q.; Xuan, C.; Wang, L.; Li, T.; Liu, J. The effects of oleanolic acid on atherosclerosis in different animal models. Acta Biochim. Biophys. Sin. (Shanghai) 2017, 49, 349–354. [Google Scholar] [CrossRef]
- Pan, Y.; Zhou, F.; Song, Z.; Huang, H.; Chen, Y.; Shen, Y.; Jia, Y.; Chen, J. Oleanolic acid protects against pathogenesis of atherosclerosis, possibly via FXR-mediated angiotensin (Ang)-(1–7) upregulation. Biomed. Pharmacother. 2018, 97, 1694–1700. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Liu, R.; Zhang, W.; Zhang, X.; Liao, N.; Wang, Z.; Li, W.; Qin, X.; Hai, C. Oleanolic acid improves hepatic insulin resistance via antioxidant, hypolipidemic and anti-inflammatory effects. Mol. Cell. Endocrinol. 2013, 376, 70–80. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, J.; Gu, T.; Yamahara, J.; Li, Y. Oleanolic acid supplement attenuates liquid fructose-induced adipose tissue insulin resistance through the insulin receptor substrate-1/phosphatidylinositol 3-kinase/Akt signaling pathway in rats. Toxicol. Appl. Pharmacol. 2014, 277, 155–163. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.S.; Kim, H.M.; Kang, J.S.; Lee, E.Y.; Yadav, D.; Kwon, M.-H.; Kim, Y.M.; Kim, H.S.; Chung, C.H. Oleanolic acid and N -acetylcysteine ameliorate diabetic nephropathy through reduction of oxidative stress and endoplasmic reticulum stress in a type 2 diabetic rat model. Nephrol. Dial. Transplant. 2016, 31, 391–400. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Chen, Y.; Abdelkader, D.; Hassan, W.; Sun, H.; Liu, J. Combination Therapy with Oleanolic Acid and Metformin as a Synergistic Treatment for Diabetes. J. Diabetes Res. 2015, 2015, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Gamede, M.; Mabuza, L.; Ngubane, P.; Khathi, A. The Effects of Plant-Derived Oleanolic Acid on Selected Parameters of Glucose Homeostasis in a Diet-Induced Pre-Diabetic Rat Model. Molecules 2018, 23, 794. [Google Scholar] [CrossRef] [PubMed]
- Djeziri, F.Z.; Belarbi, M.; Murtaza, B.; Hichami, A.; Benammar, C.; Khan, N.A. Oleanolic acid improves diet-induced obesity by modulating fat preference and inflammation in mice. Biochimie 2018, 152, 110–120. [Google Scholar] [CrossRef]
- Nakajima, K.; Maeda, N.; Oiso, S.; Kariyazono, H. Decreased Plasma Octanoylated Ghrelin Levels in Mice by Oleanolic Acid. J. Oleo Sci. 2019, 68, 103–109. [Google Scholar] [CrossRef]
- Su, S.; Wu, G.; Cheng, X.; Fan, J.; Peng, J.; Su, H.; Xu, Z.; Cao, M.; Long, Z.; Hao, Y.; et al. Oleanolic acid attenuates PCBs-induced adiposity and insulin resistance via HNF1b-mediated regulation of redox and PPARγ signaling. Free Radic. Boil. Med. 2018, 124, 122–134. [Google Scholar] [CrossRef]
- Wang, S.; Du, L.-B.; Jin, L.; Wang, Z.; Peng, J.; Liao, N.; Zhao, Y.-Y.; Zhang, J.-L.; Pauluhn, J.; Hai, C.-X.; et al. Nano-oleanolic acid alleviates metabolic dysfunctions in rats with high fat and fructose diet. Biomed. Pharmacother. 2018, 108, 1181–1187. [Google Scholar] [CrossRef]
- An, Q.; Hu, Q.; Wang, B.; Cui, W.; Wu, F.; Ding, Y. Oleanolic acid alleviates diabetic rat carotid artery injury through the inhibition of NLRP3 inflammasome signaling pathways. Mol. Med. Rep. 2017, 16, 8413–8419. [Google Scholar] [CrossRef] [PubMed]
- Matumba, M.G.; Ayeleso, A.O.; Nyakudya, T.; Erlwanger, K.; Chegou, N.N.; Mukwevho, E. Long-Term Impact of Neonatal Intake of Oleanolic Acid on the Expression of AMP-Activated Protein Kinase, Adiponectin and Inflammatory Cytokines in Rats Fed with a High Fructose Diet. Nutrients 2019, 11, 226. [Google Scholar] [CrossRef] [PubMed]
- Nyakudya, T.T.; Mukwevho, E.; Erlwanger, K.H. The protective effect of neonatal oral administration of oleanolic acid against the subsequent development of fructose-induced metabolic dysfunction in male and female rats. Nutr. Metab. 2018, 15, 82. [Google Scholar] [CrossRef] [PubMed]
- Nyakudya, T.T.; Mukwevho, E.; Nkomozepi, P.; Erlwanger, K.H. Neonatal intake of oleanolic acid attenuates the subsequent development of high fructose diet-induced non-alcoholic fatty liver disease in rats. J. Dev. Orig. Heal. Dis. 2018, 9, 500–510. [Google Scholar] [CrossRef] [PubMed]
- Nyakudya, T.T.; Isaiah, S.; Ayeleso, A.; Ndhlala, A.R.; Mukwevho, E.; Erlwanger, K.H. Short-Term Neonatal Oral Administration of Oleanolic Acid Protects against Fructose-Induced Oxidative Stress in the Skeletal Muscles of Suckling Rats. Molecules 2019, 24, 661. [Google Scholar] [CrossRef] [PubMed]
- Luo, H.-Q.; Shen, J.; Chen, C.-P.; Ma, X.; Lin, C.; Ouyang, Q.; Xuan, C.-X.; Liu, J.; Sun, H.-B.; Liu, J. Lipid-lowering effects of oleanolic acid in hyperlipidemic patients. Chin. J. Nat. Med. 2018, 16, 339–346. [Google Scholar] [CrossRef]
- Kim, H.Y.; Cho, K.W.; Kang, D.G.; Lee, H.S. Oleanolic acid increases plasma ANP levels via an accentuation of cardiac ANP synthesis and secretion in rats. Eur. J. Pharmacol. 2013, 710, 73–79. [Google Scholar] [CrossRef]
- Tappy, L.; Lê, K.-A. Metabolic Effects of Fructose and the Worldwide Increase in Obesity. Physiol. Rev. 2010, 90, 23–46. [Google Scholar] [CrossRef] [PubMed]
- Morelli, N.R.; Scavuzzi, B.M.; Miglioranza, L.H.D.S.; Lozovoy, M.A.B.; Simão, A.N.C.; Dichi, I. Metabolic syndrome components are associated with oxidative stress in overweight and obese patients. Arch. Endocrinol. Metab. 2018, 62, 309–318. [Google Scholar] [CrossRef]
- Mineo, C.; Deguchi, H.; Griffin, J.H.; Shaul, P.W. Endothelial and Antithrombotic Actions of HDL. Circ. Res. 2006, 98, 1352–1364. [Google Scholar] [CrossRef]
- Manna, P.; Jain, S.K. Obesity, Oxidative Stress, Adipose Tissue Dysfunction, and the Associated Health Risks: Causes and Therapeutic Strategies. Metab. Syndr. Relat. Disord. 2015, 13, 423–444. [Google Scholar] [CrossRef] [PubMed]
- Tangvarasittichai, S. Oxidative stress, insulin resistance, dyslipidemia and type 2 diabetes mellitus. World J. Diabetes 2015, 6, 456–480. [Google Scholar] [CrossRef] [PubMed]
- Von Frankenberg, A.D.; Reis, A.F.; Gerchman, F. Relationships between adiponectin levels, the metabolic syndrome, and type 2 diabetes: A literature review. Arch. Endocrinol. Metab. 2017, 61, 614–622. [Google Scholar] [CrossRef] [PubMed]
- Awazawa, M.; Ueki, K.; Inabe, K.; Yamauchi, T.; Kubota, N.; Kaneko, K.; Kobayashi, M.; Iwane, A.; Sasako, T.; Okazaki, Y.; et al. Adiponectin Enhances Insulin Sensitivity by Increasing Hepatic IRS-2 Expression via a Macrophage-Derived IL-6-Dependent Pathway. Cell Metab. 2011, 13, 401–412. [Google Scholar] [CrossRef] [PubMed]
- De Melo, C.L.; Queiroz, M.G.R.; Fonseca, S.G.; Bizerra, A.M.; Lemos, T.L.; Melo, T.S.; Santos, F.A.; Rao, V.S. Oleanolic acid, a natural triterpenoid improves blood glucose tolerance in normal mice and ameliorates visceral obesity in mice fed a high-fat diet. Chem. Interact. 2010, 185, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, K.; Oiso, S.; Uto, T.; Morinaga, O.; Syoyama, Y.; Kariyazono, H. Triterpenes suppress octanoylated ghrelin production in ghrelin-expressing human gastric carcinoma cells. Biomed. Res. 2016, 37, 343–349. [Google Scholar] [CrossRef] [PubMed]
- Hurrle, S.; Hsu, W.H. The etiology of oxidative stress in insulin resistance. Biomed. J. 2017, 40, 257–262. [Google Scholar] [CrossRef]
- Mukundwa, A.; Mukaratirwa, S.; Masola, B. Effects of oleanolic acid on the insulin signaling pathway in skeletal muscle of streptozotocin-induced diabetic male Sprague-Dawley rats. J. Diabetes 2016, 8, 98–108. [Google Scholar] [CrossRef]
- Li, W.; Wang, P.; Li, H.; Li, T.-Y.; Feng, M.; Chen, S. Oleanolic acid protects against diabetic cardiomyopathy via modulation of the nuclear factor erythroid 2 and insulin signaling pathways. Exp. Ther. Med. 2017, 14, 848–854. [Google Scholar] [CrossRef]
- Xue, S.; Yin, J.; Shao, J.; Yu, Y.; Yang, L.; Wang, Y.; Xie, M.; Fussenegger, M.; Ye, H. A Synthetic-Biology-Inspired Therapeutic Strategy for Targeting and Treating Hepatogenous Diabetes. Mol. Ther. 2017, 25, 443–455. [Google Scholar] [CrossRef]
- De Vries, R.B.M.; Hooijmans, C.R.; Langendam, M.W.; Van Luijk, J.; Leenaars, M.; Ritskes-Hoitinga, M.; Wever, K.E.; Ritskes-Hoitinga, M. A protocol format for the preparation, registration and publication of systematic reviews of animal intervention studies. Evid. Based Preclin. Med. 2015, 2, 1–9. [Google Scholar] [CrossRef]
- Buscemi, N.; Hartling, L.; VanderMeer, B.; Tjosvold, L.; Klassen, T.P. Single data extraction generated more errors than double data extraction in systematic reviews. J. Clin. Epidemiol. 2006, 59, 697–703. [Google Scholar] [CrossRef] [PubMed]
- Beltrán, Ó.A. Revisiones sistemáticas de la literatura. Rev. Colomb. Gastroenterol. 2005, 20, 60–69. [Google Scholar]
| Author/Year | Subjects | Sample Size (n) | Intervention | Dosage | Duration | Results |
|---|---|---|---|---|---|---|
| Ahn YM et al. [27] (2017) | Hypertensive (HTA) and normotensive rats | 31 | Oleanolic acid (OA) by oral gavage | 30 mg/kg/day | 7 weeks (OA last 3 weeks) | ↓ SBP (p < 0.001) in OA-treated HTA rats vs. HTA control |
| Bachhav SS et al. [28] (2015) | L-NAME, during the intervention, induced hypertensive rats | 34 | Oral administration of OA | 60 mg/kg/day | 4 weeks | ↓ SBP (p < 0.001) and MAP (p < 0.05), ↑ urine excretion and urine sodium vs. L-NAME control group; ↓ non-significant (ns) of body weight and ↑ ns of serum NOx vs. L-NAME control group. |
| Madlala HP et al. [29] (2015) | Normotense, DSS and SHR rats | 18 | Oral administration of OA | 30, 60 and 120 mg/kg twice every three days | 9 weeks | ↓ MAP (p < 0.05), ↑ urine sodium excretion in DSS and SHR rats; ↓MDA in al tissues, and ↑ SOD and GSH-Px activities in liver and kidney in DSS and SHR rats, only in OA60 |
| Chen S et al. [31] (2017) | HFD-fed mice and diabetic db/db mice | 20 | Intraperitoneal injection of OA | 20 mg/kg b.w/day | 4 weeks | ↓ TG, TC, LDL, HDL (p < 0.05) in OA-treated diabetic mice vs. non-OA-treated diabetic mice |
| Jiang Q et al. [32] (2015) | HFD-fed quails | 120 | OA via gavage | 25, 50 and 100 mg/kg/day | 10 weeks | ↓ serum TG, TC, LDL and MDA, and ↑ HDL (4.05 ± 0.31 vs. 2.63 ± 0.52 mM, p < 0.05), NO (37.60 ± 9.15 vs. 29.49 ± 7.47 µM, p < 0.05), SOD, CAT, GSH and GSH-Px vs. HFD control group, especially with 100 mg/kg of OA. |
| Luo H et al. [33] (2017) | 32 rabbits 32 C57BL/6J mice 24 LDLR−/− mice | 88 | OA administration to animals fed with atherogenic diet | 10 (rabbits) and 25 (mice) mg/kg/day | 12 weeks (last 5 weeks OA) | ↓ TG, TC, LDL vs. non-OA-treated rabbits ↓ TG, LDL, ↑ HDL vs. non-OA-treated LDLR−/− mice ↓ TC, LDL vs. non-OA-treated C57BL/6J mice. |
| Pan Y et al. [34] (2018) | HFD-fed rabbits | 24 | OA via gavage | 50 mg/kg/day | 12 weeks (last 4 weeks OA) | ↓ TG (p < 0.001), TC (p < 0.001), LDL (p < 0.05) and HDL (p < 0.01); ↓ serum levels of IL-1β, IL-6 (p < 0.001), and TNFα (p < 0.001) vs. HFD control group. |
| Molepo M et al. [12] (2018) | Pups rats. | 96 | OA via oral gavage | 60 mg/kg/day | 16 weeks (2nd week OA) | ↓ saturated FFA, and ↑ mono/polyunsaturated FFA vs. control group |
| Wang X et al. [35] (2013) | Non-diabetic rats and diabetic mice | 34 | Intraperitoneal injection of OA | 20 mg/kg/day | 2 weeks | ↓ FBG, and FSI; ↓ body weight (36.4 ± 2.3 vs. 41.7 ± 4.1 g); ↓ TG, TC, LDL, FFA, IL-1β, IL-6, and TNFα, and ↑ HDL both in serum and ↓ liver;↓ AUC of IPGTT and IPITT. All changes (p < 0.05) vs. non-OA-treated diabetic mice. |
| Li Y et al. [36] (2014) | Fructose induced insulin resistant rats | 24 | Oral administration of OA | 5 and 25 mg/kg/day | 10 weeks | ↓ FSI, HOMA-IR and Adipo-IR vs. non-OA-treated insulin resistant rats; ↓ AUC of FFA and ↓ non-significant of glucose in the OGTT vs. non-insulin resistant rats. These changes (p < 0.05) only with OA 25 mg. |
| Lee ES et al. [37] (2016) | Non-diabetic and T2DM rats | - | OA via oral gavage | 100 mg/kg/day | 20 weeks | ↓ Body weight vs. non-diabetic rats control group. ↑ Insulinemia, HOMA-β and serum SOD, and ↓ TG vs. non-OA-treated diabetic rats. |
| Wang X et al. [38] (2015) | Diabetic mice | 24 | Intragastric administration of OA | 250 mg/kg/day | 4 weeks | ↓ FBG (p < 0.001), HOMA-IR (p < 0.05) and HDL (7.54 ± 0.82 vs. 9.02 ± 0.97 mM/l, p < 0.01), improved glucose AUC of OGTT and ↓ non-significant of FSI vs. control group |
| Gamede M et al. [39] (2018) | HFHC diet induced prediabetic rats | 36 | Oral administration of OA | 80 mg/kg/3days | 12 weeks | ↓ Body weight (p < 0.05), glycemia in the OGTT (p < 0.05), HOMA2-IR (60.35 ± 2.05 vs. 128.26 ± 2.98, p < 0.05), HbA1c, ghrelin, hepatic and muscular glycogen concentration vs. non-OA-treated prediabetic rats. |
| Gamede M et al. [30] (2019) | HFHC diet induced prediabetic rats | 36 | Oral administration of OA | Not mentioned | 12 weeks | ↓ Body weight (516.75 ± 8.28 vs. 679.75 ± 78.52 g), FBG, MAP, and plasma levels of TG, LDL, IL-6 and TNF-α, ↑ plasma level of HDL (1.88 ± 0.02 vs. 0.85 ± 0.04 mM/l), SOD and GSH-Px, and ↓ heart MDA concentration vs. prediabetic control group. All changes p < 0.05 |
| Djeziri FZ et al. [40] (2018) | HFD induced obese mice | 18 | Oral administration of OA | Not mentioned | 16 weeks | ↓ Glycemia in the IPGTT; and ↓ gene expression of IL-1β, IL-6, and TNFα vs. HFD control group |
| Nakajima K et al. [41] (2019) | STD, HFD or HGD-fed mice | 18 | OA by oral gavage | 20 and 40 mg/kg/day | 1 week | ↓ plasma octanoylated ghrelin levels and body weight gain in STD-fed rats vs. non-OA-treated STD-fed rats |
| Su S et al. [42] (2018) | PCBs-induced metabolic disfunction in mice | 40 | Oral administration of OA | 50 mg/kg/3days | 10 weeks | ↓ FBG (132 ± 14 vs. 191 ± 16 mg/dl), HOMA-IR (1.02 ± 0.17 vs. 1.79 ± 0.35) and serum levels of TG, FFA, cholesterol and FSI (1.35 ± 0.41 vs. 2.8 ± 0.56 ng/dl); ↓ Glucose level in IPGTT and IPITT. All changes (p < 0.05) vs. non-OA-treated PCBs-induced mice |
| Wang S et al. [43] (2018) | HFF diet-fed rats | 36 | OA and Nano-OA by gavage | 25mg/kg/day | 12 weeks (last 6 weeks OA) | ↓ BW, FBG and serum NO level, ↑ serum CAT activity in OA and nano-OA groups. ↓ serum levels of FSI, TG and MDA, ↑ ISI and serum SOD activity in nano-OA group. All changes (p < 0.05) vs. non-treated insulin resistant rats. |
| An Q et al. [44] (2017) | Streptozotocin-induced diabetic rats. | 18 | Oleanolic acid | 100 mg/kg/day | 12 weeks (last 6 weeks OA) | ↓ FBG, serum levels of IL-1β (p < 0,001), IL-6 (p < 0.05), and TNFα (p < 0.01); ↑ serum NO level (p < 0.01) vs. non-OA-treated diabetic rats. |
| Matumba MG et al. [45] (2019) | Pups rats | 40 | Neonatal OA administration by orogastric gavage | 60 mg/kg/day | 16 weeks (2nd week OA) | ↑ Adiponectin (1,5 fold, p < 0.01); ↓ IL-6 (p < 0.01) and TNFα plasma concentration; and ↓ gene expression of IL-6 (p < 0.0001) and TNFα p < 0.0001) vs. non-OA-treated HF-fed rats |
| Nyakudya TT et al. [46] (2018) | Pups rats. High fructose to half of the rats | 112 | Neonatal OA administration | 60 mg/kg/day b.w. | 16 weeks (2nd week OA) | ↓ AUC in the OGTT, and of the HOMA-IR index in the rats treated with OA. |
| Nyakudya et al. [47] (2018) | Pups rats | 112 | Neonatal OA administration | 60 mg/kg/day b.w. | 16 weeks (2nd week OA) | ↑ hepatic lipid content in male rats, and in terminal body mass in female rats fed with HF as neonates and as a adults vs. OA-treated rats. |
| Nyakudya et al. [48] (2019) | Pups rats in their second postnatal week | 30 | Neonatal OA administration by orogastric gavage | 60 mg/kg/day b.w. | 1 week | ↑ level of GSH and CAT activity, ↓ MDA concentration in skeletal muscle tissue vs. HF-fed rats. |
| Author/Year | Subjects | Sample Size (n) | Intervention | Dosage | Duration | Results |
|---|---|---|---|---|---|---|
| Luo HQ et al. [49] (2018) | Hyperlipidemic patients | 15 | Oleanolic acid | Not mentioned | 4 weeks | ↓ TC, TG, LDL, glucose and FSI; |
| ↑ HDL and Leptin; slight ↓ of HbA1c |
| Items of the tool | Ahn YM et al. (2017) [27] | Bachhav SS et al. (2015) [28] | Madlala HP et al. (2015) [29] | Chen S et al. (2017) [31] | Jiang Q et al. (2015) [32] | Luo H et al. (2017) [33] | Pan Y et al. (2018) [34] | Molepo M et al. (2018) [12] | Wang X et al. (2013) [35] | Li Y et al. (2014) [36] | Lee ES et al. (2016) [37] | Wang X et al. (2015) [38] | Gamede M et al. (2018) [39] | Gamede M et al. (2019) [30] | Djeziri FZ et al. (2018) [40] | Nakajima K et al. (2019) [41] | Su S et al. (2018) [42] | Wang S et al. (2018) [43] | An Q et al. (2017) [44] | Matumba MG et al. (2019) [45] | Nyakudya TT et al. (2018) [46] | Nyakudya TT et al. (2018) [47] | Nyakudya TT et al. (2019) [48] |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. Was the allocation sequence adequately generated and applied? | ? | ? | ? | ? | + | ? | ? | ? | ? | ? | ? | ? | ? | — | ? | ? | ? | ? | ? | ? | ? | + | + |
| 2. Were the groups similar at baseline or were they adjusted for confounders in the analysis? | + | + | + | + | + | ? | + | ? | ? | + | ? | + | + | + | + | ? | ? | + | ? | ? | + | + | + |
| 3. Was the allocation to the different groups adequately concealed during? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? |
| 4. Were the animals randomly housed during the experiment? | ? | + | ? | ? | ? | + | ? | ? | + | + | + | + | ? | ? | ? | ? | ? | ? | ? | ? | ? | + | + |
| 5. Were the caregivers and/or investigators blinded from knowledge which intervention each animal received during the experiment? | ? | ? | + | + | ? | ? | ? | ? | ? | + | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? |
| 6. Were animals selected at random for outcome assessment? | ? | ? | ? | ? | ? | + | ? | + | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? |
| 7. Was the outcome assessor blinded? | ? | + | ? | ? | + | + | ? | + | ? | + | ? | + | + | + | + | + | + | + | + | + | ? | + | + |
| 8. Were incomplete outcome data adequately addressed? | + | ? | ? | ? | ? | + | + | + | ? | + | ? | + | + | + | + | ? | + | + | + | + | ? | + | + |
| 9. Are reports of the study free of selective outcome reporting? | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + |
| 10. Was the study apparently free of other problems that could result in high risk of bias? | ? | + | ? | ? | ? | + | + | ? | ? | + | + | + | + | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? |
| Authors (Year) | Randomization | Method of Randomization | Double Blinding | Method of Blinding | Dropouts/Withdrawals | Jadad Score |
|---|---|---|---|---|---|---|
| Luo HQ et al. (2018) [49] | No | No | No | No | Yes | 1 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fernández-Aparicio, Á.; Schmidt-RioValle, J.; Perona, J.S.; Correa-Rodríguez, M.; Castellano, J.M.; González-Jiménez, E. Potential Protective Effect of Oleanolic Acid on the Components of Metabolic Syndrome: A Systematic Review. J. Clin. Med. 2019, 8, 1294. https://doi.org/10.3390/jcm8091294
Fernández-Aparicio Á, Schmidt-RioValle J, Perona JS, Correa-Rodríguez M, Castellano JM, González-Jiménez E. Potential Protective Effect of Oleanolic Acid on the Components of Metabolic Syndrome: A Systematic Review. Journal of Clinical Medicine. 2019; 8(9):1294. https://doi.org/10.3390/jcm8091294
Chicago/Turabian StyleFernández-Aparicio, Ángel, Jacqueline Schmidt-RioValle, Javier S. Perona, María Correa-Rodríguez, Jose M. Castellano, and Emilio González-Jiménez. 2019. "Potential Protective Effect of Oleanolic Acid on the Components of Metabolic Syndrome: A Systematic Review" Journal of Clinical Medicine 8, no. 9: 1294. https://doi.org/10.3390/jcm8091294
APA StyleFernández-Aparicio, Á., Schmidt-RioValle, J., Perona, J. S., Correa-Rodríguez, M., Castellano, J. M., & González-Jiménez, E. (2019). Potential Protective Effect of Oleanolic Acid on the Components of Metabolic Syndrome: A Systematic Review. Journal of Clinical Medicine, 8(9), 1294. https://doi.org/10.3390/jcm8091294








