Patients with ANCA-Associated Glomerulonephritis and Connective Tissue Diseases: A Comparative Study from the Maine-Anjou AAV Registry
Abstract
:1. Introduction
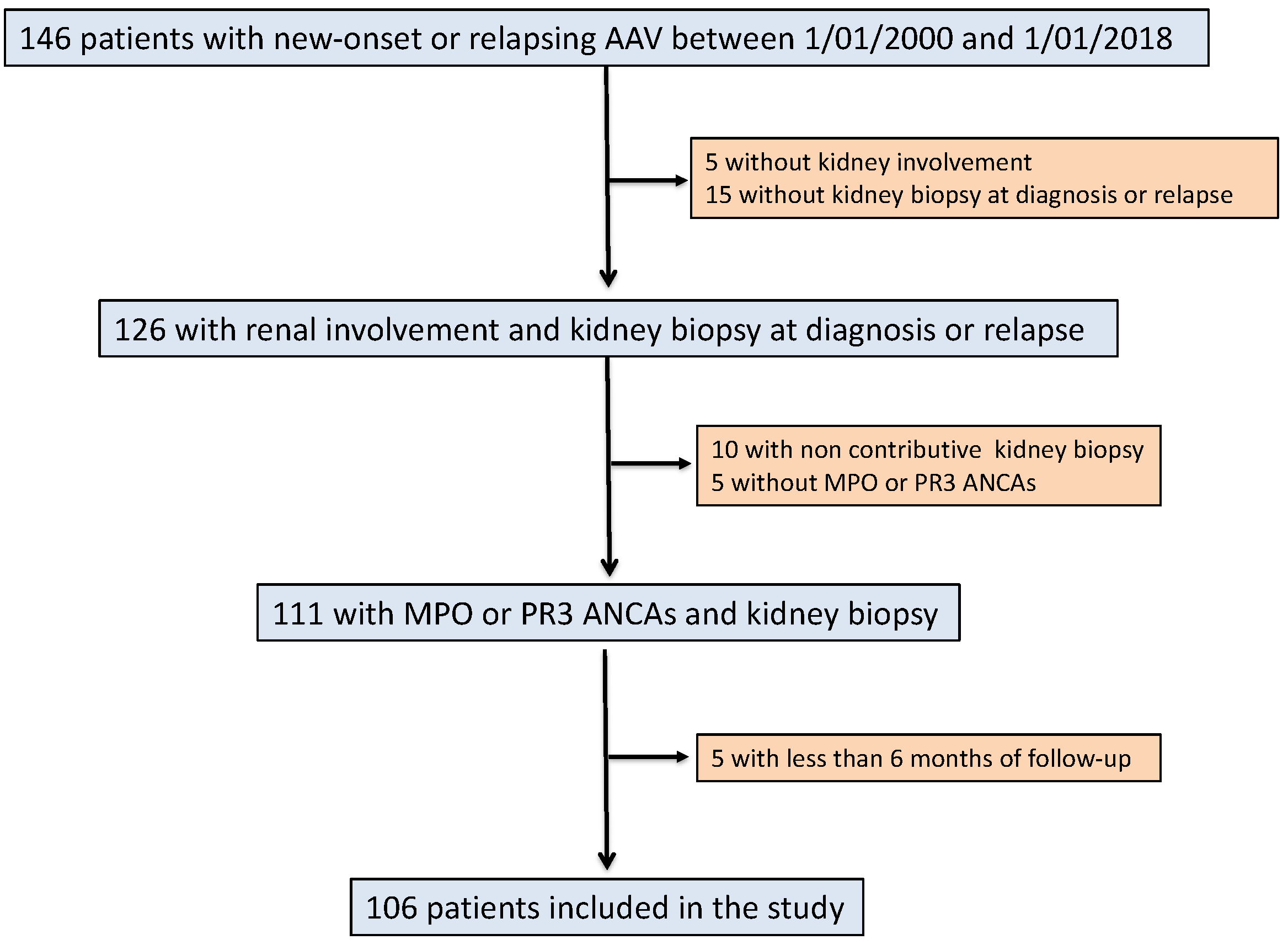
2. Materials and Methods
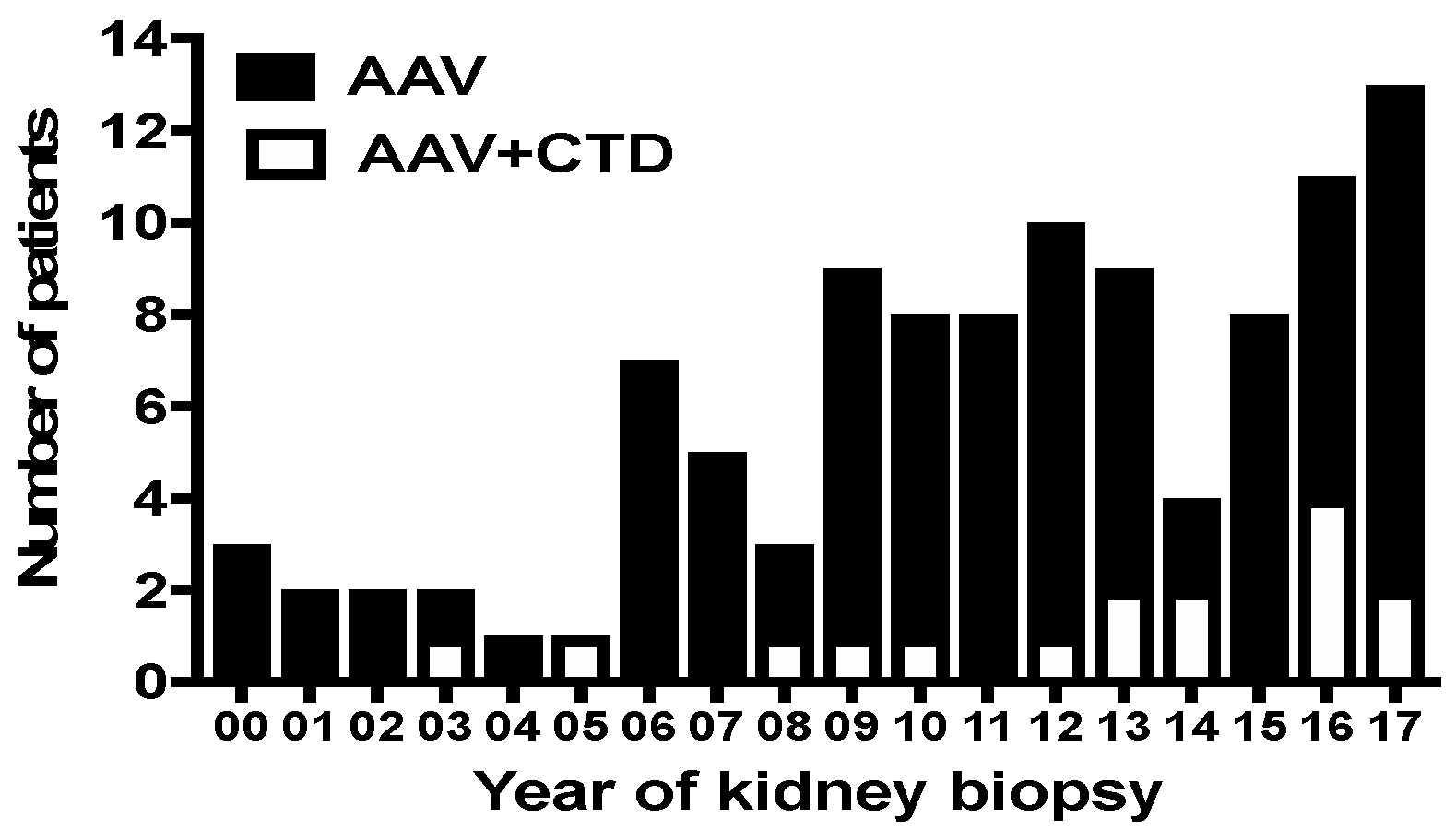
2.1. Study Population
2.2. Data Collection
2.3. Definitions
2.4. Renal Histopathology
2.5. Statistical Analysis
3. Results
3.1. Baseline Characteristics of Patients of the CTD Group
3.2. Baseline Characteristics of AAV Patients According to the Presence or Absence of CTD
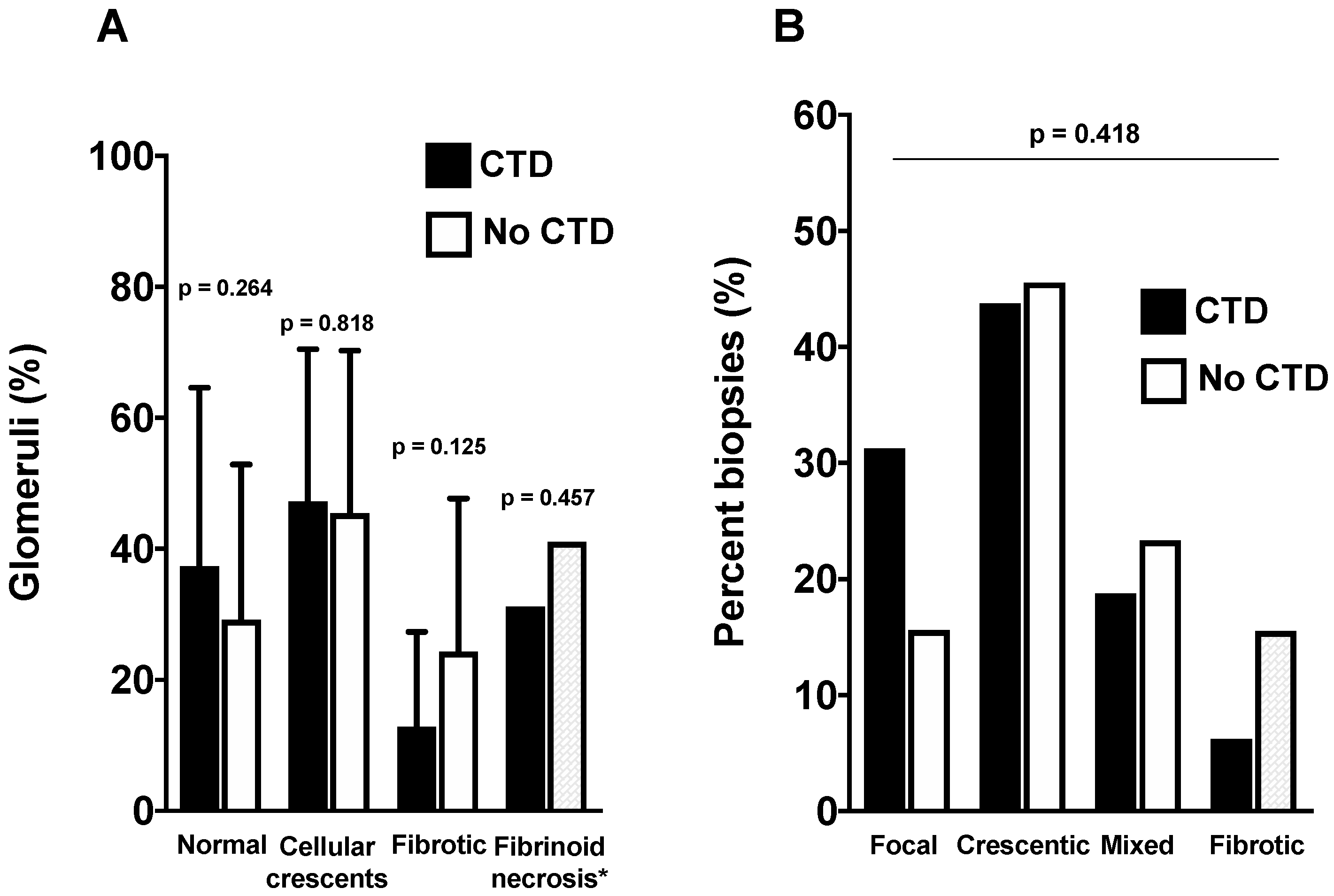
3.3. Comparison of Renal Presentation According to Groups
3.4. Comparison of Outcomes According to Groups
4. Discussion
5. Conclusions
Author Contributions
Conflicts of Interest
References
- Jennette, J.C.; Falk, R.J.; Hu, P.; Xiao, H. Pathogenesis of antineutrophil cytoplasmic autoantibody-associated small-vessel vasculitis. Annu. Rev. Pathol. 2013, 8, 139–160. [Google Scholar] [CrossRef] [PubMed]
- Jennette, J.C.; Falk, R.J. Small-vessel vasculitis. N. Engl. J. Med. 1997, 337, 1512–1523. [Google Scholar] [CrossRef] [PubMed]
- Beauvillain, C.; Delneste, Y.; Renier, G.; Jeannin, P.; Subra, J.F.; Chevailler, A. Antineutrophil cytoplasmic autoantibodies: How should the biologist manage them? Clin. Rev. Allergy Immunol. 2008, 35, 47–58. [Google Scholar] [CrossRef] [PubMed]
- Jennette, J.C.; Falk, R.J.; Bacon, P.A.; Basu, N.; Cid, M.C.; Ferrario, F.; Flores-Suarez, L.F.; Gross, W.L.; Guillevin, L.; Hagen, E.C.; et al. 2012 revised international chapel hill consensus conference nomenclature of vasculitides. Arthritis Rheum. 2013, 65, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Watts, R.A.; Mahr, A.; Mohammad, A.J.; Gatenby, P.; Basu, N.; Flores-Suarez, L.F. Classification, epidemiology and clinical subgrouping of antineutrophil cytoplasmic antibody (anca)-associated vasculitis. Nephrol. Dial. Transpl. 2015, 30, 14–22. [Google Scholar] [CrossRef] [PubMed]
- Jennette, J.C.; Nachman, P.H. Anca glomerulonephritis and vasculitis. Clin. J. Am. Soc. Nephrol. 2017, 12, 1680–1691. [Google Scholar] [CrossRef] [PubMed]
- Quemeneur, T.; Mouthon, L.; Cacoub, P.; Meyer, O.; Michon-Pasturel, U.; Vanhille, P.; Hatron, P.Y.; Guillevin, L.; Hachulla, E. Systemic vasculitis during the course of systemic sclerosis: Report of 12 cases and review of the literature. Med. Baltim. 2013, 92, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Guellec, D.; Cornec-Le Gall, E.; Groh, M.; Hachulla, E.; Karras, A.; Charles, P.; Dunogue, B.; Abad, S.; Alvarez, F.; Gerard, F.; et al. Anca-associated vasculitis in patients with primary sjogren’s syndrome: Detailed analysis of 7 new cases and systematic literature review. Autoimmun. Rev. 2015, 14, 742–750. [Google Scholar] [CrossRef]
- Jarrot, P.A.; Chiche, L.; Hervier, B.; Daniel, L.; Vuiblet, V.; Bardin, N.; Bertin, D.; Terrier, B.; Amoura, Z.; Andres, E.; et al. Systemic lupus erythematosus and antineutrophil cytoplasmic antibody-associated vasculitis overlap syndrome in patients with biopsy-proven glomerulonephritis. Med. Baltim. 2016, 95, e3748. [Google Scholar] [CrossRef] [PubMed]
- Martin-Nares, E.; Zuniga-Tamayo, D.; Hinojosa-Azaola, A. Prevalence of overlap of antineutrophil cytoplasmic antibody associated vasculitis with systemic autoimmune diseases: An unrecognized example of poliautoimmunity. Clin. Rheumatol. 2018, 38, 97–106. [Google Scholar] [CrossRef] [PubMed]
- Draibe, J.; Salama, A.D. Association of anca associated vasculitis and rheumatoid arthritis: A lesser recognized overlap syndrome. Springerplus 2015, 4, 50. [Google Scholar] [CrossRef] [PubMed]
- Luqmani, R.A.; Bacon, P.A.; Moots, R.J.; Janssen, B.A.; Pall, A.; Emery, P.; Savage, C.; Adu, D. Birmingham vasculitis activity score (bvas) in systemic necrotizing vasculitis. QJM Mon. J. Assoc. Physicians 1994, 87, 671–678. [Google Scholar]
- Levey, A.S.; Bosch, J.P.; Lewis, J.B.; Greene, T.; Rogers, N.; Roth, D. A more accurate method to estimate glomerular filtration rate from serum creatinine: A new prediction equation. Modification of diet in renal disease study group. Ann. Intern. Med. 1999, 130, 461–470. [Google Scholar] [CrossRef] [PubMed]
- Watts, R.; Lane, S.; Hanslik, T.; Hauser, T.; Hellmich, B.; Koldingsnes, W.; Mahr, A.; Segelmark, M.; Cohen-Tervaert, J.W.; Scott, D. Development and validation of a consensus methodology for the classification of the anca-associated vasculitides and polyarteritis nodosa for epidemiological studies. Ann. Rheum. Dis. 2007, 66, 222–227. [Google Scholar] [CrossRef] [PubMed]
- Aletaha, D.; Neogi, T.; Silman, A.J.; Funovits, J.; Felson, D.T.; Bingham, C.O., 3rd; Birnbaum, N.S.; Burmester, G.R.; Bykerk, V.P.; Cohen, M.D.; et al. 2010 rheumatoid arthritis classification criteria: An american college of rheumatology/european league against rheumatism collaborative initiative. Arthritis Rheum. 2010, 62, 2569–2581. [Google Scholar] [CrossRef] [PubMed]
- Hochberg, M.C. Updating the american college of rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1997, 40, 1725. [Google Scholar] [CrossRef]
- Tan, E.M.; Cohen, A.S.; Fries, J.F.; Masi, A.T.; McShane, D.J.; Rothfield, N.F.; Schaller, J.G.; Talal, N.; Winchester, R.J. The 1982 revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1982, 25, 1271–1277. [Google Scholar] [CrossRef]
- Shiboski, C.H.; Shiboski, S.C.; Seror, R.; Criswell, L.A.; Labetoulle, M.; Lietman, T.M.; Rasmussen, A.; Scofield, H.; Vitali, C.; Bowman, S.J.; et al. 2016 american college of rheumatology/european league against rheumatism classification criteria for primary sjogren’s syndrome: A consensus and data-driven methodology involving three international patient cohorts. Arthritis Rheumatol. 2017, 69, 35–45. [Google Scholar] [CrossRef]
- Van den Hoogen, F.; Khanna, D.; Fransen, J.; Johnson, S.R.; Baron, M.; Tyndall, A.; Matucci-Cerinic, M.; Naden, R.P.; Medsger, T.A., Jr.; Carreira, P.E.; et al. 2013 classification criteria for systemic sclerosis: An american college of rheumatology/european league against rheumatism collaborative initiative. Arthritis Rheum. 2013, 65, 2737–2747. [Google Scholar] [CrossRef]
- Berden, A.E.; Ferrario, F.; Hagen, E.C.; Jayne, D.R.; Jennette, J.C.; Joh, K.; Neumann, I.; Noel, L.H.; Pusey, C.D.; Waldherr, R.; et al. Histopathologic classification of anca-associated glomerulonephritis. J. Am. Soc. Nephrol. 2010, 21, 1628–1636. [Google Scholar] [CrossRef]
- Lyons, P.A.; Rayner, T.F.; Trivedi, S.; Holle, J.U.; Watts, R.A.; Jayne, D.R.; Baslund, B.; Brenchley, P.; Bruchfeld, A.; Chaudhry, A.N.; et al. Genetically distinct subsets within anca-associated vasculitis. N. Engl. J. Med. 2012, 367, 214–223. [Google Scholar] [CrossRef] [PubMed]
- Stanford, S.M.; Bottini, N. Ptpn22: The archetypal non-hla autoimmunity gene. Nat. Rev. Rheumatol. 2014, 10, 602–611. [Google Scholar] [CrossRef] [PubMed]
- Braun, M.G.; Csernok, E.; Schmitt, W.H.; Gross, W.L. Incidence, target antigens, and clinical implications of antineutrophil cytoplasmic antibodies in rheumatoid arthritis. J. Rheumatol. 1996, 23, 826–830. [Google Scholar] [PubMed]
- Rother, E.; Schochat, T.; Peter, H.H. Antineutrophil cytoplasmic antibodies (anca) in rheumatoid arthritis: A prospective study. Rheumatol. Int. 1996, 15, 231–237. [Google Scholar] [CrossRef] [PubMed]
- Manolova, I.; Dancheva, M.; Halacheva, K. Antineutrophil cytoplasmic antibodies in patients with systemic lupus erythematosus: Prevalence, antigen specificity, and clinical associations. Rheumatol. Int. 2001, 20, 197–204. [Google Scholar] [PubMed]
- Schnabel, A.; Csernok, E.; Isenberg, D.A.; Mrowka, C.; Gross, W.L. Antineutrophil cytoplasmic antibodies in systemic lupus erythematosus. Prevalence, specificities, and clinical significance. Arthritis Rheum. 1995, 38, 633–637. [Google Scholar] [CrossRef] [PubMed]
- Khanna, D.; Aggarwal, A.; Bhakuni, D.S.; Dayal, R.; Misra, R. Bactericidal/permeability-increasing protein and cathepsin g are the major antigenic targets of antineutrophil cytoplasmic autoantibodies in systemic sclerosis. J. Rheumatol. 2003, 30, 1248–1252. [Google Scholar] [PubMed]
- Nishiya, K.; Chikazawa, H.; Hashimoto, K.; Miyawaki, S. Antineutrophil cytoplasmic antibody in patients with primary sjogren’s syndrome. Clin. Rheumatol. 1999, 18, 268–271. [Google Scholar] [CrossRef] [PubMed]
- Ramos-Casals, M.; Nardi, N.; Brito-Zeron, P.; Aguilo, S.; Gil, V.; Delgado, G.; Bove, A.; Font, J. Atypical autoantibodies in patients with primary sjogren syndrome: Clinical characteristics and follow-up of 82 cases. Semin. Arthritis Rheum. 2006, 35, 312–321. [Google Scholar] [CrossRef] [PubMed]
- Moon, J.S.; Lee, D.D.; Park, Y.B.; Lee, S.W. Rheumatoid factor false positivy in patients with ANCA-associated vasculitis not having medical conditions producing rheumatoid factor. Clin. Rheumatol. 2018, 37, 2771–2779. [Google Scholar] [CrossRef] [PubMed]
- Van Vollenhoven, R.F. Sex differences in rheumatoid arthritis: More than meets the eye. BMC Med. 2009, 7, 12. [Google Scholar] [CrossRef] [PubMed]
| Patients (P) | Gender | CTD Diagnosis | Age at CTD | Delay * | CTD-Related Organ Involvement | IS ** | Nature of IS | IS at AAV Onset |
|---|---|---|---|---|---|---|---|---|
| P1 | Female | RA | 54 | 10 y | Joints | Yes | MTX | No |
| P2 | Female | RA | 72 | 2 y | Joints | Yes | Steroids | Yes (steroids) |
| P3 | Male | RA | 41 | 8 y | Joints | Yes | Steroids, MTX, anti-TNF | Yes (anti-TNF) |
| P4 | Female | RA | 65 | 6 y | Joints | Yes | Steroids, MTX, anti-TNF | Yes (anti-TNF) |
| P5 | Female | RA | 35 | 10 y | Joints | Yes | Steroids, MTX, anti-TNF | Yes (anti-TNF) |
| P6 | Female | Sarcoidosis | 57 | 9 y | Lung, nodes, salivary glands | Yes | Steroids | No |
| P7 | Male | SSc | 71 | 1 y | Skin, Sicca syndrome | No | / | No |
| P8 | Female | SSc + SS | 61 | 3 y | Skin, lung, Sicca syndrome | Yes | Steroids | Yes (steroids) |
| P9 | Female | SSc + SS | 49 | 6 y | Skin, joints, Raynaud, Sicca syndrome | No | / | No |
| P10 | Male | SS | 52 | 25 y | Lung | Yes | Steroids | No |
| P11 | Female | SLE | 44 | 5 y | Joints | Yes | Steroids, HCQ | Yes (steroids) |
| P12 | Female | PR | 74 | 3 y | Joints | Yes | Steroids, MTX | Yes (steroids+MTX) |
| P13 | Female | PR | 60 | 2 y | Joints | Yes | Steroids | Yes (steroids) |
| P14 | Female | PR | 74 | 1 y | Joints | Yes | Steroids | Yes (steroids) |
| P15 | Male | RP | 60 | 7 y | Joints, ear, nose | Yes | Steroids, MTX | Yes (steroids+MTX) |
| P16 | Female | PA | 50 | 14 y | Joints, skin | Yes | Methotrexate | No |
| Variables | All, n = 106 | Connective Tissue Diseases | ||
|---|---|---|---|---|
| Yes, n = 16 | No, n = 90 | p | ||
| Baseline characteristics | ||||
| Sex (M/F) | 67/39 | 4/12 | 63/27 | 0.001 |
| Age (years) | 63.4 ± 14.0 | 65 ± 10.3 | 63.1 ± 14.6 | 0.619 |
| ANCA-associated vasculitis characteristics | ||||
| Clinical diagnosis, n (%) | ||||
| GPA/MPA | 36 (34)/70 (66.6) | 7 (43.7)/9 (56.3) | 29 (32.2)/61 (67.8) | 0.37 |
| Newly diagnosed | 98 (92.4) | 15 (93.7) | 83 (92.2) | 0.831 |
| ANCA type, n (%) | ||||
| c-ANCA/p-ANCA, n (%) | 32 (30.2)/74 (69.8) | 7 (43.7)/9 (56.3) | 25 (27.8)/65 (72.2) | 0.2 |
| PR3-ANCA/MPO-ANCA, n (%) | 32 (30.2)/74 (69.8) | 7 (43.7)/9 (56.3) | 25 (27.8)/65 (72.2) | 0.2 |
| BVAS at kidney biopsy | 17.3 ± 5.7 | 15.6 ± 5.3 | 17.6 ± 5.8 | 0.2 |
| Organ involvement at diagnosis, n (%) | ||||
| Cutaneous signs | 20 (18.9) | 3 (18.7) | 17 (18.9) | 1 |
| Ear, nose, throat | 34 (32.1) | 6 (37.5) | 28 (31.1) | 0.772 |
| Heart | 6 (5.7) | 1 (6.2) | 5 (5.6) | 1 |
| Digestive | 4 (3.7) | 1 (6.2) | 3 (3.3) | 0.486 |
| Lung | 40 (37.7) | 4 (25) | 36 (40) | 0.401 |
| Renal (at kidney biopsy) | - | |||
| Serum creatinine, µmol/L | 350.2 ± 296 | 264.7 ± 188 | 364.9 ± 309 | 0.227 |
| eGFR, mL/min/1.73 m2 | 32.5 ± 34.7 | 35.0 ± 30.2 | 32.1 ± 35.5 | 0.766 |
| Need for renal replacement therapy, n (%) | 13 (12.3) | 0 (0.0) | 13 (15.5) | 0.21 |
| Neurological | 14 (13.2) | 2 (12.5) | 12 (13.3) | 0.928 |
| Induction therapy, n (%) | ||||
| Cyclophosphamide | 97 (91.5) | 14 (87.5) | 83 (92.2) | 0.663 |
| Rituximab | 3 (2.8) | 1 (6.2) | 2 (2.2) | - |
| Other | 6 (5.7) | 1 (6.2) | 5 (5.5) | - |
| Plasma exchange | 31 (29.2) | 5 (31.2) | 26 (28.8) | 0.848 |
| Connective Tissue Disease | |||
|---|---|---|---|
| Yes, n = 16 | No, n = 90 | p | |
| Antinuclear antibody | |||
| Screened patients, n (%) | 15 (93.7) | 80 (88.8) | 0.556 |
| Positives (≥ 1/100), n (%) | 12 (80) | 38 (47.5) | 0.021 |
| ≥ 1/200, n (%) | 8 (50) | 25 (31.2) | 0.036 |
| Antigen specificity, number of patients | 4 | 2 | / |
| Anti-SSA, n | 1 | 0 | / |
| Anti-SSB, n | 0 | 0 | / |
| Anti-centromere, n | 2 | 1 | / |
| Anti-mitochondrial, M2 subtype, n | 1 | 1 | / |
| Anti-GBM antibody | |||
| Screened patients, n (%) | 15 (93.7) | 71 (78.8) | 0.161 |
| Positives patients, n (%) | 1 (6.3) | 1 (1.1) | 0.32 |
| Cryoglobulin | |||
| Screened patients, n (%) | 8 (50) | 35 (38.9) | 0.402 |
| Positive, n (%) | 3 (37.5) | 2 (5.7) | 0.011 |
| Rheumatoid factor | |||
| Screened patients, n (%) | 5 (31.2) | 18 (20) | 0.314 |
| Positive, n (%) | 4 (80) | 7 (38.9) | 0.103 |
| Immunofluorescence Study | All, n = 106 | Connective Tissue Disease | ||
|---|---|---|---|---|
| Yes, n = 16 | No, n = 90 | p | ||
| IgG deposits | 0.12 ± 0.4 | 0 ± 0 | 0.14 ± 0.5 | 0.24 |
| ≥2+, n (%) | 2 (1.9) | 0 (0) | 2 (2.2) | 1 |
| IgA deposits | 0.14 ± 0.5 | 0 ± 0 | 0.17 ± 0.5 | 0.229 |
| ≥2+, n (%) | 2 (1.9) | 0 (0) | 2 (2.2) | 1 |
| IgM deposits | 0.89 ± 0.5 | 1 ± 0.5 | 0.86 ± 0.5 | 0.343 |
| ≥2+, n (%) | 7 (6.6) | 2 (12.5) | 5 (5.5) | 0.284 |
| C1q deposits | 0.2 ± 0.5 | 0.2 ± 0.4 | 0.17 ± 0.5 | 0.821 |
| ≥2+, n (%) | 1 (0.9) | 0 (0) | 1 (1.1) | 1 |
| C3 deposits | 0.63 ± 0.8 | 0.4 ± 0.7 | 0.67 ± 0.7 | 0.202 |
| ≥2+, n (%) | 11 (10.4) | 2 (12.5) | 9 (10) | 0.67 |
| Variables | All, n = 106 | Connective Tissue Disease | ||
|---|---|---|---|---|
| Yes, n = 16 | No, n = 90 | p | ||
| Relapses, all, n (%) | 23 (21.7) | 6 (37.5) | 17 (18.8) | 0.096 |
| Mean delay (months) | 43.6 ± 43.2 | 29.5 ± 30.1 | 46.1 ± 44.8 | 0.158 |
| Non-renal relapse, n (%) | 11 (10.4) | 4 (25) | 7 (7.7) | 0.037 |
| Mean delay (months) | 35.4 ± 39 | 24.6 ± 34 | 41.6 ± 42.9 | 0.518 |
| Renal relapse, n (%) | 12 (11.3) | 2 (12.5) | 10 (11.1) | 1 |
| Mean delay (months) | 49.1 ± 37.7 | 35.7 ± 12.3 | 51.7 ± 40.9 | 0.607 |
| Steroids | ||||
| Steroids at Month 6 | 12.5 ± 8.9 | 12.3 ± 4.9 | 12.5 ± 9.6 | 0.935 |
| Steroids at Year 1 | 5.5 ± 5.4 | 4.5 ± 4 | 5.7 ± 5.6 | 0.486 |
| Steroids at Year 2 | 3.4 ± 9.5 | 1.93 ± 2.9 | 3.7 ± 10.1 | 0.638 |
| Steroid withdrawal | 72 (67.9) | 12 (75.0) | 60 (66.6) | 0.753 |
| Mean delay | 18.4 ± 19.1 | 15.7 ± 15.3 | 19.0 ± 19.9 | 0.604 |
| Maintenance regimen, n (%) | ||||
| Azathioprine | 68 (64.2) | 10 (62.5) | 58 (64.4) | 0.881 |
| Rituximab | 33 (31.1) | 6 (37.5) | 27 (30) | 0.55 |
| Other | 5 (4.7) | 0 (0) | 5 (5.6) | - |
| Events | All, n = 106 | Connective Tissue Disease | ||
|---|---|---|---|---|
| Yes, n = 16 | No, n = 90 | p | ||
| Death, n (%) | 16 (15.1) | 2 (12.5) | 14 (15.5) | 1 |
| Mean delay (months) | 32 ± 28.2 | 37.8 ± 13.2 | 31.2 ± 29.8 | 0.767 |
| Renal function | ||||
| eGFR at year 1, mL/min/1.73 m2 | 42.1 ± 28.4 | 38.8 ± 27.3 | 42.6 ± 28.7 | 0.709 |
| eGFR at year 3, mL/min/1.73 m2 | 44.2 ± 32.6 | 55.1 ± 14.6 | 42.9 ± 34.1 | 0.435 |
| End-stage renal disease, n (%) | 33 (31.1) | 5 (31.3) | 28 (31.1) | 0.991 |
| Mean delay (months) | 19.5 ± 27.4 | 22.6 ± 20.6 | 18.9 ± 28.7 | 0.789 |
| Severe infectious events, n (%) | 48 (45.3) | 7 (43.7) | 41 (45.5) | 0.894 |
| Mean delay (months) | 23.8 ± 39.1 | 13.7 ± 16 | 25.7 ± 41.9 | 0.461 |
| Cardiovascular events, n (%) | 12 (11.3) | 1 (6.25) | 11 (12.2) | 0.688 |
| Mean delay (months) | 57.1 ± 56.1 | - | 62.3 ± 55.9 | - |
| Myocardial infarction, n (%) | 1 (0.9) | 1 (6.25) | 0 (0) | 0.151 |
| Stroke, n (%) | 2 (1.9) | 0 (0) | 2 (2.2) | 1 |
| Others, n (%) | 9 (8.5) | 0 (0) | 9 (10) | 0.349 |
| Cancer, n (%) | 13 (12.3) | 1 (6.2) | 12 (13.3) | 0.686 |
| Mean delay (months) | 40.9 ± 40 | - | 40.9 ± 40 | - |
| Solid cancer, n (%) | 6 (5.7) | 0 (0) | 6 (6.7) | 0.588 |
| Skin cancer, n (%) | 7 (6.6) | 1 (6.2) | 6 (6.7) | 0.934 |
| Thrombotic events, n (%) | 14 (14.2) | 5 (31.2) | 9 (10) | 0.021 |
| Mean delay (months) | 47.2 ± 11.9 | 17.1 ± 17.7 | 9.03 ± 16.4 | 0.461 |
| At least one event, n (%) * | 66 (62.3) | 11 (68.7) | 55 (61.1) | 0.302 |
| Mean follow-up (months) | 55.6 ± 52.2 | 40.6 ± 40.6 | 58.3 ± 53.8 | 0.213 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guibert, F.; Garnier, A.-S.; Wacrenier, S.; Piccoli, G.; Djema, A.; Gansey, R.; Demiselle, J.; Brilland, B.; Cousin, M.; Besson, V.; et al. Patients with ANCA-Associated Glomerulonephritis and Connective Tissue Diseases: A Comparative Study from the Maine-Anjou AAV Registry. J. Clin. Med. 2019, 8, 1218. https://doi.org/10.3390/jcm8081218
Guibert F, Garnier A-S, Wacrenier S, Piccoli G, Djema A, Gansey R, Demiselle J, Brilland B, Cousin M, Besson V, et al. Patients with ANCA-Associated Glomerulonephritis and Connective Tissue Diseases: A Comparative Study from the Maine-Anjou AAV Registry. Journal of Clinical Medicine. 2019; 8(8):1218. https://doi.org/10.3390/jcm8081218
Chicago/Turabian StyleGuibert, Fanny, Anne-Sophie Garnier, Samuel Wacrenier, Giorgina Piccoli, Assia Djema, Renaud Gansey, Julien Demiselle, Benoit Brilland, Maud Cousin, Virginie Besson, and et al. 2019. "Patients with ANCA-Associated Glomerulonephritis and Connective Tissue Diseases: A Comparative Study from the Maine-Anjou AAV Registry" Journal of Clinical Medicine 8, no. 8: 1218. https://doi.org/10.3390/jcm8081218





