Prediction of Transplant-Free Survival through Albumin-Bilirubin Score in Primary Biliary Cholangitis
Abstract
:1. Introduction
2. Experimental Section
2.1. Ethics
2.2. Patients
2.3. Clinical Data
2.4. Histopathological Analysis
2.5. Statistical Analyses
3. Results
3.1. Characteristics of the Patients
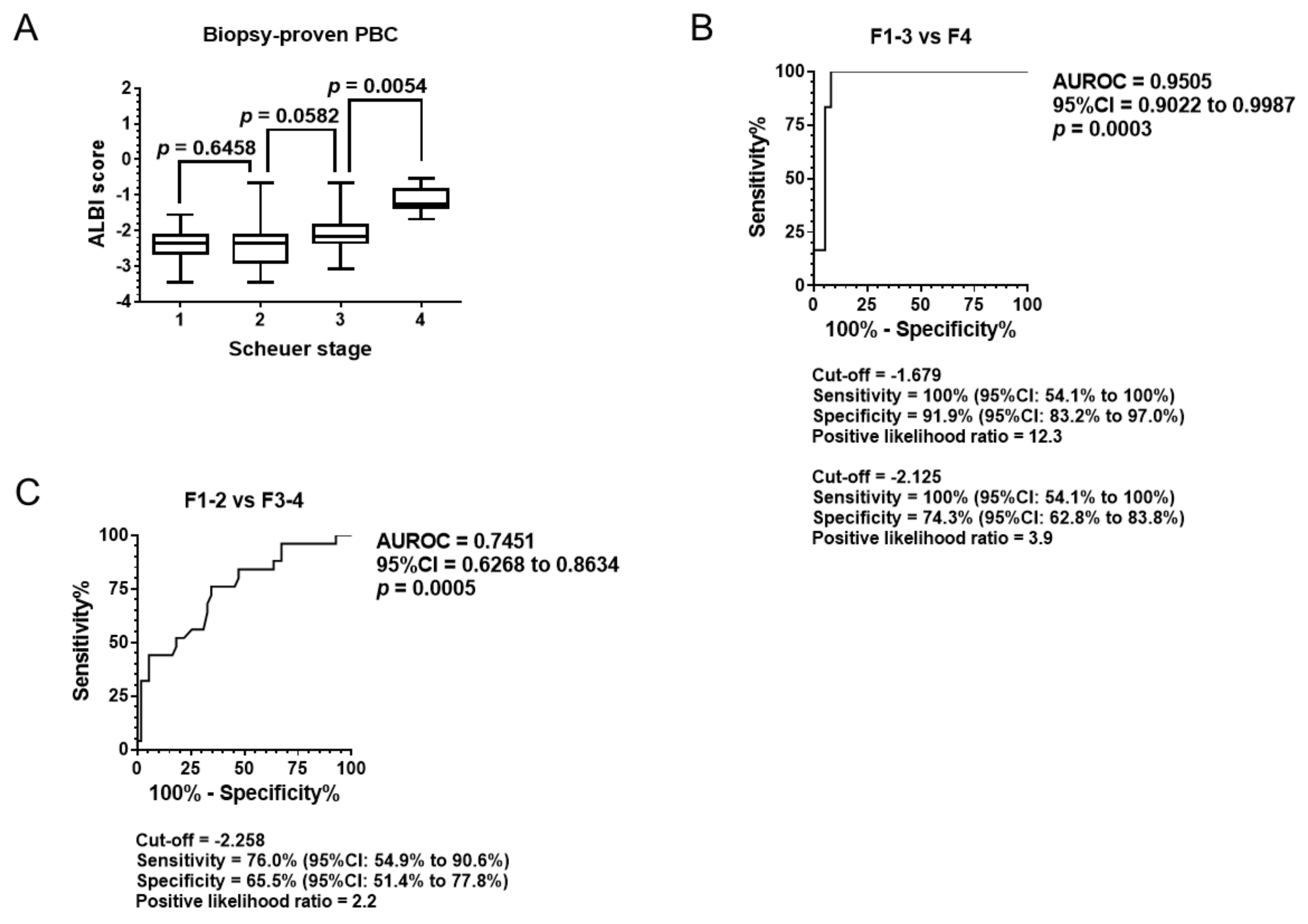
3.2. Diagnostic Ability of Pathological Stage
3.3. Baseline ALBI Score and Transplant-Free Survival
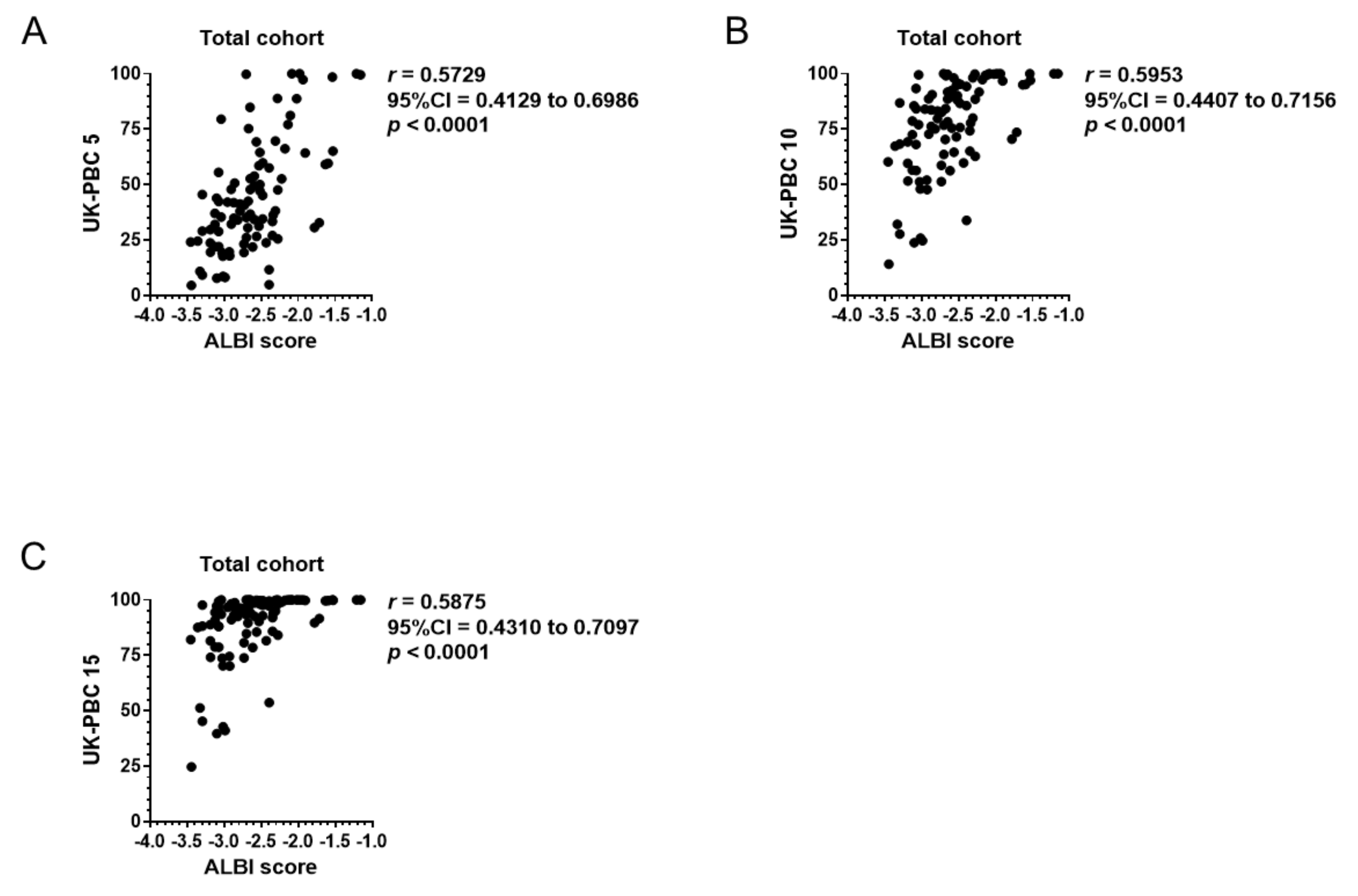
3.4. Correlation of Baseline ALBI Score with GLOBE Score, UK-PBC Score, and MELD Score
3.5. Transplant-Free Survival in Patients under UDCA Treatment
3.6. Prediction of Transplant-Free Survival by APRI
4. Discussion
5. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- European Association for the Study of the Liver. EASL Clinical Practice Guidelines: The diagnosis and management of patients with primary biliary cholangitis. J. Hepatol. 2017, 67, 145–172. [Google Scholar] [CrossRef] [PubMed]
- Hirschfield, G.M.; Dyson, J.K.; Alexander, G.J.M.; Chapman, M.H.; Collier, J.; Hübscher, S.; Patanwala, I.; Pereira, S.P.; Thain, C.; Thorburn, D.; et al. The British Society of Gastroenterology/UK-PBC primary biliary cholangitis treatment and management guidelines. Gut 2018, 67, 1568–1594. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, A.; Fritze, D.; Mansouri, M.; Lopez, R.; Poordad, F.; Lawitz, E.; Cigarroa, F.; Halff, G.; Alkhouri, N. Characteristics and Outcomes of Liver Transplantation for Primary Biliary Cholangitis (PBC) in Young patients: Analysis of the United Network for Organ Sharing Database. Transplantation 2019, 103, 1191–1198. [Google Scholar] [CrossRef] [PubMed]
- Poupon, R.E.; Balkau, B.; Eschwège, E.; Poupon, R. A multicenter, controlled trial of ursodiol for the treatment of primary biliary cirrhosis. UDCA-PBC Study Group. N. Engl. J. Med. 1991, 324, 1548–1554. [Google Scholar] [CrossRef] [PubMed]
- Poupon, R.E.; Poupon, R.; Balkau, B. Ursodiol for the long-term treatment of primary biliary cirrhosis. The UDCA-PBC Study Group. N. Engl. J. Med. 1994, 330, 1342–1347. [Google Scholar] [CrossRef] [PubMed]
- Parés, A.; Caballería, L.; Rodés, J. Excellent long-term survival in patients with primary biliary cirrhosis and biochemical response to ursodeoxycholic Acid. Gastroenterology 2006, 130, 715–720. [Google Scholar] [CrossRef] [PubMed]
- Ter Borg, P.C.; Schalm, S.W.; Hansen, B.E.; Van Buuren, H.R.; Group DPS. Prognosis of ursodeoxycholic Acid-treated patients with primary biliary cirrhosis. Results of a 10-yr cohort study involving 297 patients. Am. J. Gastroenterol. 2006, 101, 2044–2050. [Google Scholar] [CrossRef] [PubMed]
- Trivedi, P.J.; Bruns, T.; Cheung, A.; Li, K.K.; Kittler, C.; Kumagi, T.; Shah, H.; Corbett, C.; Al-Harthy, N.; Acarsu, U.; et al. Optimising risk stratification in primary biliary cirrhosis: AST/platelet ratio index predicts outcome independent of ursodeoxycholic acid response. J. Hepatol. 2014, 60, 1249–1258. [Google Scholar] [CrossRef]
- Hayashi, M.; Abe, K.; Fujita, M.; Okai, K.; Takahashi, A.; Ohira, H. Changes in serum levels of leucine-rich α2-glycoprotein predict prognosis in primary biliary cholangitis. Hepatol. Res. 2019, 49, 385–393. [Google Scholar] [CrossRef]
- Lammers, W.J.; Hirschfield, G.M.; Corpechot, C.; Nevens, F.; Lindor, K.D.; Janssen, H.L.; Floreani, A.; Ponsioen, C.Y.; Mayo, M.J.; Invernizzi, P.; et al. Development and Validation of a Scoring System to Predict Outcomes of Patients With Primary Biliary Cirrhosis Receiving Ursodeoxycholic Acid Therapy. Gastroenterology 2015, 149, 1804–1812.e1804. [Google Scholar] [CrossRef]
- Carbone, M.; Sharp, S.J.; Flack, S.; Paximadas, D.; Spiess, K.; Adgey, C.; Griffiths, L.; Lim, R.; Trembling, P.; Williamson, K.; et al. The UK-PBC risk scores: Derivation and validation of a scoring system for long-term prediction of end-stage liver disease in primary biliary cholangitis. Hepatology 2016, 63, 930–950. [Google Scholar] [CrossRef]
- Johnson, P.J.; Berhane, S.; Kagebayashi, C.; Satomura, S.; Teng, M.; Reeves, H.L.; O’Beirne, J.; Fox, R.; Skowronska, A.; Palmer, D.; et al. Assessment of liver function in patients with hepatocellular carcinoma: A new evidence-based approach-the ALBI grade. J. Clin. Oncol. 2015, 33, 550–558. [Google Scholar] [CrossRef]
- Association, W.M. World Medical Association Declaration of Helsinki: Ethical principles for medical research involving human subjects. JAMA 2013, 310, 2191–2194. [Google Scholar]
- Vellinga, A.; Cormican, M.; Hanahoe, B.; Bennett, K.; Murphy, A.W. Opt-out as an acceptable method of obtaining consent in medical research: A short report. BMC Med. Res. Methodol. 2011, 11, 40. [Google Scholar] [CrossRef]
- Montoy, J.C.; Dow, W.H.; Kaplan, B.C. Patient choice in opt-in, active choice, and opt-out HIV screening: Randomized clinical trial. BMJ 2016, 532, h6895. [Google Scholar] [CrossRef]
- Gershwin, M.E.; Rowley, M.; Davis, P.A.; Leung, P.; Coppel, R.; Mackay, I.R. Molecular biology of the 2-oxo-acid dehydrogenase complexes and anti-mitochondrial antibodies. Prog. Liver Dis. 1992, 10, 47–61. [Google Scholar]
- Nakamura, M.; Kondo, H.; Mori, T.; Komori, A.; Matsuyama, M.; Ito, M.; Takii, Y.; Koyabu, M.; Yokoyama, T.; Migita, K.; et al. Anti-gp210 and anti-centromere antibodies are different risk factors for the progression of primary biliary cirrhosis. Hepatology 2007, 45, 118–127. [Google Scholar] [CrossRef]
- Yang, F.; Yang, Y.; Wang, Q.; Wang, Z.; Miao, Q.; Xiao, X.; Wei, Y.; Bian, Z.; Sheng, L.; Chen, X.; et al. The risk predictive values of UK-PBC and GLOBE scoring system in Chinese patients with primary biliary cholangitis: The additional effect of anti-gp210. Aliment. Pharmacol. Ther. 2017, 45, 733–743. [Google Scholar] [CrossRef]
- Wai, C.T.; Greenson, J.K.; Fontana, R.J.; Kalbfleisch, J.D.; Marrero, J.A.; Conjeevaram, H.S.; Lok, A.S.F. A simple noninvasive index can predict both significant fibrosis and cirrhosis in patients with chronic hepatitis C. Hepatology 2003, 38, 518–526. [Google Scholar] [CrossRef] [Green Version]
- Scheuer, P. Primary biliary cirrhosis. Proc. R. Soc. Med. 1967, 60, 1257–1260. [Google Scholar]
- Scheuer, P.J. Ludwig Symposium on biliary disorders—Part II. Pathologic features and evolution of primary biliary cirrhosis and primary sclerosing cholangitis. Mayo Clin. Proc. 1998, 73, 179–183. [Google Scholar] [CrossRef]
- Malinchoc, M.; Kamath, P.S.; Gordon, F.D.; Peine, C.J.; Rank, J.; Ter Borg, P.C. A model to predict poor survival in patients undergoing transjugular intrahepatic portosystemic shunts. Hepatology 2000, 31, 864–871. [Google Scholar] [CrossRef]
- Kamath, P.S.; Wiesner, R.H.; Malinchoc, M.; Kremers, W.; Therneau, T.M.; Kosberg, C.L.; D’Amico, G.; Dickson, E.R.; Dickson, E.R. A model to predict survival in patients with end-stage liver disease. Hepatology 2001, 33, 464–470. [Google Scholar] [CrossRef]
- Hens, N.; Aerts, M.; Molenberghs, G. Model selection for incomplete and design-based samples. Stat. Med. 2006, 25, 2502–2520. [Google Scholar] [CrossRef] [Green Version]
- Kanda, Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transpl. 2013, 48, 452–458. [Google Scholar] [CrossRef]
- Fujita, K.; Oura, K.; Yoneyama, H.; Ting Ting, S.; Takuma, K.; Nakahara, M.; Tadokoro, T.; Nomura, T.; Morishita, A.; Tsutsui, K. Albumin-bilirubin score indicates liver fibrosis staging and prognosis in chronic hepatitis C patients. Hepatol. Res. 2019. [Google Scholar] [CrossRef]
- Zhang, Z.Q.; Xiong, L.; Zhou, J.J.; Miao, X.Y.; Li, Q.L.; Wen, Y.; Zou, H. Ability of the ALBI grade to predict posthepatectomy liver failure and long-term survival after liver resection for different BCLC stages of HCC. World J. Surg. Oncol. 2018, 16, 208. [Google Scholar] [CrossRef]
- Toyoda, H.; Lai, P.B.; O’Beirne, J.; Chong, C.C.; Berhane, S.; Reeves, H.; Manas, D.; Fox, R.P.; Yeo, W.; Mo, F.; et al. Long-term impact of liver function on curative therapy for hepatocellular carcinoma: Application of the ALBI grade. Br. J. Cancer 2016, 114, 744–750. [Google Scholar] [CrossRef]
- Ogasawara, S.; Chiba, T.; Ooka, Y.; Suzuki, E.; Kanogawa, N.; Saito, T.; Motoyama, T.; Tawada, A.; Kanai, F.; Yokosuka, O. Liver function assessment according to the Albumin-Bilirubin (ALBI) grade in sorafenib-treated patients with advanced hepatocellular carcinoma. Investig. New Drugs 2015, 33, 1257–1262. [Google Scholar] [CrossRef]
- Gui, B.; Weiner, A.A.; Nosher, J.; Lu, S.E.; Foltz, G.M.; Hasan, O.; Kim, S.K.; Gendel, V.; Mani, N.B.; Carpizo, D.R.; et al. Assessment of the Albumin-Bilirubin (ALBI) Grade as a Prognostic Indicator for Hepatocellular Carcinoma Patients Treated with Radioembolization. Am. J. Clin. Oncol. 2018, 41, 861–866. [Google Scholar] [CrossRef]
- Carling, U.; Røsok, B.; Line, P.D.; Dorenberg, E.J. ALBI and P-ALBI grade in Child-Pugh A patients treated with drug eluting embolic chemoembolization for hepatocellular carcinoma. Acta Radiol. 2018. [Google Scholar] [CrossRef]
- Chan, A.W.; Chan, R.C.; Wong, G.L.; Wong, V.W.; Choi, P.C.; Chan, H.L.; To, K.F. New simple prognostic score for primary biliary cirrhosis: Albumin-bilirubin score. J. Gastroenterol. Hepatol. 2015, 30, 1391–1396. [Google Scholar] [CrossRef]
- Guilford, J.P. Fundamental Statistics in Psychology and Education; McGraw-Hill: New York, NY, USA, 1956. [Google Scholar]
- Oikonomou, T.; Goulis, L.; Doumtsis, P.; Tzoumari, T.; Akriviadis, E.; Cholongitas, E. ALBI and PALBI Grades Are Associated with the Outcome of Patients with Stable Decompensated Cirrhosis. Ann. Hepatol. 2019, 18, 126–136. [Google Scholar] [CrossRef]
- Olmez, S.; Sayar, S.; Avcioglu, U.; Tenlik, İ.; Ozaslan, E.; Koseoglu, H.T.; Altiparmak, E. The relationship between liver histology and noninvasive markers in primary biliary cirrhosis. Eur. J. Gastroenterol. Hepatol. 2016, 28, 773–776. [Google Scholar] [CrossRef]



| Biopsy-Proven Cohort | Serologically Diagnosed Cohort | p Value | Total | |
|---|---|---|---|---|
| Patient number | 80 | 101 | - | 181 |
| Follow up period < 1 year (n) | 13 | 11 | - | 24 |
| Follow up period > 1 year (n) | 67 | 90 | 0.3780 | 157 |
| UDCA therapy during the observation (n) | 50 | 89 | <0.0001 | 139 |
| Blood exam data after one year of UDCA treatment (n) | 15 | 78 | <0.0001 | 93 |
| Prescription of corticosteroid (n) | 5 | 16 | 0.0607 | 21 |
| Follow up period >1 year (year) | 11 (7–20) | 7 (4–11) | <0.0001 | 8 (4–14) |
| Age (year) | 57 (51–65) | 62 (55–72) | 0.0014 | 60 (54–68) |
| Sex (Male/Female, n) | 12/68 | 6/95 | 0.0490 | 18/163 |
| Albumin (g/L) | 36 (33–38) | 40 (36–43) | <0.0001 | 38 (34–42) |
| AST (U/L) | 57 (37–82) | 38 (29–61) | 0.0061 | 46 (31–71) |
| ALT (U/L) | 49 (36–83) | 35 (24–62) | 0.0019 | 43 (26–73) |
| T-Bil (μmol/L) | 13.8 (10.3–18.9) | 10.3 (8.6–13.8) | 0.0012 | 12.0 (8.6–15.5) |
| Platelet count (×109/L) | *230 (154–275) | 204 (159–245) | 0.0790 | †218 (159–249) |
| APRI | *0.868 (0.479–1.428) | 0.562 (0.402–0.983) | 0.0535 | †0.648 (0.408–1.208) |
| Fibrosis stage 1/2/3/4 (n) | 34/21/19/6 | - | - | - |
| Positive for anti-mitochondrial Ab (n) | 50 | 42 | 0.0069 | 92 |
| Positive for anti-mitochondrial M2 Ab (n) | 46 | 91 | <0.0001 | 137 |
| Negative for both Abs (n) | 14 | 5 | 0.0074 | 19 |
| Infection with HBV/HCV (n) | 0/0 | 1/2 | 1.0000/0.5040 | 1/2 |
| HCC incidence (n) | 3 | 3 | 1.0000 | 6 |
| Deaths or liver transplantation (n) | 16 | 3 | 0.0003 | 19 |
| Overall deaths (n) | 14 | 3 | 0.0014 | 17 |
| Liver-related deaths (HCC/Hepatic failure, n) | 11 (3/8) | 0 | <0.0001 (0.0845/0.0012) | 11 (3/8) |
| Liver transplantation (n) | 2 | 0 | 0.1940 | 2 |
| Total | |
|---|---|
| Patient number | 93 |
| Data at the beginning of UDCA therapy | |
| Albumin (g/L) | 39 (36–43) |
| Creatinine (mg/dl) | 0.59 (0.50–0.70) |
| T-Bil (μmol/L) | 10.3 (8.6–13.8) |
| PT/INR | 0.97 (0.93–1.04) |
| Platelet count (×109/L) | 214 (161–242) |
| Data after one year of UDCA therapy | |
| Albumin (g/L) | 41 (39–43) |
| ALT (U/L) | 23 (15–34) |
| ALP (U/L) | 302 (239–418) |
| T-Bil (μmol/L) | 10.3 (6.9–12.0) |
| Platelet count (×109/L) | 206 (156–254) |
| Positive for anti-centromere Ab (n) | 30 |
| Hazard Ratio | 95% Confidence Interval | p Value | |
|---|---|---|---|
| ALBI score < −2.258 | 0.2669 | 0.0716–0.9947 | 0.0491 |
| APRI < 0.76 | 0.5807 | 0.1755–1.9220 | 0.3734 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fujita, K.; Nomura, T.; Morishita, A.; Shi, T.; Oura, K.; Tani, J.; Kobara, H.; Tsutsui, K.; Himoto, T.; Masaki, T. Prediction of Transplant-Free Survival through Albumin-Bilirubin Score in Primary Biliary Cholangitis. J. Clin. Med. 2019, 8, 1258. https://doi.org/10.3390/jcm8081258
Fujita K, Nomura T, Morishita A, Shi T, Oura K, Tani J, Kobara H, Tsutsui K, Himoto T, Masaki T. Prediction of Transplant-Free Survival through Albumin-Bilirubin Score in Primary Biliary Cholangitis. Journal of Clinical Medicine. 2019; 8(8):1258. https://doi.org/10.3390/jcm8081258
Chicago/Turabian StyleFujita, Koji, Takako Nomura, Asahiro Morishita, Tingting Shi, Kyoko Oura, Joji Tani, Hideki Kobara, Kunihiko Tsutsui, Takashi Himoto, and Tsutomu Masaki. 2019. "Prediction of Transplant-Free Survival through Albumin-Bilirubin Score in Primary Biliary Cholangitis" Journal of Clinical Medicine 8, no. 8: 1258. https://doi.org/10.3390/jcm8081258
APA StyleFujita, K., Nomura, T., Morishita, A., Shi, T., Oura, K., Tani, J., Kobara, H., Tsutsui, K., Himoto, T., & Masaki, T. (2019). Prediction of Transplant-Free Survival through Albumin-Bilirubin Score in Primary Biliary Cholangitis. Journal of Clinical Medicine, 8(8), 1258. https://doi.org/10.3390/jcm8081258





