Anti-Tumor Effects of Low Dose Zoledronate on Lung Cancer-Induced Spine Metastasis
Abstract
:1. Introduction
2. Results
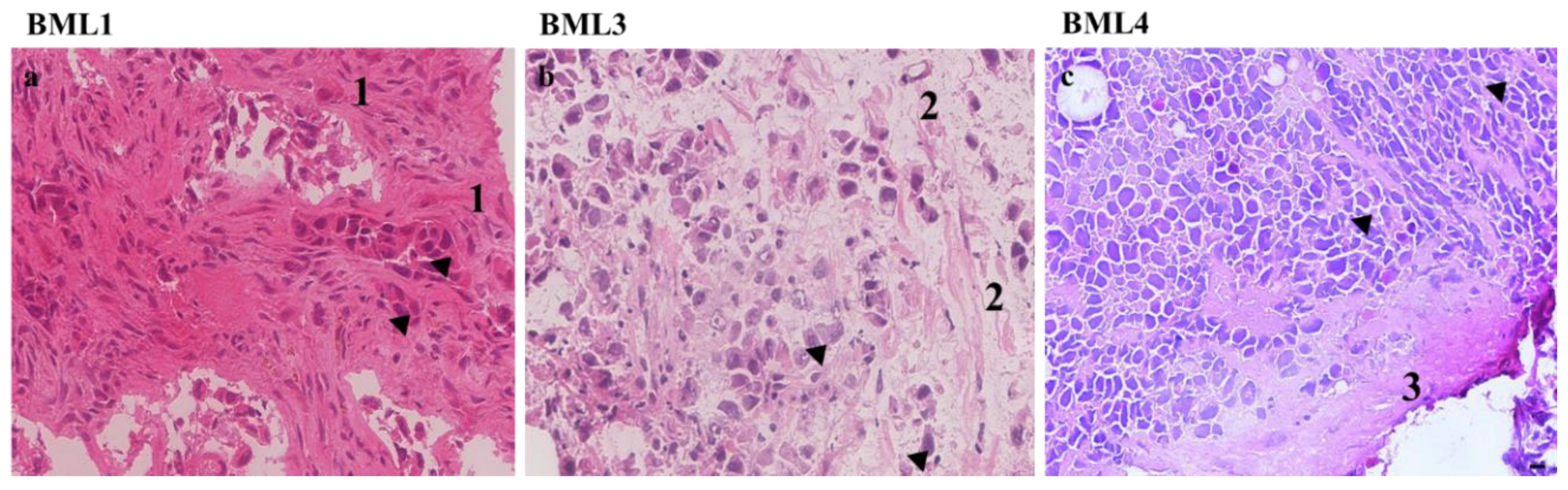
2.1. Analysis of Lung Cancer-Induced Bone Metastasis Tissues
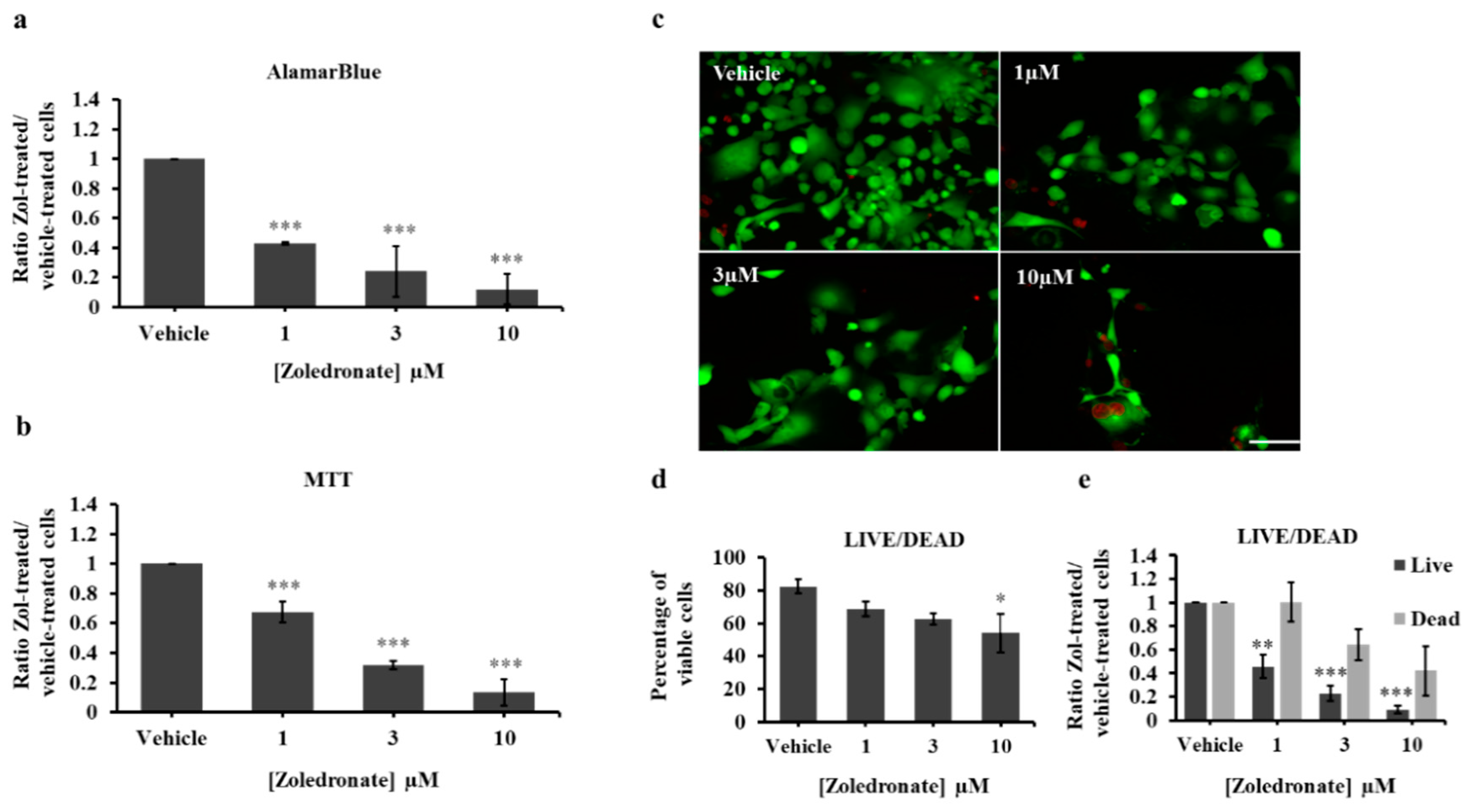
2.2. Zoledronate Decreases Lung Cancer Cell Line Proliferation
2.3. Zoledronate Decreases the Proliferation of Lung Cancer-Induced Bone Metastasis Cells
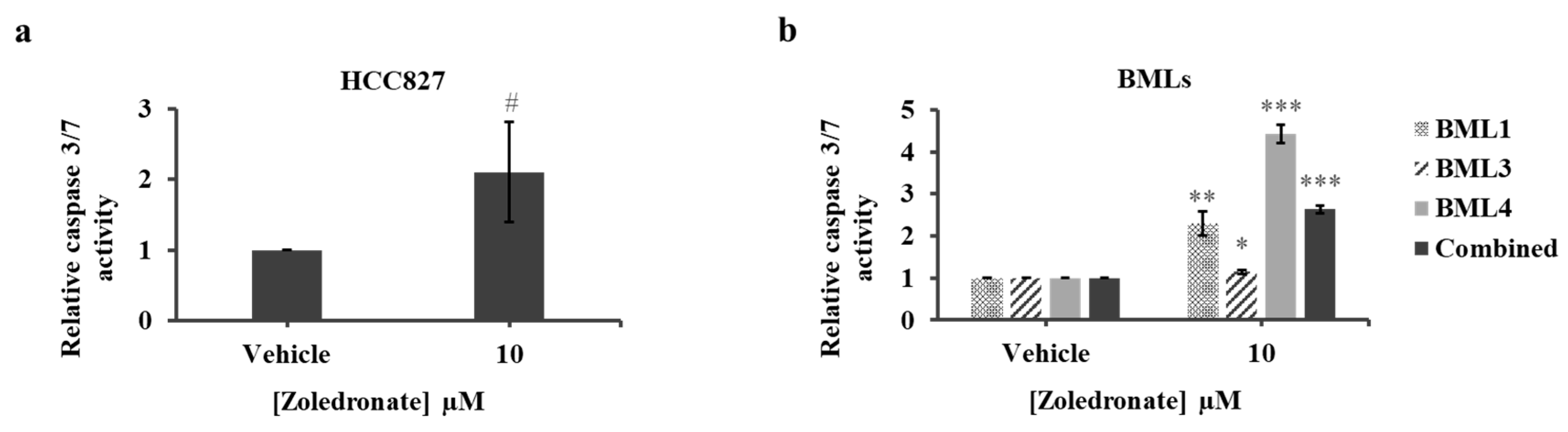
2.4. Zoledronate Increases Apoptosis of HCC827 and Lung Cancer-Induced Bone Metastasis Cells
2.5. Zoledronate Affects the Migration of HCC827 and Lung Cancer-Induced Bone Metastasis Cells
2.6. Zoledronate Affects the Invasion of HCC827 and Lung Cancer-Induced Bone Metastasis Cells
3. Discussion
4. Materials and Methods
4.1. Lung Cell Line and Lung Cancer-Induced Bone Metastasis Cells
4.2. Proliferation Assays
4.3. Live/Dead Cell Viability Assay
4.4. Caspase 3/7 Activity Assay
4.5. Transwell Cell Migration Assay
4.6. 3D Matrix Invasion Assay
4.7. Statistics
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer Statistics, 2017. CA Cancer J. Clin. 2017, 67, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Ferlay, J.; Soerjomataram, I.; Dikshit, R.; Eser, S.; Mathers, C.; Rebelo, M.; Parkin, D.M.; Forman, D.; Bray, F. Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. Int. J. Cancer 2015, 136, E359–E386. [Google Scholar] [CrossRef] [PubMed]
- PDQ® Adult Treatment Editorial Board. Non-Small Cell Lung Cancer Treatment (PDQ®): Health Professional Version. In PDQ Cancer Information Summaries; Bethesda: Rockville, MD, USA, 2019. [Google Scholar]
- Alberg, A.J.; Ford, J.G.; Samet, J.M. Epidemiology of lung cancer: ACCP evidence-based clinical practice guidelines (2nd edition). Chest 2007, 132, 29S–55S. [Google Scholar] [CrossRef] [PubMed]
- Tulunay, O.E.; Hecht, S.S.; Carmella, S.G.; Zhang, Y.; Lemmonds, C.; Murphy, S.; Hatsukami, D.K. Urinary metabolites of a tobacco-specific lung carcinogen in nonsmoking hospitality workers. Cancer Epidemiol. Prev. Biomark. 2005, 14, 1283–1286. [Google Scholar] [CrossRef] [PubMed]
- Berrington de Gonzalez, A.; Kim, K.P.; Berg, C.D. Low-dose lung computed tomography screening before age 55: Estimates of the mortality reduction required to outweigh the radiation-induced cancer risk. J. Med. Screen. 2008, 15, 153–158. [Google Scholar] [CrossRef] [PubMed]
- Raaschou-Nielsen, O.; Andersen, Z.J.; Beelen, R.; Samoli, E.; Stafoggia, M.; Weinmayr, G.; Hoffmann, B.; Fischer, P.; Nieuwenhuijsen, M.J.; Brunekreef, B.; et al. Air pollution and lung cancer incidence in 17 European cohorts: Prospective analyses from the European Study of Cohorts for Air Pollution Effects (ESCAPE). Lancet Oncol. 2013, 14, 813–822. [Google Scholar] [CrossRef]
- Guo, Y.; Zeng, H.; Zheng, R.; Li, S.; Barnett, A.G.; Zhang, S.; Zou, X.; Huxley, R.; Chen, W.; Williams, G. The association between lung cancer incidence and ambient air pollution in China: A spatiotemporal analysis. Environ. Res. 2016, 144, 60–65. [Google Scholar] [CrossRef] [PubMed]
- Shanker, M.; Willcutts, D.; Roth, J.A.; Ramesh, R. Drug resistance in lung cancer. Lung Cancer (Auckl) 2010, 1, 23–36. [Google Scholar] [PubMed]
- Gustafsson, B.I.; Kidd, M.; Chan, A.; Malfertheiner, M.V.; Modlin, I.M. Bronchopulmonary neuroendocrine tumors. Cancer 2008, 113, 5–21. [Google Scholar] [CrossRef] [PubMed]
- Pignon, J.P.; Arriagada, R.; Ihde, D.C.; Johnson, D.H.; Perry, M.C.; Souhami, R.L.; Brodin, O.; Joss, R.A.; Kies, M.S.; Lebeau, B.; et al. A meta-analysis of thoracic radiotherapy for small-cell lung cancer. N. Engl. J. Med. 1992, 327, 1618–1624. [Google Scholar] [CrossRef] [PubMed]
- Janne, P.A.; Freidlin, B.; Saxman, S.; Johnson, D.H.; Livingston, R.B.; Shepherd, F.A.; Johnson, B.E. Twenty-five years of clinical research for patients with limited-stage small cell lung carcinoma in North America. Cancer 2002, 95, 1528–1538. [Google Scholar] [CrossRef] [PubMed]
- Riihimaki, M.; Hemminki, A.; Fallah, M.; Thomsen, H.; Sundquist, K.; Sundquist, J.; Hemminki, K. Metastatic sites and survival in lung cancer. Lung Cancer 2014, 86, 78–84. [Google Scholar] [CrossRef] [PubMed]
- Coleman, R.E.; McCloskey, E.V. Bisphosphonates in oncology. Bone 2011, 49, 71–76. [Google Scholar] [CrossRef] [PubMed]
- Sciubba, D.M.; Goodwin, C.R.; Yurter, A.; Ju, D.; Gokaslan, Z.L.; Fisher, C.; Rhines, L.D.; Fehlings, M.G.; Fourney, D.R.; Mendel, E.; et al. A Systematic Review of Clinical Outcomes and Prognostic Factors for Patients Undergoing Surgery for Spinal Metastases Secondary to Breast Cancer. Glob. Spine J. 2016, 6, 482–496. [Google Scholar] [CrossRef] [PubMed]
- Santini, D.; Barni, S.; Intagliata, S.; Falcone, A.; Ferrau, F.; Galetta, D.; Moscetti, L.; La Verde, N.; Ibrahim, T.; Petrelli, F.; et al. Natural History of Non-Small-Cell Lung Cancer with Bone Metastases. Sci. Rep. 2015, 5, 18670. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsuya, A.; Kurata, T.; Tamura, K.; Fukuoka, M. Skeletal metastases in non-small cell lung cancer: A retrospective study. Lung Cancer 2007, 57, 229–232. [Google Scholar] [CrossRef] [PubMed]
- Singh, T.; Kaur, V.; Kumar, M.; Kaur, P.; Murthy, R.S.; Rawal, R.K. The critical role of bisphosphonates to target bone cancer metastasis: An overview. J. Drug Target. 2015, 23, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Nogues, X.; Martinez-Laguna, D. Update on osteoporosis treatment. Med. Clin. (Barc) 2018, 150, 479–486. [Google Scholar] [CrossRef] [PubMed]
- Garganta, M.D.; Jaser, S.S.; Lazow, M.A.; Schoenecker, J.G.; Cobry, E.; Hays, S.R.; Simmons, J.H. Cyclic bisphosphonate therapy reduces pain and improves physical functioning in children with osteogenesis imperfecta. BMC Musculoskelet. Disord. 2018, 19, 344. [Google Scholar] [CrossRef]
- Aapro, M.; Abrahamsson, P.A.; Body, J.J.; Coleman, R.E.; Colomer, R.; Costa, L.; Crino, L.; Dirix, L.; Gnant, M.; Gralow, J.; et al. Guidance on the use of bisphosphonates in solid tumours: Recommendations of an international expert panel. Ann. Oncol. 2008, 19, 420–432. [Google Scholar] [CrossRef]
- Coleman, R.E. Risks and benefits of bisphosphonates. Br. J. Cancer 2008, 98, 1736–1740. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, E.H.; Kim, M.S.; Lee, K.H.; Koh, J.S.; Jung, W.G.; Kong, C.B. Zoledronic acid is an effective radiosensitizer in the treatment of osteosarcoma. Oncotarget 2016, 7, 70869–70880. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Conry, R.M.; Rodriguez, M.G.; Pressey, J.G. Zoledronic acid in metastatic osteosarcoma: Encouraging progression free survival in four consecutive patients. Clin. Sarcoma Res. 2016, 6, 6. [Google Scholar] [CrossRef] [PubMed]
- Lacerna, L.; Hohneker, J. Zoledronic acid for the treatment of bone metastases in patients with breast cancer and other solid tumors. Semin. Oncol. 2003, 30, 150–160. [Google Scholar] [CrossRef] [PubMed]
- Akoury, E.; Ahangar, P.; Nour, A.; Lapointe, J.; Guerard, K.P.; Haglund, L.; Rosenzweig, D.H.; Weber, M.H. Low-dose zoledronate for the treatment of bone metastasis secondary to prostate cancer. Cancer Cell Int. 2019, 19, 28. [Google Scholar] [CrossRef]
- Selvaggi, G.; Scagliotti, G.V. Management of bone metastases in cancer: A review. Crit. Rev. Oncol. Hematol. 2005, 56, 365–378. [Google Scholar] [CrossRef] [PubMed]
- Umunakwe, O.C.; Herren, D.; Kim, S.J.; Kohanim, S. Diffuse ocular and orbital inflammation after zoledronate infusion-case report and review of the literature. Digit. J. Ophthalmol. 2017, 23, 18–21. [Google Scholar] [CrossRef]
- Bobyn, J.D.; McKenzie, K.; Karabasz, D.; Krygier, J.J.; Tanzer, M. Locally delivered bisphosphonate for enhancement of bone formation and implant fixation. J. Bone Jt. Surg. Am. 2009, 91, 23–31. [Google Scholar] [CrossRef]
- Miettinen, S.S.; Jaatinen, J.; Pelttari, A.; Lappalainen, R.; Monkkonen, J.; Venesmaa, P.K.; Kroger, H.P. Effect of locally administered zoledronic acid on injury-induced intramembranous bone regeneration and osseointegration of a titanium implant in rats. J. Orthop. Sci. 2009, 14, 431–436. [Google Scholar] [CrossRef]
- Cattalini, J.P.; Boccaccini, A.R.; Lucangioli, S.; Mourino, V. Bisphosphonate-based strategies for bone tissue engineering and orthopedic implants. Tissue Eng. Part B Rev. 2012, 18, 323–340. [Google Scholar] [CrossRef]
- Verron, E.; Pissonnier, M.L.; Lesoeur, J.; Schnitzler, V.; Fellah, B.H.; Pascal-Moussellard, H.; Pilet, P.; Gauthier, O.; Bouler, J.M. Vertebroplasty using bisphosphonate-loaded calcium phosphate cement in a standardized vertebral body bone defect in an osteoporotic sheep model. Acta Biomater. 2014, 10, 4887–4895. [Google Scholar] [CrossRef] [PubMed]
- Nooh, A.; Zhang, Y.L.; Sato, D.; Rosenzweig, D.H.; Tabaries, S.; Siegel, P.; Barralet, J.E.; Weber, M.H. Intra-tumor delivery of zoledronate mitigates metastasis-induced osteolysis superior to systemic administration. J. Bone Oncol. 2017, 6, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Akoury, E.; Weber, M.H.; Rosenzweig, D.H. 3D-Printed Nanoporous Scaffolds Impregnated with Zoledronate for the Treatment of Spinal Bone Metastases. MRS Adv. 2019, 4, 1245–1251. [Google Scholar] [CrossRef]
- Matsumoto, S.; Kimura, S.; Segawa, H.; Kuroda, J.; Yuasa, T.; Sato, K.; Nogawa, M.; Tanaka, F.; Maekawa, T.; Wada, H. Efficacy of the third-generation bisphosphonate, zoledronic acid alone and combined with anti-cancer agents against small cell lung cancer cell lines. Lung Cancer 2005, 47, 31–39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, J.W.; Hsieh, J.J.; Shen, Y.C.; Yeh, K.Y.; Wang, C.H.; Li, Y.Y.; Hsu, T. Bisphosphonate zoledronic acid enhances the inhibitory effects of gefitinib on EGFR-mutated non-small cell lung carcinoma cells. Cancer Lett. 2009, 278, 17–26. [Google Scholar] [CrossRef] [PubMed]
- Di Salvatore, M.; Orlandi, A.; Bagala, C.; Quirino, M.; Cassano, A.; Astone, A.; Barone, C. Anti-tumour and anti-angiogenetic effects of zoledronic acid on human non-small-cell lung cancer cell line. Cell Prolif. 2011, 44, 139–146. [Google Scholar] [CrossRef] [PubMed]
- Rosen, L.S.; Gordon, D.; Tchekmedyian, N.S.; Yanagihara, R.; Hirsh, V.; Krzakowski, M.; Pawlicki, M.; De Souza, P.; Zheng, M.; Urbanowitz, G.; et al. Long-term efficacy and safety of zoledronic acid in the treatment of skeletal metastases in patients with nonsmall cell lung carcinoma and other solid tumors: A randomized, Phase III, double-blind, placebo-controlled trial. Cancer 2004, 100, 2613–2621. [Google Scholar] [CrossRef]
- Saad, F.; Gleason, D.M.; Murray, R.; Tchekmedyian, S.; Venner, P.; Lacombe, L.; Chin, J.L.; Vinholes, J.J.; Goas, J.A.; Chen, B.; et al. A randomized, placebo-controlled trial of zoledronic acid in patients with hormone-refractory metastatic prostate carcinoma. J. Natl. Cancer Inst. 2002, 94, 1458–1468. [Google Scholar] [CrossRef]
- Carteni, G.; Bordonaro, R.; Giotta, F.; Lorusso, V.; Scalone, S.; Vinaccia, V.; Rondena, R.; Amadori, D. Efficacy and safety of zoledronic acid in patients with breast cancer metastatic to bone: A multicenter clinical trial. Oncologist 2006, 11, 841–848. [Google Scholar] [CrossRef]
- Ibrahim, A.; Scher, N.; Williams, G.; Sridhara, R.; Li, N.; Chen, G.; Leighton, J.; Booth, B.; Gobburu, J.V.; Rahman, A.; et al. Approval summary for zoledronic acid for treatment of multiple myeloma and cancer bone metastases. Clin. Cancer Res. 2003, 9, 2394–2399. [Google Scholar]
- Rosen, L.S.; Gordon, D.; Tchekmedyian, S.; Yanagihara, R.; Hirsh, V.; Krzakowski, M.; Pawlicki, M.; de Souza, P.; Zheng, M.; Urbanowitz, G.; et al. Zoledronic acid versus placebo in the treatment of skeletal metastases in patients with lung cancer and other solid tumors: A phase III, double-blind, randomized trial--the Zoledronic Acid Lung Cancer and Other Solid Tumors Study Group. J. Clin. Oncol. 2003, 21, 3150–3157. [Google Scholar] [CrossRef]
- Fidler, I.J. Critical determinants of metastasis. Semin. Cancer Biol. 2002, 12, 89–96. [Google Scholar] [CrossRef]
- Liotta, L.A.; Kohn, E.C. The microenvironment of the tumour-host interface. Nature 2001, 411, 375–379. [Google Scholar] [CrossRef]
- Kenessey, I.; Koi, K.; Horvath, O.; Cserepes, M.; Molnar, D.; Izsak, V.; Dobos, J.; Hegedus, B.; Tovari, J.; Timar, J. KRAS-mutation status dependent effect of zoledronic acid in human non-small cell cancer preclinical models. Oncotarget 2016, 7, 79503–79514. [Google Scholar] [CrossRef] [Green Version]
- Hiraga, T.; Williams, P.J.; Mundy, G.R.; Yoneda, T. The bisphosphonate ibandronate promotes apoptosis in MDA-MB-231 human breast cancer cells in bone metastases. Cancer Res. 2001, 61, 4418–4424. [Google Scholar]
- Hauschka, P.V.; Mavrakos, A.E.; Iafrati, M.D.; Doleman, S.E.; Klagsbrun, M. Growth factors in bone matrix. Isolation of multiple types by affinity chromatography on heparin-Sepharose. J. Biol. Chem. 1986, 261, 12665–12674. [Google Scholar]
- Pfeilschifter, J.; Mundy, G.R. Modulation of type beta transforming growth factor activity in bone cultures by osteotropic hormones. Proc. Natl. Acad. Sci. USA 1987, 84, 2024–2028. [Google Scholar] [CrossRef]
- Kostenuik, P.J.; Singh, G.; Suyama, K.L.; Orr, F.W. Stimulation of bone resorption results in a selective increase in the growth rate of spontaneously metastatic Walker 256 cancer cells in bone. Clin. Exp. Metastasis 1992, 10, 411–418. [Google Scholar] [CrossRef]
- Yin, J.J.; Selander, K.; Chirgwin, J.M.; Dallas, M.; Grubbs, B.G.; Wieser, R.; Massague, J.; Mundy, G.R.; Guise, T.A. TGF-beta signaling blockade inhibits PTHrP secretion by breast cancer cells and bone metastases development. J. Clin. Investig. 1999, 103, 197–206. [Google Scholar] [CrossRef]
- Mundy, G.R. Metastasis to bone: Causes, consequences and therapeutic opportunities. Nat. Rev. Cancer 2002, 2, 584–593. [Google Scholar] [CrossRef]
- Futamura, N.; Urakawa, H.; Arai, E.; Kozawa, E.; Ishiguro, N.; Nishida, Y. Hyaluronan synthesis inhibitor supplements the inhibitory effects of zoledronic acid on bone metastasis of lung cancer. Clin. Exp. Metastasis 2013, 30, 595–606. [Google Scholar] [CrossRef]
- Ishiguro, T.; Ohata, H.; Sato, A.; Yamawaki, K.; Enomoto, T.; Okamoto, K. Tumor-derived spheroids: Relevance to cancer stem cells and clinical applications. Cancer Sci. 2017, 108, 283–289. [Google Scholar] [CrossRef] [Green Version]
- Edmondson, R.; Broglie, J.J.; Adcock, A.F.; Yang, L. Three-dimensional cell culture systems and their applications in drug discovery and cell-based biosensors. Assay Drug Dev. Technol. 2014, 12, 207–218. [Google Scholar] [CrossRef]
- Jagdev, S.P.; Coleman, R.E.; Shipman, C.M.; Rostami, H.A.; Croucher, P.I. The bisphosphonate, zoledronic acid, induces apoptosis of breast cancer cells: Evidence for synergy with paclitaxel. Br. J. Cancer 2001, 84, 1126–1134. [Google Scholar] [CrossRef]
- Lin, J.H. Bisphosphonates: A review of their pharmacokinetic properties. Bone 1996, 18, 75–85. [Google Scholar] [CrossRef]
- Benyettou, F.; Alhashimi, M.; O’Connor, M.; Pasricha, R.; Brandel, J.; Traboulsi, H.; Mazher, J.; Olsen, J.C.; Trabolsi, A. Sequential Delivery of Doxorubicin and Zoledronic Acid to Breast Cancer Cells by CB [7]-Modified Iron Oxide Nanoparticles. ACS Appl. Mater. Interfaces 2017, 9, 40006–40016. [Google Scholar] [CrossRef]
- Ramanlal Chaudhari, K.; Kumar, A.; Megraj Khandelwal, V.K.; Ukawala, M.; Manjappa, A.S.; Mishra, A.K.; Monkkonen, J.; Ramachandra Murthy, R.S. Bone metastasis targeting: A novel approach to reach bone using Zoledronate anchored PLGA nanoparticle as carrier system loaded with Docetaxel. J. Control. Release 2012, 158, 470–478. [Google Scholar] [CrossRef]
- Koto, K.; Murata, H.; Sawai, Y.; Ashihara, E.; Horii, M.; Kubo, T. Cytotoxic effects of zoledronic acid-loaded hydroxyapatite and bone cement in malignant tumors. Oncol. Lett. 2017, 14, 1648–1656. [Google Scholar] [CrossRef] [Green Version]
- Lu, Y.; Li, M.; Li, L.; Wei, S.; Hu, X.; Wang, X.; Shan, G.; Zhang, Y.; Xia, H.; Yin, Q. High-activity chitosan/nano hydroxyapatite/zoledronic acid scaffolds for simultaneous tumor inhibition, bone repair and infection eradication. Mater. Sci. Eng. C Mater. Biol. Appl. 2018, 82, 225–233. [Google Scholar] [CrossRef]
- Ahangar, P.; Akoury, E.; Ramirez Garcia Luna, A.S.; Nour, A.; Weber, M.H.; Rosenzweig, D.H. Nanoporous 3D-Printed Scaffolds for Local Doxorubicin Delivery in Bone Metastases Secondary to Prostate Cancer. Materials 2018, 11, 1485. [Google Scholar] [CrossRef]
- Chen, M.; Le, D.Q.; Hein, S.; Li, P.; Nygaard, J.V.; Kassem, M.; Kjems, J.; Besenbacher, F.; Bunger, C. Fabrication and characterization of a rapid prototyped tissue engineering scaffold with embedded multicomponent matrix for controlled drug release. Int. J. Nanomed. 2012, 7, 4285–4297. [Google Scholar] [CrossRef] [Green Version]
- Jalani, G.; Naccache, R.; Rosenzweig, D.H.; Haglund, L.; Vetrone, F.; Cerruti, M. Photocleavable Hydrogel-Coated Upconverting Nanoparticles: A Multifunctional Theranostic Platform for NIR Imaging and On-Demand Macromolecular Delivery. J. Am. Chem. Soc. 2016, 138, 1078–1083. [Google Scholar] [CrossRef] [Green Version]
- Yang, N.; Chen, H.; Han, H.; Shen, Y.; Gu, S.; He, Y.; Guo, S. 3D printing and coating to fabricate a hollow bullet-shaped implant with porous surface for controlled cytoxan release. Int. J. Pharm. 2018, 552, 91–98. [Google Scholar] [CrossRef]
- Xue, W.; Bandyopadhyay, A.; Bose, S. Polycaprolactone coated porous tricalcium phosphate scaffolds for controlled release of protein for tissue engineering. J. Biomed. Mater. Res. B Appl. Biomater. 2009, 91, 831–838. [Google Scholar] [CrossRef]
- Rosenzweig, D.H.; Carelli, E.; Steffen, T.; Jarzem, P.; Haglund, L. 3D-Printed ABS and PLA Scaffolds for Cartilage and Nucleus Pulposus Tissue Regeneration. Int. J. Mol. Sci. 2015, 16, 15118–15135. [Google Scholar] [CrossRef] [Green Version]
- Hong, M.-H.; Hong, H.J.; Pang, H.; Lee, H.-J.; Yi, S.; Koh, W.-G. Controlled Release of Growth Factors from Multilayered Fibrous Scaffold for Functional Recoveries in Crushed Sciatic Nerve. ACS Biomater. Sci. Eng. 2018, 4, 576–586. [Google Scholar] [CrossRef]
- Fairag, R.; Rosenzweig, D.H.; Ramirez-Garcialuna, J.L.; Weber, M.H.; Haglund, L. Three-Dimensional Printed Polylactic Acid Scaffolds Promote Bone-like Matrix Deposition in vitro. ACS Appl. Mater. Interfaces 2019, 11, 15306–15315. [Google Scholar] [CrossRef]




© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Akoury, E.; Ramirez Garcia Luna, A.S.; Ahangar, P.; Gao, X.; Zolotarov, P.; Weber, M.H.; Rosenzweig, D.H. Anti-Tumor Effects of Low Dose Zoledronate on Lung Cancer-Induced Spine Metastasis. J. Clin. Med. 2019, 8, 1212. https://doi.org/10.3390/jcm8081212
Akoury E, Ramirez Garcia Luna AS, Ahangar P, Gao X, Zolotarov P, Weber MH, Rosenzweig DH. Anti-Tumor Effects of Low Dose Zoledronate on Lung Cancer-Induced Spine Metastasis. Journal of Clinical Medicine. 2019; 8(8):1212. https://doi.org/10.3390/jcm8081212
Chicago/Turabian StyleAkoury, Elie, Ana Sofia Ramirez Garcia Luna, Pouyan Ahangar, Xiaoya Gao, Pylyp Zolotarov, Michael H. Weber, and Derek H. Rosenzweig. 2019. "Anti-Tumor Effects of Low Dose Zoledronate on Lung Cancer-Induced Spine Metastasis" Journal of Clinical Medicine 8, no. 8: 1212. https://doi.org/10.3390/jcm8081212
APA StyleAkoury, E., Ramirez Garcia Luna, A. S., Ahangar, P., Gao, X., Zolotarov, P., Weber, M. H., & Rosenzweig, D. H. (2019). Anti-Tumor Effects of Low Dose Zoledronate on Lung Cancer-Induced Spine Metastasis. Journal of Clinical Medicine, 8(8), 1212. https://doi.org/10.3390/jcm8081212





